Simultaneous Reduction of CO2 and Brilliant Green Dye Removal Electrochemically in Bicarbonates of Sodium and Potassium Salts
- 1. Department of Chemical Engineering, Indian Institute of Technology Guwahati, India
ABSTRACT
A new approach of experimental studies for simultaneous CO2 reduction and Brilliant Green (BG) dye removal electrochemically was studied on Zinc (Zn) and Tin (Sn) cathodes and a common Cobalt oxide (Co3 O4 ) anode in 0.5M (10 ppm BG) KHCO3 , NaHCO3 electrolytes. Formic acid was the only product formed along with removal of BG dye was observed at different applied voltages. High Faradaic efficiencies were observed at low applied voltages with low BG dye removal. Conversely, high voltages were favorable for maximum dye removal with low HCOOH acid Faradaic efficiencies. For Sn as cathode maximum Faradaic efficiencies of HCOOH in NaHCO3 is obtained to be 76.3% (5 min) – 2 V, BG dye removal using Sn as electrocatalyst in NaHCO3 electrolytes was obtained to be 94.9 % (25 min) – 3.8 V. The present studies demonstrate the most effective process for conversion of atmospheric CO2 to HCOOH and the remedy for purification of impure water.
KEYWORDS
• CO2 reduction
• Faradaic efficiency
• Brilliant Green (BG) dye
• Tin (Sn) • Zinc (Zn)
• Formic acid (HCOOH)
CITATION
Yadav VSK, Purkait MK (2017) Simultaneous Reduction of CO2 and Brilliant Green Dye Removal Electrochemically in Bicarbonates of Sodium and Potassium Salts. Chem Eng Process Tech 3(1): 1033.
INTRODUCTION
Global warming effect was caused by the combustion of fossil fuels, which releases greenhouse gases during energy generation which causes great damage to the society. The different gases like methane, nitrous oxide, other halocarbons and CO2 were evolved during the process. Of which CO2 is the main contributor to the global warming effect [1-3]. There is a need to reduce these CO2 before it causes damage to the environment [4,5]. Several methods were in existence towards reduction of CO2 to different products, the main focus went on electrochemical CO2 reduction because of its several advantages [6,7]. The studies reported that reduction depends on different conditions like electrocatalyst, electrolytes, and applied voltages [8-10]. Irabein et al., studied the work on different copper based electrocatalysts for liquid product methanol generation with high efficiencies [35,36]. Copper is accepted as a best electrocatalyst in reducing CO2 to hydrocarbons, but due to multiple product formation the system becomes complex [11,12]. Some studies were done using Zn, Sn and Pb selectively form HCOOH as main product reduces the system complexity [13-17]. Lu et al., given a review in which discussed a reaction mechanism with effective experimental conditions on electrocatalyst type, reactor design and applied conditions for reduction of CO2 towards HCOOH formation electrochemically [18]. The effect of CO2 reduction to HCOOH was studied on synthesized lead oxide electrocatalyst at different applied voltages in KHCO3 and NaHCO3 electrolytes. Guerra et al., studied the continuous reduction process for HCOOH formation using a lead cathode in ambient conditions. It was reported that electrolyte type used for reaction medium may also improve the process. Mainly, ionic liquids, place a major role in future investigations as it has a high CO2 solubility and best electrochemical properties [20,37] Wang et al., studied the CO2 reduction on Sn electrocatalyst for HCOOH formation in microbial cell. It was reported that Sn loaded gas diffusion electrodes were favorable towards the process with low energy consumption with purification of water [21]. Synthesis of Sn and Zn electrocatalysts by electrodeposition method and their applications for electrochemical reduction of CO2 to HCOOH in sodium and potassium based