Sustainable Optimized Procedures for Bioactive Phytochemicals from Turmeric using Deep Eutectic Solvents and their Activities and Applications
- 1. Former Professor of AcSIR and Chief Scientist, CSIR-Central Food Technological Research Institute, India
Abstract
Turmeric is a root spice consumed owing to its colour, bioactivity and flavour. It contains curcuminoids as major bioactive phytochemicals. Their sensitivity to light and poor aqueous solubility restricts application. Deep eutectic solvents are produced by uniting hydrogen bond donor and hydrogen bond acceptor. Natural deep eutectic solvents (NADES) are formed from primary metabolites. These are considered as green solvents. Extraction and estimation periods are reduced with the use of DES / NADES. Activity coefficients of curcuminoids in DESs are one of the important factors to determine the extractability and solubility in respective solvents. Solubility of bioactive molecules in the aqueous NADES is increased. The stability of bioactive compounds to light is increased due to NADES intervention. Solvent volumes for processing are decreased. Mechanisms of DES / NADES extractions and analytical methods indicate the reasons for their higher efficiencies. Ultimately energy is saved as the periods and volume of NADES usage is decreased. Bioavailability of bioactive phytochemicals is increased in NADES extracts. Turmeric extracts from NADES could be used directly in pharmaceutical products and functional foods, as the side effects and toxicity are minimal than the extracts from organic solvents. Turmeric NADES extracts are applied in antimicrobial photodynamic therapy, edible food packaging, antidiabetic drugs adsorption, textile dyeing, and trapping of methylglyoxal. Studies on the use of extract from NADES as drug carriers are initiated. Scale up studies on extraction is commenced. DES / NADES as alternate solvents in turmeric processing for various purposes is increasing gradually 3.
Highlights
1. DES can become excellent sustainable solvents for processing of turmeric.
2. Periods and quantities of substrates are reduced for extraction and estimation of curcuminoids.
3. Stability, solubility and bioavailability of the curcuminoids in these extracts is increased.
4. Energy is saved for extraction owing to shorter periods and reduced volumes.
5. Applications are improving due to the increased stability of bioactive phytochemicals.
Keywords
• Turmeric; Curcuma Longa; Deep Eutectic Solvents; Curcuminoids; Bioactive Compounds; Extraction; Analysis; Solubility; Stability; Bioactivity; Bioavailability; Applications Drug Delivery.
Citation
Jaganmohanrao L (2025) Sustainable Optimized Procedures for Bioactive Phytochemicals from Turmeric using Deep Eutectic Solvents and their Activities and Applications. Chem Eng Process Tech 10(3): 1106.
ABBREVIATIONS
AT: Ambient temperature; BDMC: Bisdemethoxycurcumin; CA: Citric Acid; ChCl: Choline Chloride; COSMOS: RS: Conductor-like Screening Model for Realistic Solvents; CUR: Curcumin; DES: Deep Eutectic Solvent; DFT: Density Functional Theory; DLLME SFO: Dispersive liquid-liquid microextraction based on solidification of floating organic drop; DLS: Dynamic Light Scattering study; DMC: Demethoxycurcumin; DPPH 2,2: Diphenyl-1-picrylhydrazyl radical; EFs: Enrichment Factors; ESP: Electrostatic Potential Analysis; FRAP: Ferric Reducing Antioxidant Power; FT-IR: Fourier-transform Infrared Spectroscopy; HD: Hydro Distillation; HPLC: High Performance Liquid Chromatography; LA: Lactic acid; LOD: Limit of Detection; LOQ: Limit of Quantification;MAE: Microwave Assisted Extraction; MAHD: Microwave-assisted Hydro Distillation; M.P.: Microwave Power; NADES: Natural deep eutectic solvent; PEG: Polyethylene Glycol; RS-DES-DLLME: Reusable and Switchable DESs: based Dispersive Liquid-Liquid Microextraction; SEM: Scanning Electron Microscopy; SeS2 NPS: Preparation of Selenium sulphide Nano Particles; S/L-Solid Liquid Ratio; SPE: Solid Phase Extraction; STLLME: Solvent-terminated liquid-liquid Microextraction; Temp.: Temperature; TFC: Total Flavonoids content; UAE: Ultrasonication Assisted Extraction; U.temp: Ultrasonic bath Temperature; U.time: Ultrasonic Extraction Time; UNIQUAC: Universal Quasi-Chemical; U.P.: Ultrasound Power; UPLC-MS-MS: Ultra Performance liquid Chromatography-tandem Mass Spectrometry; UV Vis: Ultra Violet and Visible Spectroscopy; VA-DES-ELLME: Vortex assisted Deep Eutectic Solvent Emulsification liquid-liquid microextraction; VA-DES-ME: Vortex assisted Deep Eutectic Solvent Microextraction; VA: Vortex Assisted; WSFME: Water and surfactant free micro emulsion.
INTRODUCTION
Turmeric (curcuma longae) is one of the major root spice consumed throughout the globe for its biological and pharmaceutical activities, flavour, colour and nutrition. It is used in the kitchen for cooking since hundreds of years. Turmeric powder imparts colour and flavour to the food. Turmeric is obtained from rhizomes of plant Curcuma longa, a member of the Zingiberaceae family. Role of turmeric in traditional medicinal systems (i.e., ayurveda, siddha, and unani) is noted since ancient days to treat gynaecological problems, gastric problems, hepatic disorders, cough, sore throat, respiratory ailments, infectious diseases, and blood disorders [1,2]. Turmeric may fight against human immunodeficiency viruses (HIV) / acquired immune deficiency syndrome (AIDS). Antimicrobial, antimutagenic, anti-cancer, insecticidal, larvicidal, and radio protector properties of turmeric are revealed in cell studies.
Curcuminoids (yellow in colour), which include mainly curcumin (diferuloylmethane / curcumin - CUR), demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC) are major components of turmeric solvent extracts [1-3]. Other classes of components reported are starch, essential elements, proteins, vitamins, volatile oils, polyphenols, alkaloids, diterpenes, sesquiterpenes, triterpenoids, and sterols. Pleiotropic curcumin and its analogues show strong anti-oxidative, anti inflammatory (viz., reduced white blood cell, neutrophil, eosinophil numbers, caring properties on serum levels of inflammatory mediators and total protein in various inflammatory disorders), anticancer, and antiandrogenic effects due to their molecular structure [4]. These effects are paramount when curcumin is united with selected molecules (viz., carbohydrates and piperine) as these increases its bioavailability [5]. Curcumin enhances the antioxidant enzymes activities (i.e., superoxide dismutase, catalase, glutathione peroxidase and heme oxygenase-1) and control the level of lipid peroxidation and reduce the oxidative damage in the tissues. Curcumin can moderate multiple cellular targets. Curcumin found to be chemo preventive and possesses therapeutic potential. Pharmacological activities (in vivo and in vitro) made turmeric as valuable drug and helpful in the treatment of several diseases from cancer to Alzheimer's disease [6,7]. Turmeric extracts and curcumin provide therapeutic effect on respiratory, allergy, immunological problems, metabolic syndrome, arthritis, anxiety, hyperlipidaemia and management of muscle pain and inflammation [8]. Anticancer activity of curcumin is through multiple mechanisms viz., interfering with different cellular pathways and inducing / inhibiting the production of various types of cytokines, growth factors, lipid mediators, eicosanoids and proteolytic enzymes [2]. The anticancer applications are constrained due to little water solubility and low chemical stability of curcumin. Consequences are low cellular uptake and poor oral bioavailability. Various methods such as structural modification and the use of drug delivery systems are being tried. The therapeutic activities (i.e., antimicrobial, antitumor, antioxidant, anti-inflammatory, antidiabetic and neuroprotective properties) of curcumin and its synthesized derivatives are reviewed [9]. However, curcumin derivatives are not more efficient than curcumin. Nanoparticles from dried turmeric rhizome reported to provide higher cytotoxic effect on human hepatoma cells when compared to free curcumin and could be a delivery system for cancer treatment [10]. Curcumin is effective in patients with rheumatoid arthritis, inflammatory eye diseases, inflammatory bowel disease, chronic pancreatitis, psoriasis, hyperlipidaemia, post operative inflammation and cancers in preliminary studies. Clinical trials are required to find the complete potential. Clinical studies are encouraged for several disorders such as diabetes, Alzheimer, cardiovascular, liver injury, cancer and osteoarthritis. The beta-diketone moiety, the phenyl rings, and the substituent groups on them are vital for the biological activity. Chemical changes of these moieties provide curcumin derivatives with greater ability and / or improved water solubility or stability. Natural or synthetic polymers, lipids, proteins are being developed to deliver curcumin to cancer cells as well as to improve cellular uptake. Curcumin can provide health benefits for people who do not have diagnosed health conditions, when used in enough quantities. Superior bioavailability and efficacy of ‘free’ curcuminoids from curcumagalactomannoside, curcumin formulation, when compared to unformulated curcumin is reported [11]. Curcumin from turmeric stimulate weight loss by monitoring lipid metabolism; curcumin exercises direct influence on fat, pancreas and muscle cells, helping to reduce insulin resistance. Although many research studies are already reported, further research is required for medication development. Suitable delivery methods of curcumin are required to target specific tissues for more effectiveness. Essential oils, extracts and selected components of turmeric showed strong antibacterial activity [against food (i.e., meat) spoilage and human as well as plant pathogenic bacteria] and antifungal activity.