salts at different applied voltages were reported [22,23]. Zhang et al., studied the effective CO2 reduction on different types of nano structured Sn electrocatalysts for HCOOH formation. High Faradaic efficiency for HCOOH formation from reduction of CO2 at low over potential on Pd-Pt electro catalyst particles were reported [24]. Peng et al., studied the simultaneous CO2 reduction and the methyl orange dye removal using nitrogen doped tin oxide, Pt and copper as electro catalysts [25,26]. The removal of BG dye removal along with CO2 reduction is extremely significant because dye water from textile industries can be used as a reactant for H+ generation instead of using pure water for oxidation reaction. This gives better solution for purification of water by removing the dye from the waste water by oxidation reaction at anode. Platinum was widely accepted as anode catalyst for water oxidation reaction to generate protons, however, in the present studies was done with Co3 O4 as an anode which is cheap alternate electro catalyst [27-30]. From the literature studies, it was clearly confirmed that bicarbonate based solutions were favorable for CO2 reduction due to high solubility of CO2 [14-17]. The present study reports for the first time on simultaneous CO2 reduction and BG dye removal in bicarbonate solutions using Sn and Zn as cathode with Co3 O4 anode. The effect of Zn and Sn electrocatalyst were studied separately for reduction and dye removal were studied with respect to time and different applied voltages. All studies were done using a 2-electrode glass cell and respective optimized results were reported clearly. The studies done here may be useful to initiate the process for CO2 reduction to HCOOH with water purification using the selected catalyst combination for future applications.
MATERIALS AND METHODS
Materials Graphite plates (1.5 x 2.5) cm2 as a base for catalyst from Sunrise Enterprises, Mumbai. NaHCO3 , KHCO3 , iso-propyl alcohol and Brilliant green dye - Merck, India, Nafion (5wt %) - DuPont, USA. Chemicals (without any purification) and deionized water were used here for all experimental studies.
Preparation of catalyst coated graphite electrodes for cathode and anode
The electrocatalysts were synthesized using electrodeposition method by applying a constant current between the two electrodes which were reported in our earlier studies [22,23]. The catalyst ink was prepared using synthesized electrocatalysts and coated on the graphite plates to prepare cathode and anode electrodes [22]. Adding 7.5 mg of the electrocatalysts in a binder solution [1:5 (nafion + IPA (Iso propyl alcohol) of 200 µl] and sonicated for 30 min to get catalyst ink. Further the prepared ink was coated on graphite plate surface at the 80o C (2hr) at 2 mg/ cm2 catalyst loading to form an electrode. The same procedure was followed for all different catalyst loading.
Electrochemical studies on electrochemical CO2 reduction and BG dye removal
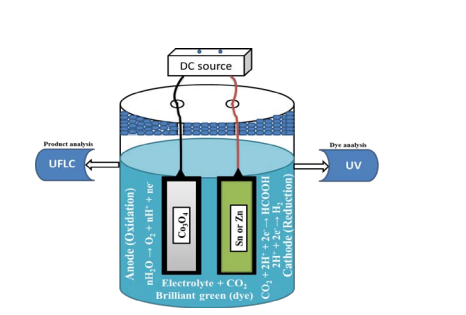
A 2-electrode glass cell was used to study the electrochemical CO2 reduction and BG dye removal. The setup used for the studies was shown in (Figure 1).
Figure 1: BG dye removal and CO2 reduction experimental setup.
80 ml [0.5 M (10 ppm MB)] solution was bubbled with CO2 for 50 min to get completely saturated CO2 electrolyte solution and used the solution for the whole reaction without further bubbling. The anode and cathode were dipped in an electrolyte solution which was connected to a DC source for energy source. The studies were done at different applied voltages of 2, 2.3, 2.5, 2.8, 3.3 and 3.8 V with each voltage at reaction times of 0-5, 10, 15, 20 and 25 min [19]. Faradaic efficiency was measured by using charge utilize for a product (HCOOH) to the total charge for the whole reaction.
FE= nFZ/Q, where n is moles of HCOOH formed, F-faradaic constant, Z is number of electrons involved in HCOOH formation reaction, Q-total charge of reaction
Analysis of product and BG dye analysis
Ultra-fast liquid chromatography (UFLC) - [Shimadzu LC20AD, UV-detector of deuterium lamp (SPD-20A)] is used at 205 nm wavelength for HCOOH analysis by using C-18 column (10X4 mm). 5mM Tetrabutyl ammonium hydrogen sulfate is used as mobile phase at 1 ml/min flow rate. MB dye removal analysis [UV-Visible Spectrophotometer (Perkin Elmer, Model: Lambda 35)].
RESULTS AND DISCUSSION
CO2 reduction and BG dye removal on Sn (cathode) and Co3O4 (anode) electrocatalysts
The reduction of CO2 and BG dye removal was studied using the Sn (cathode) and Co3 O4 (anode) electrocatalysts in bicarbonate based electrolytes. Maximum dye removal was observed along with higher Faradaic efficiencies for different applied voltages and the results were shown clearly. For all reaction studies, 0.5 M (10 ppm BG) concentration was used as electrolyte solution to conduct reaction.
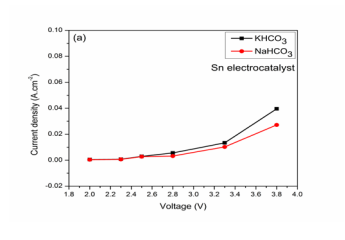
Current density on applied voltage using Sn cathode: Bases on the applied voltages, current densities were shown which corresponds to the rate of reaction. The results in different electrolyte solutions were shown in (Figure 2A).
Figure 2: (a) Effect on Voltage on Current density,
Current densities were observed to be increasing with increasing voltages. That shows that the reaction rate is more at higher applied voltages which may be due to CO2 reduction or hydrogen evolution. The effect of current density with different voltages shows the ability of selected electrocatalysts towards BG dye removal CO2 reduction. CO2 reduction and BG dye removal in KHCO3 solution: The experimental results in KHCO3 solution was shown in (Figure 2B).
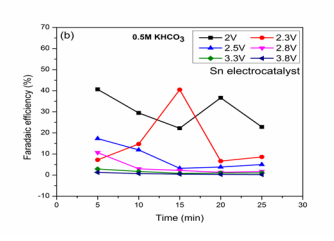
Figure (2b): FE (HCOOH) vs. Time
(Figure 2D).
Figure (2d): BG Removal (%) vs. Time on Sn electro catalyst.
For all applied voltages HCOOH was obtained as product with maximum dye removal. The reaction at 2V for reaction time of 5,10,15,20 and 25 min shows high efficiencies of 40.6, 29.4, 22.1, 36.6 and 22.8 % were obtained with dye removal (94.1, 94.6, 94.3, 94.3 and 94.2 %) were respectively. The variation in product concentrations with respect to time may due to the oxidation of HCOOH at anode [17]. At 2.3 V, Faradaic efficiencies (7.2, 14.7, 40.4, 6.64 and 8.6 %) and BG dye removal (93, 93.1, 93.1 and 93.44 %) were observed with the optimized condition for HCOOH formation is 40.4 % for reaction of 15 min. Lv et al., studied on HCOOH formation from reduction of CO2 on Sn electrode in KHCO3 solution without any dye using Pt anode [17]. For reaction at 2.5 V, the decrease in efficiencies may be due to the evolution of hydrogen at cathode due to excess proton generation at anode. At 2.8 V reactions, though high removal was observed, but efficiencies were reduced with the optimized condition of 10.6 % for 5 min reaction. Koleli et al., studied the reduction studies electrochemically on Sn electrocatalyst using potassium based electrolytes and reported the HCOOH at different applied voltages without any dye solution [14].Very low efficiencies were observed at higher voltages (3.3 and 3.8 V) with high BG dye removal. The high applied voltages were not favorable for CO2 reduction may be due to low CO2 molecule concentration than hydrogen ions [23]. High efficiencies were observed at 2V which is the most optimized condition for simultaneous CO2 reduction effective dye removal.