STATUS OF EXISTING SOLVENTS
Solvents are essential in most of the techniques related to extraction, analysis, synthesis and dissolution of bioactive phytochemicals from various sources. Solvents can interact, hinder, degenerate, moderate any substance and separate out phytochemicals from diverse materials. In general, water is abundant and widely used from ancient periods due to its extraordinary and appropriate characteristics (i.e., viscosity, density, surface tension, easily transferable, crystallisable) at ambient temperature. It is available in pure form. Further, it is cheaper, harmless, dissolve / suspend variety of substances. Water does not pollute air or soil, and its supercritical conditions are easily reached at ambient temperature, owing to its physicochemical properties; water-based formulations are commonly found in traditional medicine. Nevertheless, many substances are disinclined to water and require other solvents. Conventional organic solvents (petrochemical industry origin i.e., n-hexane, petroleum ether, diethyl ether, ethyl acetate, chloroform, dichloromethane, acetone, n-butanol, ethanol and methanol) are being used, where ever water is not suitable. In general, polar solvents (i.e., ethanol, methanol and their aqueous mixtures) are generally used for processing of polar compounds, in addition to water. Apolar solvents (i.e., hexane and petroleum ether) are used for separation of non-polar compounds. The remaining solvents are used for the extraction of bioactive compounds of intermediate polarity. However, organic solvents are flammable, dangerous, volatile, vaporise at ambient temperatures, unpredictable, dilapidation of bioactive phytochemicals owing to constant heat extraction and the damage to reach high concentration of the target phytochemicals, low extraction efficiency, complicated operation, contribution of large amount of waste to water, air and soil, high energy consumption and noxious to the health of human beings and other organisms. These organic solvents create environmental and ecological problems. The methods using traditional organic solvents are incapable of meeting the requirements for manufacturing activities [12-14]. Therefore, green solvents are necessary for the separation and purification of bioactive compounds from plant sources particularly that are applicable for food, beverage, nutraceutical, pharmaceutical, and cosmetic industries. These should be engineered based on to reduce energy consumption, produce non-denatured extract without contaminants, not produce waste, and reduce unit operations. Green solvents should be less harmful, biodegradable, non pollutants and reusable [14]. Green solvents are necessary for the extraction of bioactive compounds from plant sources particularly that are related to food, nutraceutical, pharmaceutical, and cosmetic industries, as the traditional organic solvents are toxic. Value-added products related to above industries need to be safe for organisms.
DEVELOPMENT OF DEEP EUTECTIC SOLVENTS AS ALTERNATE GREEN SOLVENTS
Deep eutectic solvents (DES) are easily prepared and found to be less harmful for organisms as these are low toxic, when compared to organic solvents. These are homogenous liquids / solvents and are formed by combining [through heating and stirring (suitable technique for laboratory), vacuum evaporation, lyophilization or freeze-drying, grinding, extrusion, ultrasound-assisted irradiation, and microwave-assisted heating with mixing / grinding / freeze drying] a hydrogen bond acceptor (HBA - e.g., such as quaternary ammonium) and a hydrogen bond donor (HBD- e.g., urea, carboxylic acids or amine) at a specified stoichiometric ratio [14,15]. Hence, DES are solutions of Lewis or Bronsted acids and bases. Deep eutectic solvents are easily adaptable / designable by changing the structure or ratio of parent components as per the need of the applications including extraction, isolation, separation, analysis, use as drug carrier of target compounds as well as enhancement of their solubility and stability. The components of deep eutectic solvents involve in a complex hydrogen bonding and Van der Waals interactions [16]. This results in depression of melting point of formed DES, compared to their parent compounds. DESs are non flammable with low vapour pressures. 3
A review on the isolation of bioactive compounds (viz., phenolic acid, flavonoids, tanshinone, keratin, tocols, terpenoids, carrageenans, xanthones, isoflavones, alpha-mangostin, genistin, apigenin etc.) from different matrices using deep eutectic solvents is reported [16]. The extraction of bioactive compounds using DESs in terms of consumption of energy, extraction periods, quantity of sample and amount of solvent and their future necessity are discussed [15]. The separation of value-added compounds from the wastes of agro, forest and food sectors using DESs is presented [17]. A review on the separation of organic and inorganic analytes from aqueous surroundings using hydrophobic DESs is reported [18]. Extraction of bioactive compounds from medicinal and herbal plants using DESs is described in a review [19].
Selected DESs are prepared using primary metabolites (e.g., carbohydrates, organic acids, alcohols, amino acids) of plants. These are called as natural deep eutectic solvents (NADES). A systematic study and insight on the toxicity of cholinium based deep eutectic solvents is described and observed it is minimum [20]. These cholinium based eutectic solvents are reported to be decomposable. Various applications and changes from ionic liquids, to DES and NADES are discussed [21]. In general, NADESs possess low toxicity and minimum conservational impact. An opinion on the toxicity of NADES basing on the earlier studies is presented [22]. NADES have the advantage to reproduce the natural recovery way for water-insoluble primary and secondary metabolites in plants. NADES usage improved positively to isolate bioactive compounds from natural sources [23]. Researchers studied the physicochemical properties, functionalities, molecular interactions and solubilizing capabilities of NADES to explore the opportunities, in particular for hydrophilic NADES [24]. The key parameters of NADES are its constituents, molar ratio, density, viscosity, polarity, pH, stability and toxicity, to develop an excellent extraction process for isolation of bioactive compounds. NADES can be included directly in food products, when these are used to extract as well as to dissolve bioactive compounds (e.g., antioxidants, antibacterials and flavorants) including food additives. Due to advantages (i.e., low volatility, high boiling points, non-inflammable, water solubility / miscibility, economic viability, reusability, extensive liquid range, biodegradability, renewable sources, eco friendly, high stability, sustainability, easy preparation / synthesis, insignificant toxicity compare to conventional solvents), DES/NADES are emerging as alternate solvents in the processing of turmeric. DES / NADES are associated to the United Nations Agenda for 2030 for sustainable development of energy efficient technologies [25].
EXISTING METHODOLOGY USED IN PROCESSING OF TURMERIC
Conventional extraction methods (e.g., solvent extraction, Soxhlet extraction, hydro/steam distillation) novel extraction technologies (e.g., ultra-sonication assisted extraction, microwave assisted extraction, enzyme assisted extraction, pressurized liquid extraction, subcritical water extraction, supercritical fluid extraction, subcritical water extraction and ionic liquids-based extraction) for isolation of bioactive compounds from turmeric are reported. Conventional separation techniques (i.e., column chromatography, semi-preparative high performance liquid chromatography) and supplementary modern techniques (i.e., high-speed counter-current chromatography, supercritical fluid chromatography, etc.) are reported for purification of bioactive compounds from turmeric. Application on large scale is meagre. Priority is being given to develop highly efficient, safe, environment friendly extraction and purification methods on large scale for industrial use [26]. Vibrational, molecular, chemical and structural properties of curcumin-selected natural deep eutectic solvent (menthol, myristic acid) mixtures using computational, experimental and theoretical procedures is reported [27]. However, sustainable extraction of turmeric, separation, purification and estimation of bioactive compounds from turmeric using DES / NADES and their applications has not been consolidated and discussed to the author’s knowledge. The aim of this review is to provide the above information using DES / NADES.