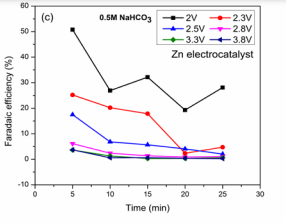
CO2 reduction and BG dye removal in NaHCO3 solution: Figure (2C)
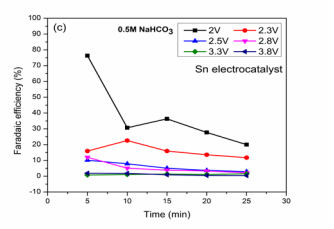
Figure (2c): FE (HCOOH) vs. Time
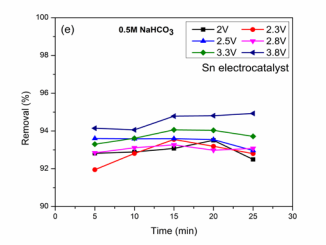
Figure (2e): BG Removal (%) vs. Time on Sn electro catalyst.
shows the most effective results in sodium based electrolyte solution. It was observed that high Faradaic efficiencies were obtained for HCOOH formation compared with Potassium based solution with maximum dye removal. This is due to the higher solubility of CO2 in this solution that participates in reduction reaction at the cathode surface than hydrogen evolution due to proton [15]. Faradaic efficiencies (76.3, 30.6, 36.3, 27.7 and 20.1 %) with BG removal of 92.8, 92.8, 93, 93.5 and 92.4 % were obtained at 2 V reactions. The optimized reaction for the maximum HCOOH formation is 76.3 % for reaction time 5 min. Chen et al., studied the effect of CO2 reduction on Sn based electrocatalyst in CO2 saturated NaHCO3 solution for HCOOH generation [15]. At 2.3 V, the variation of Faradaic efficiencies may be due to formed product oxidation at Co3 O4 which leads to hydrogen evolution at the cathode [22,31]. At 3.3 V, low Faradaic efficiencies of 0.78, 1.05, 1.3, 1.1 and 1.5 % (Figure 2C) with simultaneous BG removal 93.3, 93.6, 94.06, 94.03 and 93.7 % were obtained. Zhang et al., studied the reduction process for HCOOH formation on nano Sn electrocatalysts using sodium based electrolyte [32]. The low efficiencies for HCOOH may be due to high hydrogen evolution at Sn electrode [19]. Faradaic efficiency (1.89, 1.8, 0.9, 0.46 and 0.39 %), BG removal (94.1, 94.06, 94.7, 94.8 and 94.9 %) were shown for reaction at 3.8 V. The optimized condition was 5 min reaction at which maximum efficiency of 1.89 % was obtained. The efficiencies were high in sodium based solution with effective reduction in low applied voltages and dye removal.
CO2 reduction and BG dye removal on Zn (cathode) and Co3O4 (anode) electrocatalysts
The simultaneous CO2 reduction with BG dye removal was studied using Zn electrocatalyst as cathode. The studies were done in bicarbonate electrolyte solutions at different applied voltages to study the effective, optimized conditions towards maximum CO2 reduction along with dye removal.
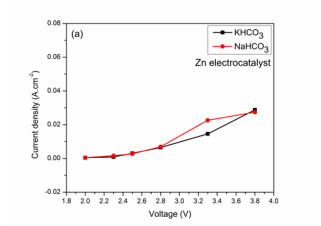
Current density on applied voltage using Zn cathode:The results for current density with respect to applied voltages for HCOOH Faradaic efficiencies in both electrolyte solutions were shown in (Figure 3). In particular the Current densities were increasing with applied voltage increase as shown in (Figure 3A).
Figure 3: (a) Effect on Voltage on Current density,
Electrolyte concentration of 0.5 M (10 ppm CV) was used to conduct electrochemical studies for all applied conditions. The current densities represent high reaction rates with maximum in NaHCO3 electrolyte solution than KHCO3 .