SUSTAINABLE EXTRACTION, SEPARATION AND ANALYTICAL METHODS FOR PROCESSING OF TURMERIC FOR ITS CONSTITUENTS USING DEEP EUTECTIC SOLVENTS AND THEIR APPLICATIONS
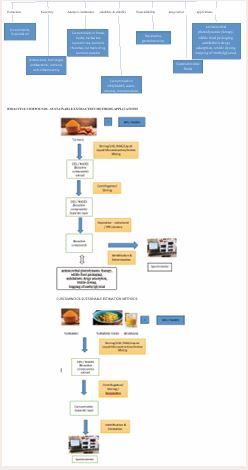
In this review, the details of recent optimized extraction, separation processes and analytical methods using DES / NADES, which involve turmeric as well as curcuminoids are collected and discussed. The solubility and stability of bioactive compounds are also increased with the use of deep eutectic solvents. It covered the processing of turmeric using DES / NADES in combination with microwave (MA-DES / NADES-MAE) and ultra-sonic energies (UA-DES / NADES-UAE) also. Methods using vortex-assisted deep eutectic solvent microextraction (VA-DES-ME), vortex assisted deep eutectic solvent emulsification liquid-liquid Microextraction (VA-DES ELLME), water and surfactant free microemulsion (WSFME) and deep eutectic solvent dispersive liquid liquid microextraction based on solidification of floating organic drop (DES-DLLME-SFO) are also discussed. The solubility and stability of bioactive compounds are also increased with the use of DES / NADESs. Results / conclusions are provided in table 1 along with details of matrix, DES / NADESs composition (HBA and HBD molar ratio as well as water content), optimized parameters (viz., extraction time, temperature, microwave energy and time, ultrasonication energy and extraction time, stirring speeds and times, centrifugation speed and times etc.) for methods of extraction, determination as well as identification of target compounds. Outcomes of the DES / NADES extracts of turmeric and its bioactive phytochemicals are provided in supplementary information (Scheme 1) .
Scheme 1 Turmeric – curcuminoids - DES / NADES extracts sustainable outcomes
Extraction of bioactive compounds from turmeric
Curcuminoids from turmeric are extracted using natural deep eutectic solvents (NADES-citric acid to glucose-1:1 with 15% water-selected). Optimum conditions standardized are type of NADES, HBD and HBA of NADES, molar ratio, water content in NADES, temperature, solid to liquid ratio, and extraction time (Table 1, No.2) to achieve higher extraction yields of three curcuminoids (BDMC, DMC and CUR). NADES is selected based on its moderate viscosity due to the hydrogen bonds and Vander Waals interactions [28]. Water quantity is optimized with reference to viscosity of DES as well as solubility of target components. Requisite quantities of NADES and turmeric powder are stirred for required period at optimized temperature under low light conditions. During stirring, solution mixture is vortexed for 1 minute, for every 10 minutes. Mixture is centrifuged and supernatant is diluted before HPLC analysis. Extraction yields are higher than routine organic alcoholic solvents. DPPH radical scavenging activity of NADES extract (87%) is reported. Curcuminoids are found to be more stable in NADES extract, when compared to organic alcoholic extract. Curcuminoids are more stable (30 days) at lower temperatures (4oC) in the dark and degradation is less (~2.5%). Curcuminoids are regenerated from NADES extract, using solid phase extraction column. Their recovery is excellent (BDMC -88.5%, DMC - 94.4%, CUR - 93.2%). The selected NADES are sustainable. NADES extract are stable for storage and useful in food, biochemical, and pharmaceutical industries.
Table 1: Sustainable processing of turmeric for curcuminoids using deep eutectic solvents (DES / NADES) as alternate green solvents and their activities and applications.
|
No |
Matrix |
Compounds |
DES /NADES |
Optimized condition (HBA-HBD)* |
Method Determination |
Yields / conclusions |
Reference |
|
1. |
Food and Herbs |
Curcumin |
Choline chloride, phenol. |
Choline chloride-phenol (1:4), DES volume - 400 μL, sample-12.5mL THF volume- 400 μL pH 4.0, pre-concentration factor - 12.5, vortex time-2 min, centrifugation @ 4500 rpm, 5min, DES phase-curcumin dilution-EtOH (1mL), analysis. |
VA-DES-ELLME, UV, HPLC, LC-MS, LC-MS/MS |
LOD-2.86 μg/L, LOQ-9.44 μg/L, RSD-1.8%, Recovery-96 to 102% . PF-12.5 Sample quantities (µg/L) turmeric liquid – 380.3±37.3, herbal tea-Swedish syrup (turmeric)-54.2±1.7, herbal tea-ginger-Lemon -48.9±2.1 turmeric samples-quantities (mg/g) drug-0.37 to 0.40, powder-8.25 to 8.36, root herbal tea-7.45 to 7.60. |
[41] |
|
2. |
Turmeric |
Curcuminoids |
Glucose, citric acid, malic acid, lactic acid. |
citric acid-glucose (1:1)+15% water, S/L-0.1g/10mL, stirring, temp. - 50 °C, time - 30 min, vortex – 1min / 10 min. Recovery, SPE column |
HPLC |
Quantities-mg/g BDMC-16.54, DMC - 15.12, CUR - 21.18. Curcuminoids in DES Stable-30days@4oC, NADES extract, DPPH - 87%. recovery – SPE BDMC - 88.5%, DMC - 94.4%, CUR - 93.2%. |
[28] |
|
3 |
Food samples |
Curcumin |
Betaine hydrochloride, glycerol, choline chloride, tetra butyl ammonium chloride, 1,4-butanediol, ethylene glycol, 1,6-Hexanediol |
Betaine hydrochloride - glycerol (1:3), sample solution sample-10g, methanol – 30 mL, sequential methanol – 10 mL, until clear, centrifugation speed – 3000 rpm, time - 3min. dilution – 100 mL (with methanol). DES extraction DES-500μL sample volume-50 mL acetone (300 μL), pH-6.0 , vortex time -2 min, vortex speed -3000 rpm, centrifugation speed-4000rpm, time-3min. |
VA-DES-ME, UV-Vis |
LOD - 1.5 μg/L , LOQ - 5.0 μg/L, pre-concentration factor – 100, recovery - 90.9 to108.3 %, working range - 5 to 300 μg/L. Food samples-observed range: 13.9- 154.7 μg/L samples curcumin – (μg/L) cinnamon tea- 95.2, 94.5 anti-parasite Herbal tea-74.7, 72.5 herbal tea- 62.1, 60.9 herbal tea (turmeric -lemon)-105.7, 104.1 herbal tea ( turmeric -mint)- 39.5, 37.9 turmeric-1- 154.7, 151.7 turmeric -2- 125.8,123.4 curry-1- 137.9, 135.8 curry-2- 88.3, 86.8 cinnamon- 117.4, 105.7 sesame -15.8, 13.9. |
[42] |
|
4 |
Turmeric rhizomes, Turmeric tea |
Curcuminoids |
Low density DES, Tetrabutylammonium chloride, Undodecanol; Dodecanol; Decanoic acid |
DES:Tetrabutyl ammonium chloride-decanoic acid (1:1), sample solution sample powder-0.5g, methanol – 50 mL soaking-12h, U.time-1h, supernatant, filtered sample solution extraction sample solution + water – 10 mL DES volume-70µL, pH-3 (HCl-0.8mL), stirring temp. - 40°C, speed - 1100 rpm, time - 10 min. DES drops aggregation (curcuminoids). cooling temp. – 20 oC, time – 6 min, solidification, separation room temp. HPLC |
DES-DLLME-SFO; HPLC-UV |
LODs -7 to 9×10-5 mg/L
enrichment factors – 608 to 848, recovery - 84.6 to 114.8 %
yields: (mg/g) turmeric rhizomes BDMC-2.95, DMC-2.42, CUR-5.12, total – 10.49 mg/g. turmeric tea BDMC-1.40, DMC-1.54, CUR-4.15, total – 7.09 mg/g. |
[43] |
|
5 |
Turmeric |
Curcuminoids |
Choline chloride, lactic acid |
NADES choline chloride-lactic acid (1:1) extraction solvent: NADES/EtOH/ triacetin: 35/ 27.5/37.5; turmeric powder S/L- 1/8; stirring, speed - 1300 rpm, time - 1 h. centrifugation, speed - 4200rpm, time - 10 min. supernatant HPLC analysis Cyclic extraction S/L-1/16 1st cycle as above 2 to 7 / 8 cycles supernatant + sample as above supernatant dilution – 25 fold HPLC |
WSFME, HPLC |
Yields: (mg/g) CUR-11.80±0.29, DMC- 2.73±0.13, BDMC-3.26±0.14.