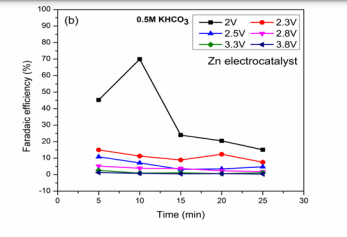
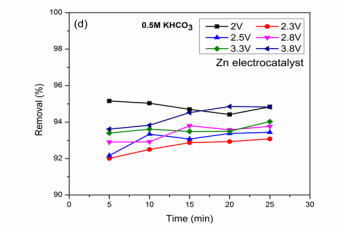
CO2 reduction and BG dye removal in KHCO3 solution:The reaction in potassium based solutions was shown in (Figure 3B).
Figure 3: (b ) FE (HCOOH) vs. Time
(Figure 3D).
Figure 3:(d ), BG Removal (%) vs. Time on Zn electrocatalyst.
Hori et al., studied the reduction of CO2 for HCOOH formation on Zn electrocatalyst in KHCO3 solution using Pt anode [31]. In all applied voltages, HCOOH was observed as an only product with maximum BG removal at the different reactions of 5, 10, 15, 20 and 25 min. At 2 V, maximum efficiencies were obtained at this voltage which is a clear sign for the best reaction condition. The change in Faradaic efficiencies with time in (Figure 3c).
Figure 3:(c) FE (HCOOH) vs. Time
(Figure 3e).
Figure 3: (e) BG Removal (%) vs. Time on Zn electrocatalyst.
may be due to oxidation of the product (HCOOH) at a anode [17,22]. For reaction at 2.3 V, The optimized condition at this voltage is 15.01 % for 5 min reaction. Faradaic efficiencies [(10.8, 7.1, 3.2, 3.5 and 4.7 %), (5.1, 3.8, 3.7, 2.3 and 1.7)] were obtained at applied voltages of 2.5 and 2.8 V with BG removal [(92.1, 93.3, 93, 93.3 and 93.4 %), (92.9, 92.9, 93.8, 93.5 and 93.7 %)] respectively. Low efficiencies were observed at these voltages may be due to high hydrogen evolution at Zn electrode [33]. At 3.3, 3.8 V, very low efficiencies were seen compared with low applied voltages which may not be a better reaction condition towards CO2 reduction in HCOOH formation. Electrochemical studies for HCOOH as product in CO2 reduction on Zn electrocatalyst without dye in potassium salts were reported [22]. From the results it may be concluded that high applied voltages were not favorable for maximum HCOOH efficiencies. The result proves the capability of electrocatalyst towards CO2 reduction to HCOOH and maximum dye removal.
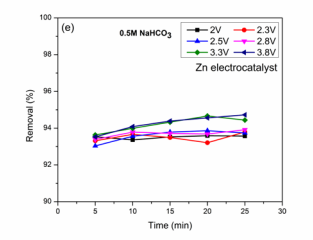
CO2 reduction and BG dye removal in NaHCO3 solution: The electrochemical studied results in NaHCO3 electrolyte solution were shown in (Figure 2C,2E). High efficiencies were observed at low applied voltages compared with higher voltages. Faradaic efficiencies (50.7, 26.9, 32.1, 19.3 and 28.1 %), BG dye removal (93.5, 93.3, 93.5, 93.5 and 93.5 %) were obtained at reaction time of 2 V. The optimized condition towards the maximum HCOOH formation is 50.7 % for reaction time of 5 min. The results show that with reaction times, efficiencies were changing may be due to HCOOH oxidation at anode [17,19]. Jin et al., studied the effect of CO2 reduction on Zn electrocatalyst for HCOOH as product was reported [34]. At 2.3 V, low efficiencies were observed when compared with 2 V which may be due to high proton generation at Co3 O4 anode which converts into hydrogen at the cathode [31]. The reactions at 2.5 V, the optimized reaction condition are 17.4 % after a reaction time of 5 min for maximum HCOOH efficiency. HCOOH as product from CO2 reduction has been reported in KHCO3 solution without dye on Zn electrocatalyst at different applied voltages [22]. Low Faradaic efficiencies were obtained at a applied voltage of 2.8 V (Figure 2D). The decrease in efficiencies may be due to low CO2 reactant concentration molecules at cathode compared with protons [31]. Very low Faradaic efficiencies were obtained in 3.3 and 3.8 V may be due to high hydrogen evolution at the cathode surface [31]. The experimental results for CO2 reduction and BG dye removal for conditions were discussed and optimized conditions for HCOOH Faradaic efficiencies and BG dye removal for different reaction times with applied voltages were shown in (Table 1).