7 / 8 cycles curcuminoids concentration 1st cycle ~35 mg. 7th cycle ~170 mg. |
[29] |
|
6 |
Curcumin |
Curcumin |
Camphor, menthol, thymol |
Camphor - menthol (1:1), pH-5.60 to 5.64, temp. - 35 to 40 oC, stirring speed-500 rpm time-1 h. kept-overnight, centrifugation speed-10,000 rpm, time-20 min. supernatant, dilution methanol-made up-10mL, diluted solution-1mL addition buffer(5.5pH)-made up -10mL, UV-Vis. |
UV |
Curcumin solubility -22.07±0.095mg/ mL. |
[47] |
|
7 |
Turmeric |
Curcumin |
Tetra-n-butyl ammonium chloride, n-decanoic acid. |
Tetra-n-butyl ammonium chloride- n-decanoic acid (1:2) HDES +60%water, triton X-100 (0.3M), curcumin addition, stirring-24h, centrifugation, supernatant-UV-Vis. |
HDES-ME, DLS, UV-Vis |
Microemulsion: curcumin solubility-51 mg/mL. solubility, stability increased. stability (degradation) sunlight – 4 h (25 %), dark - 15 days (25 %). |
[46] |
|
8 |
Turmeric |
Curcumin |
ChCl, Ethylene Glycol, Glycerol, Urea. |
ChCl- Ethylene glycol(1:2)+water |
UV-Vis |
Curcumin solubility water+DES -33,000 fold@T-45oC. e -NRTL (9.85%) > UNIQUAC (10.86%) |
[48] |
|
9 |
Turmeric |
Curcuminoids |
Choline chloride, lactic acid, sucrose, fructose.
5 NADES |
M.P.- 0 to 1500 W, temp.- 64.7 to 71.8 ?C, time-15.4 to 21.6 min. recovery-curcuminoids DES extract centrifugation speed-10000rpm, time-15 min, supernatant:water 1:20, incubation temp.-0oC, time-8h, centrifugation speed-10000rpm, time-15 min, precipitate lyophilized. |
FT-IR, HPLC, CUPRAC |
DES-1 highest curcuminoids content. (31.97 mg /g) DES-2 highest total antioxidant capacity. (0.198 mmol trolox / g) |
[30] |
|
10 |
Turmeric (rhizome, leaves, flowers) |
Curcuminoids, extracts, antioxidant, flavonoids, antimicrobial, chelation Fe2+, cholinesterase inhibition, cytotoxicity, genotoxicity |
Menthol, choline chloride, lactic Acid, acetic acid, lauric acid. |
Hydrophobic DES menthol-lactic Acid (2:1), menthol-acetic acid (1:1) S/L-1/20. U.temp.- 45 ?C, U.time- 2 to 3 h, U.P.-50 to 60 Hz, microcentrifuge speed - 9500 rpm, time - 3 min. |
UAE, TFC, DPPH, FRAP, iron chelation, antibacterial, |
Extracts from menthol-lactic acid (1:2), menthol-acetic acid(1:1) showed highest potential for all activities: antioxidant, Iron chelation and antibacterial; inhibitory activities against selected enzymes. non-toxic to Allium cepa cells -safe for human consumption. |
[31] |
|
11 |
Turmeric |
Curcuminoids |
Glycerol, ethylene glycol, 1,4-butanediol, lactic acid, malic acid, citric acid, choline chloride. |
Choline chloride-lactic acid (1:1) + 20% water. S/L-5 g / 100 mL, particle size - 0.355 mm, U.P.-70.8 W/cm2@ 60% (6 sec ON and 4 sec OFF), U.temp – 30 °C, UtTime - 20 min. centrifugation, speed – 10000 rpm, time - 15 min. DES extract recovery anti-solvent water (1:20), temp. - 0oC, time - 8h. centrifugation, speed – 10000 rpm, time - 15min. HPLC |
UA-DES, HPLC |
Curcuminoids - 77.13 mg/g, anti-solvent recovery – 42 %, purity - 82% |
[32] |
|
12 |
Turmeric |
Essential oil |
choline chloride, oxalic acid, fructose, lactic acid, glucose, malic acid. |
Pre-treatment (MW) choline chloride- oxalic acid (molar ratio 1:1), NADES-60 g, sample-30 g, M.P.- 600 W, temp.- 84 ºC, time – 5 min. Hydro distillation, M.P.- 600 W, M.temp.- 110 ºC, M.time – 5 min, boiling ready M.P.-300 W, M.temp.- 110ºC, M.time-76min |
MAHD GC-MS |
Essential oil - 0.85% (Highest yield), compounds - 49, more oxygenated compounds. flavour is superior. |
[55] |
|
13 |
Turmeric |
Curcumin |
HBAs betaine , L-carnitine, choline chloride, Tetrapropyl ammonium bromide, HBDs, lactic acid, levulinic acid, pyruvic acid, 2-oxobutanoid acid, 2–oxovaleric acid, acetylbutyric acid, L-mandelic acid, hexanoic acid. |
Levulinic acid - HBA (70%) + ethanol (30 %), curcumin stirring time-1h filter |
UV-Vis, NMR, COSMO-RS |
Curcumin solubility – 20 fold increase. cyclic confirmation between curcumin and levulinic acid and synergistic effect of DES. |
[49] |
|
14 |
Turmeric |
Curcumin, coumarin, eugenol. |
Citric acid, propanediol, betaine, proline, lactic acid, glucose. |
Citric acid- propanediol-betaine (2:2:1), S/L-1g/20g, ball mill shaking time – 1 h, shaking frequency - 10 / sec, centrifugation speed - 5000 rpm time - 10 min DES extract. preparation-SeS2NPS (red colour) SeCl4 (0.2M)-20mL+ DES spice extract-10mL+ Na2S.H2O (0.2 M), ratio - Se / S2- - 2.8, pH - 8, crystal size – 28.72 nm, particle size – 830 nm. antifungal activity, antibacterial activity, antiviral activity |
UV-Vis, TPC, TFC, DPPH, Antibacterial, antifungal, antiviral activities |
Quantities-mg/g curcumin - 140.96±11.20, coumarin - 13.60±1.83, eugenol - 0.358±0.111, cinnamal – 84.27±23.12. selenimun sulphide nanoparticles (spice NADES extract) - bioactivities, antibacterial – 117 mg /dm3, antifungal – 469 mg /dm3, antiviral influenza viruses – 99% B-coronavirus – 99%. |
[38] |
|
15 |
Turmeric + black pepper |
Curcumin, coumarin eugenol |
Citric acid, propanediol, betaine, proline, lactic acid, glucose |
Citric acid- propanediol-betaine (2:2:1), S/L-1g/20g, ball mill shaking time – 1 h, shaking frequency - 10 / sec, centrifugation speed - 5000 rpm time - 10 min DES extract. preparation-SeS2NPS (red colour) SeCl4 (0.2M)-20mL+ DES spice extract-10mL+ Na2S.H2O (0.2M) Ratio-Se / S2- -2.8, pH-8, crystal size – 28.72 nm, particle size – 830 nm. antifungal activity, antibacterial activity, antiviral activity |
UV-Vis |
Quantities-mg/g curcumin - 120.43±29.32, coumarin - 11.79±0.87, cinnamal – 69.39±18.38, eugenol - 0.348±0.088. selenimun sulphide nanoparticles (spice NADES extract) - bioactivities, antibacterial – 59 to 117 mg /dm3, antifungal – 469 mg /dm3, antiviral influenza viruses – 99% B-coronavirus – 99%. |
[38] |
|
16 |
Turmeric residues (starch free). |
Curcuminoids, antioxidant, acetylcholinesterase (AChE) inhibition |
Choline chloride, propylene glycol |
NADES, Choline chloride-propylene glycol (1:2) + 20% water, Sample turmeric (fresh) – 10 kg washing, mincing, water – 50 L, vigorous mixing, filteration, sieve-0.25 mm, filtrate (starch), turmeric residue, drying, temp. - 60oC, humidity – 8 to 10%. extraction, turmeric residue, particle size - 0.18 mm S/L-1/40, surfactant - 5mM, stirring, temp. - 50oC, time - 1h, centrifugation, speed – 5000 rpm, time –15 min filtration, size – 0.45 µm, filtrate, HPLC. NADES extract, curcuminoids recovery NADES extract-Water: 1/40. precipitate, drying, storage. |
HPLC, Antioxidant, enzyme inhibition, |
NADES extract, Curcuminoids, yield-54.2mg/g. (1.31 times higher-alcohol extract) IC50-µg/mL DPPH-25.58±0.51, AChE inhibition-19.12±0.83
Recovery of curcuminoids (anti solvent water) -99.7%, curcuminoids content -20.08±0.3%. |
[34] |
|
17 |
Turmeric |
Curcuminoids |
Choline chloride, oxalic Acid, malonic Acid, lactic acid, acetic acid, propionic acid, glycolic acid, citric acid, ascorbic acid, ethylene glycol, glycerol. |
Cholinr chloride- malonic acid (1:1) + 50 % Water S/L-1/20, stirring time – 10 min, U.P. - 50.8 W/cm2 U.temp - 363oK, U.time - 60 min. extract HPLC analysis.
Solubility shake flask curcumin - excess, NADES – known mL time – 72 h, supernatant filtration membrane - 0.45 µm filtrate, dilution, HPLC analysis |
NADES-UAE, HPLC |
NADES-UAE yield Curcuminoids quantity - 164.51 mg/g
Solubility – 5,92,667 times higher in DES (cholinr chloride- malonic acid) than water. |
[33] |
|
18 |
Turmeric |
BDMC, DMC, CUR, ar-turmerone |
Menthol, octanoic acid, decanoic acid, dodecanoic acid. |
Octanoic acid-menthol (1:3.6), S/L-10mg/1mL U.P.-37 kHz, U.time-50 min, U.temp.-25 °C, centrifugation, speed - 7155 × g time - 10 min, HPLC-UV. Microemulsions tween 20: propylene glycol (PG) (1:1, w/w), octanoic acid-menthol (1:3.6), U.P.-37 kHz, U.time-90min centrifugation speed - 7155 × g, HPLC-UV |
UA-DES, FT-IR, HPLC LC-MS/MS |
Quantities-% UA-DES BDMC-2.49±0.25, DMC-5.61±0.45, CUR-9.40±0.86, ar-turmerone-3.83±0.19. microemulsions BDMC-2.10±0.18, DMC-6.31±0.48, CUR-12.6±1.20, ar-turmerone-2.58±0.19. |
[35] |
|
19 |
Turmeric |
Curcuminoids |
ChCl, Propylene glycol, Ascorbic acid, Fructose, Menthol, Proline, Glucose, Glycerol, Urea, PEG-4000 |
ChCl-Propylene glycol (1:1) +30% water, S/L-1g/10mL, U.Temp.-70oC, U.Time-30min, Centrifugation-5000rpm/15min, Supernatant-SPE, MeOH elution. |
UPLC, HPTLC, UPLC-MS-MS. |
Total curcuminoids – 45-50 mg/g, Bioavailability – 450% (compare to organic solvent extract), Curcumin (DES) in plasma-0.6mg/µL. 31 curcumin metabolites. |
[52] |
|
20 |
Turmeric |
Curcuminoids |
Choline chloride, ethylene glycol, 1,4-butanediol, glycerol. |
Choline chloride-1,4-butanediol (1:6) +10% water, S/L-1g/15mL, U.P.-35 kHz U.temp.-AToC, U.time-2h, centrifugation recovery water addition precipitation-one day, filtration, washing, drying temp - 40oC. |
NADES-UAE, HPLC, UV COSMOS-RS |
Quantities - mg/g NADES-UAE, BDMC- 46.14±0.82, DMC- 10.63±0.35, CUR- 46.70±0.55. |
[36] |
|
21 |
Turmuric Rhizome Turmeric tea |
curcuminoids |
L-Menthol. propanoic acid, butanoic acid, decanoic acid, lactic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, undecanoic acid, dodecanoic acid |
L-Menthol-lactic acid (1:2), sample solution-200µL, NaCl solution (18%)-8mL, DES-160µL, shaking time-30s, pH-7, Demulsifier methanol-0.8mL, demulsification time-230s |
STLLME, HPLC |
LODs- 0.1 to 0.4 ng/mL, LOQs-0.4 to 1.2 ng/mL, enrichment factors – 279 to 350, recovery – 85.3 to 108.9% Quantities – mg/g turmeric BDMC- 5.18, DMC- 2.40, CUR- 6.19. turmeric tea BDMC- 2.24, DMC- 1.43, CUR- 4.70. |
[44] |
|
22 |
Turmeric Curcuma Longa |
BDMC, DMC, CUR, ar-turmerone |
Octanoic acid, menthol |
Microemulsions (ME): HDES [Octanoic acid - menthol (20 : 80, mass ratio)] /tween 80 : propylene glycol (1 : 1)/water, 25/70/5 stirring-20 min, heating @70oC Extraction S/L -50 mg/1 mL ME U.P.-37 kHz, U.time-60min centrifugation speed - 7155×g. |
NO Production inhibition; HPLC |
Yields (wt%) microemulsion BDMC- 1.69±0.06, DMC-3.04±0.01, CUR -7.36±0.20, ar-turmerone -1.39±0.01
NO production inhibition (IC50 value). HDES ME - 0.0136 % v/v. HDES ME turmeric extract - 75.2±6.7 nM, |
[39] |
|
23 |
Curcuminoids |
Curcuminoids solubility-quantum chemistry |
Choline chloride, 1,2-propanediol 1,3-propanediol ethylene glycol, glycerol |
Choline chloride -1,2-Propanediol (1:4) + water (0.03 %), DES mass fraction-0.8, temp. - 30 to 45oC, solubility curcumin in DESs stirring time-5days, centrifugation, short time, filtration, filtrate HPLC |
HPLC, ESP, DFT |
Curcuminoids aqueous DES solubility - 19.20 μg/mL 1700 times higher than water. |
[51] |
|
24 |
Turmeric |
Curcuminoids |
Choline chloride, citric acid, 1,4-Butanediol, glycerol, ethylene glycol, lactic acid, malonic acid, malic acid, urea, methyl urea, acetamide. |
Choline chloride - citric acid (1:1) + 30 % water S/L-1 g / 20 mL, particle size - 0.150 to 0.212 μm M.P.- 60 W, high stirring-soaking time - 8 min, M. time - 6 min, Recovery centrifugation, speed - 10,000 rpm, time - 15 min, supernatant, water - 20 fold, magnetic stirring, incubation, temp.- 0 oC, time – 8 h, centrifugation, speed - 10,000 rpm, time-15min, residue – air drying, HPLC |
NADES-MAE, HPLC |
Yields (mg/g) Curcuminoids - 89.87±2.21, antisolvent recovery – 43±1.78%, purity-82.3% |
[37] |
|
25 |
Turmeric rhizome |
Curcumin |
Menthol, LA, Acetic acid, |
Menthol-LA(1:2), Menthol-Acetic acid(1:1) S/L-1/20. U.temp.- 45 ?C, U.times- 2 to 3 h, U.P.- 50 to 60 Hz, microcentrifuge speed - 4000 rpm time - 10 min. Emulsion gel: sodium alginate, biopolymers, carboxymethylcellulose (1:1), turmeric extract stirring-10000 rpm time -180 to 470 s; calcium chloride -0.1M stirring-10000 rpm time – 120 to 265s |
UAE-DES, DPPH, ABTS |
Turmeric extracts DPPH 24.4 to 33.9 mg trolox/g. Stable for 30 days. ABTS 7024.7 to 8486.3 µmol trolox/L, decreased to 4455 µmol trolox/L in 10 days, Stable from 10 to 30 days. Emulsions DPPH 7 to 17 mg trolox/g. stable for 30 days. ABTS 800 to 3000 µmol trolox/L stable for 30 days. |
[40] |
|
26 |
Curcumin |
Curcuminoids; coconut oil |
D-glucose sucrose molar ratios (5:1);(3:1);(1:1); (1:3); (1:5) |
Natural DES-water-in-oil high internal phase emulsions with curcumin (NADES-HIPEW/O) preparation: sugars mixing water:Sugars (5:1) heating @ 80 ?C stirring - 6 h cooling to RT - 1 h stock solution Storage at RT curcumin - 1, 2, 4, 8, 16 or 40 mg/ mL heating @ 80 ?C stirring - 4 h cooling to RT - 1 h emulsion storage at RT. |
UV |
Curcumin loaded NDES-HIPEW/O -UV stability -72 h - residual curcumin % : coconut oil-13.44±0.21 , NADES1:1-HIPE - 31.91±0.93, NADES1:3-HIPE - 43.1±2.5, NADES1:5-HIPE - 39.7±2.1 .