Table 1: Maximum Faradaic efficiencies for HCOOH at different applied conditions.
|
Voltage |
Maximum Faradaic efficiency (time) |
|||||||
|
Sn |
Zn |
|||||||
|
KHCO3 |
NaHCO3 |
KHCO3 |
NaHCO3 |
|||||
|
(V) |
(%) |
(min) |
(%) |
(min) |
(%) |
(min) |
(%) |
(min) |
|
2 |
40.6 |
5 |
76.3 |
5 |
69.9 |
10 |
50.7 |
5 |
|
2.3 |
40.4 |
15 |
22.5 |
10 |
15 |
5 |
25.2 |
5 |
|
2.5 |
11.9 |
10 |
10.1 |
5 |
10.8 |
5 |
17.4 |
5 |
|
2.8 |
10.6 |
5 |
11.9 |
5 |
5.1 |
5 |
6.1 |
5 |
|
3.3 |
2.8 |
5 |
1.5 |
25 |
2.5 |
5 |
3.6 |
5 |
|
3.8 |
1.2 |
5 |
1.89 |
5 |
1.2 |
5 |
3.7 |
5 |
(Table 2).
Table 2: Maximum BG dye removal at different applied conditions.
|
Voltage |
BG removal (time) |
|||||||
|
Sn |
Zn |
|||||||
|
KHCO3 |
NaHCO3 |
KHCO3 |
NaHCO3 |
|||||
|
(V) |
(%) |
(min) |
(%) |
(min) |
(%) |
(min) |
(%) |
(min) |
|
2 |
94.6 |
15 |
93.5 |
20 |
95.1 |
5 |
93.58 |
20 |
|
2.3 |
93.4 |
25 |
93.5 |
15 |
93 |
25 |
93.7 |
25 |
|
2.5 |
94 |
10 |
93.6 |
5 |
93.4 |
25 |
93.8 |
20 |
|
2.8 |
93.9 |
10 |
93.2 |
20 |
93.8 |
15 |
93.9 |
25 |
|
3.3 |
94.17 |
15 |
94.06 |
15 |
94 |
25 |
94.6 |
20 |
|
3.8 |
94.6 |
25 |
94.9 |
25 |
94.85 |
20 |
94.7 |
25 |
For all the cases, low voltages were shown high Faradaic efficiencies for HCCOH formation.
CONCLUSION
A novel method for maximum BG dye removal and HCOOH Faradaic efficiencies were shown on Zn and Sn electrocatalysts with Co3 O4 (anode). In both KHCO3 and NaHCO3 solutions HCOOH was formed as the only product for CO2 reduction. Irrespective of applied voltages maximum BG dye removal was observed in all conditions for both (Zn and Sn) as electrocatalysts. HCOOH formation is favorable at low applied voltages compared with higher voltages. Maximum Faradaic efficiency of 76.3 % (5min) –2 V, BG dye removal 94.9 % (25 min) – 3.8 V for Sn electrocatalysts in NaHCO3 electrolyte solutions. For Zn as electrocatalyst, maximum Faradaic efficiency of 69.9 % (10 min)-2 V, BG dye removal 95.1 % (5 min)-2 V in KHCO3 electrolyte solution. The studies will be helpful in water purification with maximum CO2 reduction for our future energy applications.
REFERENCES
- Li L, Zhao N, Wei W, Sun Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel. 2013; 108: 112-130.