Emulsions exhibited centrifugal, thermal, freeze-thaw, and storage stability.
curcumin (%) - micelles -NADES- HIPEs: NADES1:1 -151.4±3.5, NADES1:3 -143.2±4.1, NADES1:5 - 131.5±3.0, Coconut oil -100. |
[53] |
|
27 |
Curcumin |
Food water samples turmeric drugs, turmeric powder |
HBA: octylamine (OA) HBD: succinic acid (SA) |
RS-DES-DLLME RS-DES preparation (hydrophilic and homogenous) OA:SA:W molar ratios 1:1:5, 2:1:5, 1:2:5, and 3:1:5 heating agitaion ------------------------ sample -50 mg methanol – 40.0 mL ultrasonication mixing -10 min. centrifugation, speed - 4000 rpm time - 5min. ------------------------- DLLME sample solution – 40mL (curcumin 50µg/L) DES - 2.0 mL shake-30 sec 3M NaOH - 800µL vortex – 1 min, maintain - 1 min@RT, upper layer separation (DES + analyte) 3M HCl - 900 µL shake - 30 sec upper layer methanol – 1 mL |
UV spectro photometer FT-IR NMR |
Curcumin LOD-10µg/L LOQ-35µg/L recovery - 92 to100 %, EF-38.68 curcumin content (µg/Kg) herbal tea 1 – 52±3.8 herbal tea 2 – 62±1.8 herbal tea 3 – 42±1.6 turmeric drug - 117±3.8 turmeric powder -159±2.7 |
[45] |
Extraction of curcuminoids from dried roots of Curcuma Longa and its solubilization using homogenous, transparent and low viscosity mixtures of ethanol triacetin (4:6) containing natural deep eutectic solvent (NADES) [choline chloride-lactic acid (1:1)] as an adjuvant [29]. Variable parameter optimized are composition of extracting solvent, adjuvant composition and solid to liquid ratio (Table 1, No.5) to obtain maximum yield of curcuminoids into the extract. Adjuvant NADES is selected based on its lowest melting point and miscibility with extraction solvent mixture to form micro emulsions. Required quantities of turmeric powder and extraction solvent (including NADES as adjuvant) are stirred at optimum speed for necessary time. Extracted solution is centrifuged under preferred conditions of speed and time Supernatant is filtered and subjected to HPLC analysis (curcumin - 11.80 mg/g, dimethoxy curcumin - 2.73 mg/g, bisdemethoxy curcumin - 3.26 mg /g). These quantities corresponds to 97 % with reference to soxhlet extract. Curcuminoids solubility is increased by two folds due to the formation of nanostructures of surfactant-free micro emulsions as revealed by dynamic light scattering due to the intermolecular interactions between NADES and curcuminoids and named as water and surfactant free micro emulsion (WSFME) method. Recycling of extraction solvent (supernatant) is described. Filtrate from first cycle is used for the extraction of fresh turmeric powder and procedure is repeated (second cycle) to enrich curcuminoids into filtrate. Fresh turmeric powder is treated with supernatant and enrichment of curcuminoids is sustained up to seven cycles, which can save the solvent. Curcuminoids concentration is increased extract from ~35 mg (first cycle) to ~170 mg (seventh cycle). Final supernatant is diluted to required fold and subjected to HPLC analysis. Recovery of curcuminoids from DES extract is not carried out and needs development. However, DES extract can be used directly for selected applications as claimed.
Preparation of turmeric extracts is reported using NADES (containing binary combinations of choline chloride, lactic acid, fructose, and sucrose) with microwave assistance [30]. Optimized factors are type and composition of DES, water content in DES, extraction time and temperature, microwave power, solid to liquid ratio (Table 1, No.9) using response surface methodology. Requisite quantities of turmeric powder and NADES are mixed and subjected to extraction at selected temperatures using automatic microwave power adjustments for various time periods. Extracts are filtered and stored for further analysis. Temperature is found to be important parameter, as solubility of the curcuminoids increases with temperature upto certain level owing to decrease in surface tension and viscosity of DES. Penetration of NADES is increased due to the above reasons. Further sufficient quantity of DES in the material facilitates easy mass transfer. Longer periods of extraction degrade the bioactive compounds. NADES [sucrose - lactic acid - water (1:5:7)] extract contained higher content of curcuminoids (31.97mg /g). However, extract with NADES [fructose - lactic acid - water (1:5:5)] exhibited high antioxidants capacity (cupric reducing antioxidant capacity - 0.198 mmol trolox/g). Recovery of curcuminoids is carried out using antisolvent water. Conditions optimized for recovery are centrifugation speed and time, ratio of DES extract supernatant and water, lyophilization temperature and time. NADES extracts are centrifuged at optimized speed for required time. Supernatant collected and necessary quantity of water is added and incubated at 0oC for suitable time for precipitation. Precipitated curcuminoids are separated by centrifugation and dried using lyophilizer. All the NADES extracts contained higher quantities of curcuminoids and exhibited higher antioxidant activity than the extracts using conventional organic alcoholic solvents. Required time for NADES-MAE is shorter and solvent requirement is small quantity, when compared to conventional solvents. Further, NADES extracts can be used directly. Advantages of this method are convenience for automation, efficient, sustainable. Product is ready for use in food, pharmaceutical and allied industries.