- Shafiei S, Salim Ra. Non-renewable and renewable energy consumption and CO2 emissions in OECD countries: A comparative analysis. Energy Policy. 2014; 66: 547-556.
- Kone AC, Buke T. Forecasting of CO2 emissions from fuel combustion using trend analysis, Renew Sust Energ Rev. 2010; 14: 2906-2915.
- An L, Chen R. Direct formate fuel cells: A review. J Power Sources. 2016; 320: 127-139.
- Surya Prakash GK, Viva FA, Olah GA. Electrochemical reduction of CO2 over Sn-Nafion coated electrode for a fuel-cell-like device. J Power Sources. 2013; 223: 68-73.
- Kim B, Hillman F, Ariyoshi M, Fujikawa S, Kenis PJA. Effects of composition of the micro porous layer and the substrate on performance in the electrochemical reduction of CO2 to CO. J Power Sources. 2015; 312: 192-198.
- Centi G, Quadrelli EA, Perathoner S. Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ Sci. 2013; 6: 1711-1731.
- Jitaru M. Electrochemical carbon dioxide reduction - fundamental and applied topics (review). J Univ Che. Technol Metallurgy. 2007; 42: 333-344.
- Jia F, Yu X, Zhang L. Enhanced selectivity for the electrochemical reduction of CO2 to alcohols in aqueous solution with nanostructure Cu-Au alloy as catalyst. J Power Sources. 2014; 252: 85-89.
- Ganesh I. Conversion of carbon dioxide into methanol – a potential liquid fuel?: Fundamental challenges and opportunities (A review ). Renew Sust Energ Rev. 2014; 31: 221–257.
- Kas R, Kortlever R, Milbrat A, Koper MTM, Mul G, Baltrusaitis J. Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: controlling the catalytic selectivity of hydrocarbons. Phys Chem Chem Phys. 2014; 16: 12194–12201.
- Ren D, Deng Y, Handoko AD, Chen CS, Malkhandi S, Yeo BS. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper (I) Oxide Catalysts. ACS Catalysis. 2015; 5: 2814–2821.
- Bumroongsakulsawat P, Kelsall GH. Effect of solution pH on CO: formate formation rates during electrochemical reduction of aqueous CO2 at Sn cathodes. Electrochim Act. 2014; 141: 216–225.
- Koleli F, Atilan T, Palamut N, Gizir AM, Aydin R, Hamann CH. Electrochemical reduction of CO2 at Pb- and Sn-electrodes in a fixed-bed reactor in aqueous K2CO3 and KHCO3 media. 2003: 447–450.
- Chen Y, Kanan MW. Tin Oxide Dependence of the CO2 Reduction Efficiency on Tin Electrodes and Enhanced Activity for Tin/Tin Oxide Thin-Film Catalysts. 2012; 143: 1986-1989.
- Innocent B, Liaigre D, Pasquier D, Ropital F, Leger JM, Kokoh KB. Electro-reduction of carbon dioxide to formate on lead electrode in aqueous medium. J Appl Electrochem. 2008; 39: 227–232.
- Lv W, Zhang R, Gao P, Lei L. Studies on the faradaic efficiency for electrochemical reduction of carbon dioxide to formate on tin electrode. J Power Sources. 2014; 253: 276–281.
- Lu X, Leung DYC, Wang H, Leung MKH, Xuan J. Electrochemical Reduction of Carbon Dioxide to Formic Acid. ChemElectroChem. 2014; 1: 836–849.
- Yadav VSK, Purkait MK. Synthesis of Pb2O electrocatalyst and its application in the electrochemical reduction of CO2 to HCOOH in various electrolytes. RSC Adv. 2015; 5: 40414–40421.
- Alvarez-Guerra M, Quintanilla S, Irabien A. Conversion of carbon dioxide into formate using a continuous electrochemical reduction process in a lead cathode. Chem Eng J. 2012; 207-208: 278–284.