Extraction of bioactive compounds from the rhizome, leaves, and flowers of Curcuma longa using deep eutectic solvents [hydrophobic DES: menthol + lactic acid (2:1) and menthol + acetic acid (1:1)-selected] with ultrasound assisted extraction is reported [31]. Variable parameters optimized are type and composition of DES and their molar ratio, solid to liquid ratio, ultrasonication power, temperature and time, centrifugation speed and time (Table 1, No.10). DESs are selected basing on their performance efficiency, density closer to water and hydrophobic nature as the target compounds are hydrophobic. Required quantities of sample powder and DESs are mixed in dark bottles and subjected to extraction using ultrasonic bath at necessary power, time and temperature. Selected hydrophobic DESs provided higher extraction yields, when compared to routine organic solvents and other DESs. DES extracts of rhizomes, leaves and flowers exhibited higher antioxidant and antibacterial activities, iron chelation activity, inhibiting activity against enzymes acetyl and butyryl cholinesterase. In general, rhizome and leaf extracts exhibited higher antioxidant activities (DPPH - 40 to 60mg trolox/g; FRAP - 25 to 65 mg ferric sulphate / g), as more quantities of flavonoids (7 to 19 mg quercetin equivalents / g) are extracted. Extracts from DES (menthol + lactic acid) exhibited higher activities with respect to antibacterial activities, inhibiting activity against enzymes acetyl cholinesterase (99%) and butyryl cholinesterase (82 to 84%), when compared to other DES. In case of iron chelation activity, pure DESs showed higher activity than the extracts due to formation of stable complexes between DES components and metal. These extracts are found to be non-toxic to Allium cepa cells and indicated to be safe for human consumption. Authors claim these hydrophobic DES extracts possesses excellent prospective for use in food, pharmaceutical and cosmetic industries. Isolation of curcuminoids from Curcuma longa using ultrasonication assisted deep eutectic solvent [choline chloride and lactic acid NADES at 1:1 M ratio with 20% water- selected] is reported [32]. Flexible parameters optimized are type and composition of NADES, water content in NADES, sample particle size, sample to solvent ratio, ultrasonication cycles and power, extraction temperature and time, centrifugation speed and time (Table 1, No.11) to obtain extract with maximum quantity of curcuminoids. DES is selected based on its moderate viscosity and high extraction efficiency towards target molecules. Requisite quantities of turmeric powder and NADES are taken and subjected to extraction using ultrasonication in pulse mode for required time. Homogeneously mixed solutions are diluted and centrifuged. Supernatant is taken and known quantity of water added, while stirring. Resultant mixture is incubated for required time. Precipitated mixture is subjected to centrifugation and curcuminoids precipitate is collected. Curcuminoids are analysed by HPLC for their purity. Sample to solvent ratio and particle size are important for proper creation of cavitation with breakage of raw material by ultrasonication, which provides suitable mass transfer of curcuminoids to NADES. Yield of curcuminoids is 77.13 mg/g under optimized conditions. Kinetics is described with the help of Peleg’s model. Curcuminoids (41.97%) are recovered using antisolvent (viz., water) with high purity (82.22%). Authors claim that the developed NADES-UAE method for extraction of curcuminoids from turmeric, is efficient, economical and green technology, when compared to routine solvent extraction.
Curcuminoids from turmeric are extracted using natural deep eutectic solvent [NADES-choline chloride - malonic acid (1:1) + 50% water-selected using Hansen solubility parameter] as well as ionic liquid [IL-1-Octyl-3 methyl imidazolium chloride+50% water-selected] with ultrasonication assistance [33]. Variable factors optimized are type and composition of DES, water content in DES, type of IL and water content, sample to solvent ratio, ultrasonication power intensity, extraction temperature (Table 1, No.17) to obtain extract containing maximum quantity of curcuminoids. NADES is selected basing on the higher solubility of curcuminoids, NADES extraction efficiency, hydrogen bonds, hydrophobic interaction, and π- π interactions and Hansen solubility parameter. Required quantities of DES and turmeric powder are taken in a glass tube and 1 mL of acetone is added. Tubes are covered with parafilm and subjected to extraction under stirring and ultrasonication for required time at suitable temperature. Solubility of curcuminoids in NADES (choline chloride - malonic acid) is determined. Excess amount of curcumin is added to known quantity of solvent (NADES / water) in a shake-flask under shaking for required time. Supernatant is filtered through the membrane. Filtrate diluted and subjected to HPLC analysis. Solubility of curcuminoids is higher (590,000 fold) in NADES than water. Yield of curcuminoids (164.51 mg/g) reported in this method is higher than earlier reports. However, recovery method is not discussed. Hansen solubility computational parameters supported the experimental results. Green and easily accessible NADES is used for extraction.
Extract (containing curcumin) from starch free turmeric residues, is isolated using NADES [choline chloride propylene glycol (1:2) + 20% water-selected] [34]. Known quantity of fresh turmeric is washed and minced with using preferred quantity of water Total contents are thoroughly mixed and filtered through required sieve to obtain filtrate (containing starch) and turmeric residue. NADES for extraction, is selected basing on its high efficiency due to moderate viscosity, hydrogen bonds and π-π interactions with target molecule. Optimized parameters are type and composition of NADES, water content in NADES, particle size, solid to liquid ratio, extraction time, and temperature (Table 1, No.16). Starch free turmeric residues is dried. Requisite quantities of turmeric residue powder, NADES and surfactant solvent are mixed and stirred at optimized temperature for required period. The extract is centrifuged to remove solids. Supernatant is filtered and subjected to HPLC analysis. Yield of curcuminoids is 54.2 mg /g and is higher (1.31 times) than alcoholic extracts. Curcuminoids (99.7%) are recovered using antisolvent water at 1:40 ratio. Curcuminoids content in recovered precipitate is 21%. IC50 values of NADES extract (containing curcuminoids) for antioxidant activity (DPPH) and acetylcholinesterase inhibition activity are 25.58 and 19.12 μg/mL respectively, which are better than traditional solvents. Sustainable green technology is developed to extract bioactive phytochemicals (i.e., curcuminoids) from starch free turmeric residue, which is useful to control environmental pollution. Bioactive compounds [(Yields (% on dry basis): bisdemethoxycurcumin-2.10, demethoxycurcumin-6.31, curcumin-9.40, and ar-turmerone-3.83] are extracted from turmeric using hydrophobic deep eutectic solvents [HDES- octanoic acid: menthol (1:3.6 molar ratio)] with ultrasonication assistance [35]. Variable parameters optimized are type and composition of HDES, solid to liquid ratio, extraction time, ultrasonication frequency, temperature and time, centrifugation rotation speed and time (Table 1, No.18) to achieve the extract with maximum quantities of target compounds. DES is selected based on its high hydrophobicity, low viscosity and high extraction efficiency of curcuminoids as well as both components of DES are food grade with GRAS status. Hydrogen bonds and Van der Waal interactions exists between both components. Requisite quantities of HDES-surfactant and turmeric powder are subjected to required ultrasonication frequency for optimized time. Extract subjected to centrifugation. Supernatant collected and analysed using HPLC-UV. HDES based micro emulsions are prepared using surfactant along with cosurfactant. HDES and surfactant mix homogenised and water is added drop wise under agitation to obtain transparent microemulsions. These micro emulsions are used for the extraction of target compounds as above and are analysed by HPLC-UV (Yields in percentage on dry basis: bisdemethoxycurcumin-2.49, demethoxycurcumin-5.61, curcumin-12.6 and ar turmerone-2.58 respectively). The yields are more when compared to hydrophobic DES itself due to lager surface areas of micro emulsions. These HDES and HDES based micro emulsions are found to be more efficient than hydrophilic DES and routine organic solvents to extract curcuminoids. These micro emulsions based extracts are well matched with various water based food and beverage applications and provide additional health benefits. HDES based microemulsions can easily solubilize the target bioactive compounds in aqueous environment as well as protect them from degradation. Authors claim that hydrophobic DES turmeric extracts with curcuminoids could be used in pharmaceutical, colouring, and food industries. Extraction of curcuminoids using NADES [choline chloride – 1,4 butane diol (1:6) + 10% water-selected] with ultra-sonication assistance from turmeric is reported [36]. Variable parameters optimized are solid to liquid ratio, solvents, NADES composition and water quantity, ultrasonication power, temperature and time (Table 1, No.20). NADES is selected based on the low-toxicity of the components, as well as its high extraction efficiency due to high selectivity of NADES towards curcuminoids and weak acidic hydroxyl groups. Optimized quantities of turmeric powder and NADES are taken and extracted using ultrasonication at necessary frequency for required time at ambient temperature. Extracts are subjected to centrifugation. Crude extracts / supernatants are dissolved in known quantity of HPLC grade methanol and subjected to HPLC analysis. Curcuminoids mixture from crude NADES extract (supernatant) is separated by adding three folds of water and kept for one day for complete precipitation. Precipitated curcuminoids are filtered, dried and preserved for further use. Extract containing curcumin (46.70 mg/g), bisdemethoxy curcumin (46.14 mg/g) and demethoxy curcumin (10.63 mg/g) is reported under optimized conditions. Significant rupture of plant cells is observed in SEM analysis, after extraction with DES. Residue from NADES contained 55.86 mg/g of total curcuminoids. Extraction phenomenon is explained through conductor-like screening model for realistic solvents (COSMO-RS) computational analysis. The higher extraction efficiency of NADES is owing to solvent interactions with the cell walls of the sample and no correlation with profiles of solvent polarity and solubility. Selected NADES extraction of curcuminoids from turmeric using ultrasonication is considered as one of the excellent technique as per CHEM21 green metrics due to higher yields, accessible, inexpensive bio-based solvent with low toxicity. Authors claim this technique possesses a good potential as sustainable and alternate.