- Wang Q, Dong H, Yu H, Yu H, Liu M. Enhanced electrochemical reduction of carbon dioxide to formic acid using a two-layer gas diffusion electrode in a microbial electrolysis cell. RSC Adv. 2015; 5: 10346–10351.
- Yadav VSK, Purkait MK. Electrochemical reduction of CO2 to HCOOH using zinc and cobalt oxide as electrocatalysts. New J Chem. 2015; 39: 7348-7354.
- Yadav VSK, Purkait MK. Electrochemical reduction of CO2 to HCOOH on a synthesized Sn electrocatalyst using Co3O4 anode. RSC Adv. 2015; 5: 68551-68557.
- Kortlever R, Peters I, Koper S, Koper MTM. Electrochemical CO2 Reduction to Formic Acid at Low Overpotential and with High Faradaic Efficiency on Carbon-Supported Bimetallic Pd–Pt Nanoparticles. ACS Catalysis. 2015; 5: 3916–3923.
- Peng Y, Ta Y, Yen P, Huang CP. A solar cell driven electrochemical process for the concurrent reduction of carbon dioxide and degradation of azo dye in dilute KHCO3 electrolyte. Sep Purif Technol. 2013; 117: 3–11.
- Peng YP, Yeh YT, Shah SI, Huang CP. Concurrent photoelectrochemical reduction of CO2 and oxidation of methyl orange using nitrogen-doped TiO2. Appl Catal B: Environ. 2012; 123-124: 414–423.
- Yang J, Walczak K, Anzenberg E, Toma FM, Yuan G, Schwartzberg A, et al. Efficient and Sustained Photoelectrochemical Water Oxidation by Cobalt Oxide/Silicon Photoanodes with Nanotextured Interfaces. J Am Chem Soc. 2014; 136: 6191–6194.
- Deng X, Tuysuz H. Cobalt-Oxide-Based Materials as Water Oxidation Catalyst: Recent Progress and Challenge. 2014; 4: 3701-3714.
- Han A, Wu H, Sun Z, Jia H, Du P. Facile deposition of nanostructured cobalt oxide catalysts from molecular cobaloximes for efficient water oxidation. Phys Chem Chem Phys.? PCCP. 2013; 15: 12534–12538.
- Blakemore JD, Gray HB, Winkler JR, Mu AM. Co3O4 Nanoparticle Water-Oxidation Catalysts Made by Pulsed-Laser Ablation in Liquids. ACS Catalysis. 2013; 3: 2497–2500.
- Hori C, Kikuchi SK, Shin FC. Production of co and ch4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogen carbonate solution. Chem Soc Japan. 1985; 1695–1698.
- Zhang S, Kang P, Meyer TJ. Nanostructured Tin Catalysts for Selective Electrochemical Reduction of Carbon Dioxide to Formate. J Am Chem Soc. 2014; 136: 1734-1737.
- Wu J. Sharma PP, Harris BH, Zhou XD. Electrochemical reduction of carbon dioxide: IV dependence of the Faradaic efficiency and current density on the microstructure and thickness of tin electrode. J Power Sources. 2014; 258: 189–194.
- Jin F, Zeng X, Liu J, Jin Y, Wang L, Zhong, H. Highly efficient and autocatalytic H? dissociation for CO? reduction into formic acid with zinc. Sci Rep. 2014; 4; 4503.
- Albo J, Saez A, Solla-Gullon J, Montiel V, Irabien A. Production of methanol from CO2 electro reduction at Cu2O andCu2O/ZnO-based electrodes in aqueous solution. Appl Catal B: Environ. 2015; 176–177; 709–717.
- Albo J, Irabien A. Cu2O-loaded gas diffusion electrodes for the continuous electrochemical reduction of CO2 to methanol. J Catal. 2016; 343: 232–239.
- Alvarez-Guerra M, Albo J, Alvarez-Guerra E, Irabien A. Ionic liquids in the electrochemical valorization of CO2. Energy Environ Sci. 2015; 8: 2574-2599.