Isolation of curcuminoids from turmeric using NADES (choline chloride – citric acid (1:1) + 30% water) with assistance of microwave energy (NADES-MAE) is described [37]. Flexible factors optimized are type and composition of NADES, water quantity in NADES, solid particle size, solid to liquid ratio, microwave power, soaking time, stirring speed / mode, extraction time (Table 1, No.24). NADES is chosen based on its lower melting point, stability under microwave conditions as well as high extraction efficiency due to the presence of more carboxyl groups to form hydrogen bonds with target molecules. Known quantities of turmeric powder and water are taken in a tube and kept for soaking for required time under microwave power. NADES (100%) is added to the above mixture to make optimum aqueous NADES. This mixture is subjected to extraction using microwave irradiation for required time. Mixture is subjected to centrifugation under optimized conditions. Supernatant is taken and diluted with Mobile phase and analysed by HPLC. NADES-MAE provided maximum quantities of target compounds (89.87 mg/g) into the extract. Curcuminoids from the extract are recovered using anti-solvent (water) precipitation. Measured volume of supernatant is taken and water (20 fold) is added slowly under magnetic stirring. Total solution is incubated for required time for precipitation of curcuminoids. Solution with precipitated curcuminoids is subjected to cooling centrifugation to separate curcuminoids. Separated curcuminoids are collected, air-dried, analysed by HPLC and preserved. Recovery of curcuminoids from NADES extract needs to be improved. Period of extraction is shorter. Cost of production for curcuminoids (1 Kg) is discussed and reported as lowest in the current process, when compared to all other reported methods, as energy required and time of extraction are minimum.
Turmeric phytochemicals - bioactivity studies
Extract containing curcumin and coumarin from turmeric using NADES (citric acid - propane diol - betaine) is isolated [38]. Optimized parameters are type and composition of NADES, sample to solvent ratio, ball mill frequency and time, centrifugation speed and time (Table 1, No.14). NADES is selected based on the highest extraction efficiency for spice specific major active compounds. Known quantities of spice powder and DES are taken and the suspension is shaken in a ball mill at specified frequency for required time to obtain the extract. Extract is subjected to centrifugation at optimized conditions and supernatant is taken for further analysis and studies. NADES extract of turmeric is analysed for curcumin (140.96 mg /g), coumarin (13.60 mg/g), cinnamal (84.27 mg/g) and eugenol (0.358 mg/g) using UV-Vis. NADES extract is evaluated for total polyphenol content (18.40 mg GAE / g) total flavonoid content (3.61 mg quercetin / g) and antioxidant activity (DPPH - 59.6 %). NADES turmeric extract is used to prepare homogenous selenium sulphide nanoparticles. Optimized conditions are concentrations of selenium chloride and sodium sulphide, pH of the solution using sodium hydroxide. Mixture of known quantities NADES spice extract and SeCl4 (specified concentration) is prepared. Selenium sulphide nanoparticles (SeS2 NPs - red) are prepared by the addition of Na2 S•H2 O (known concentration) into the mixture. SeS2 NPs are stabilised owing to the presence of spice extracts. Dimensions of homogenous selenium sulphide nanoparticles [crystallite size: 28.72 nm; particle size: ~ 830 nm] are analysed by Fourier-transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). These nanoparticles repressed the growth of pathogenic fungi (469 mg/ dm3) and bacteria (117 mg/dm3) at minimum biocidal concentrations due to increased dispersion of NADES extract. These nano particles in NADES extract suppressed (99%) influenza viruses and B-coronavirus by damaging the viral surface proteins, which controlled the viral replication. These authors also reported the isolation of extract containing curcumin, coumarin, cinnamal and eugenol from turmeric and black pepper mixture using NADES under optimized parameters (Table 1, No.15). Bioactive phytochemicals [curcumin (120.43 mg /g), coumarin (11.79 mg/g), cinnamal (69.39 mg/g) and eugenol (0.348 mg/g)] are estimated from NADES extract of mixed spice using UV-Vis. NADES extract is also analysed for total polyphenol content (36.30 mg GAE / g) total flavonoid content (1.06 mg quercetin / g) and antioxidant activity (DPPH - 49.7 %). The NADES extract is used for preparing the homogenous selenium sulphide stable nanoparticles in NADES and similar antiviral activities are reported. These nanoparticles repressed the growth of pathogenic fungi (469 mg/dm3) and bacteria (59-117 mg/dm3) at minimum biocidal concentrations owing to diffusion of NADES extract and synergistic effect of bioactive phytochemicals in NADES extract.
Extraction of turmeric using hydrophobic DES (HDES) based micro emulsion [octanoic acid - menthol (20:80, mass ratio)] / tween 80: propylene glycol (1:1) / water, 25/70/5] to obtain bioactive compounds [39]. Variable parameters optimized for micro emulsion preparation are HDES ingredients and their mass ratio as well as other components and their molar ratio, stirring time and heating temperature. HDES is selected, as both components provide assistance in anti-inflammatory mechanism. Known quantities of HDES components are mixed, while stirring on heating for required period to obtain hydrophobic DES. Requisite amounts of HDES and surfactant are mixed and homogenized. Water is added to this homogenous mixture with vigorous stirring to obtain transparent HDES microemulsions. Optimized conditions for extraction are hydrophobic DES ingredients and their molar ratio, solid to liquid ratio, ultrasonication frequency, time and centrifugation speed (Table 1, No.22). Known quantity of dried turmeric powder is extracted using HDES microemulsion under ultrasonication with specified frequency for required time. Extract is centrifuged under optimized conditions. Supernatant is taken for further analysis and studies. Yields of active compounds (bisdemethoxycurcumin-1.69, demethoxycurcumin-3.04, curcumin-7.36, and ar-turmerone-1.39 percentages on dry basis) are reported. IC50 value of HDES micro emulsion is 0.0136 %v/v, while an IC50 value of curcumin in HDES micro emulsion turmeric extract is 75.2 ±6.7 nM for NO production inhibition. However, it is 103 fold more effective than curcumin in dimethyl sulfoxide solution. These micro emulsions lowered inflammatory cytokines tumour necrosis factor-α, interleukin (IL)-6, and IL-1β production in lipopolysaccharide-activated murine macrophages simultaneously. Hence, extract obtained from turmeric using therapeutic hydrophobic deep eutectic solvent based micro emulsion enhanced the anti-inflammatory efficacy of curcuminoids and aromatic-turmerone. Further curcumin is stable for 30 days in these microemulsions. Extraction of curcumin from turmeric using Hydrophobic DES [menthol-lactic acid (1:2), menthol acetic acid (1:1)] and stabilisation of curcumin containing extract with the preparation of polymer emulsion using carboxy methyl cellulose and sodium alginate biopolymer (1:1) as wall material is reported [40]. NADESs are selected basing on their extraction efficiency of target compounds from turmeric [31]. Optimized conditions for extraction are NADES ingredients and their molar ratio, solid to liquid ratio, ultrasonication frequency, time, temperature, centrifugation speed (Table 1, No.25). Required quantities of sample powder and NADESs are mixed in dark bottles and subjected to extraction using ultrasonic bath at necessary power, time and temperature. Extracts are centrifuged under optimized conditions. Supernatant is taken for further analysis and studies. 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH) antioxidant capacity of the turmeric extracts is reported in the range of 24.4 to 33.9 mg trolox/g and stable for 30 days. ABTS antioxidant capacity of the turmeric extracts is reported in the range of 7024.7 to 8486.3 µmol trolox/L and decreased activity up to 10 days (~4455 µmol trolox/L) and stable from 10 to 30 days. Variable parameters optimized for emulsion preparation are calcium chloride concentration, time, temperature, centrifugation speed during the addition of turmeric extract as well as calcium chloride. Wall material is prepared by mixing equal quantities of sodium alginate and a mixture of the biopolymers sodium alginate and carboxy methyl cellulose in aqueous media at specified concentration under stirring at required temperature. Turmeric NADES extract taken in syringe and is added drop wise to wall material solution under agitation at optimized speed for required time for homogenization. Calcium chloride solution (specified concentration) is dripped under stirring at optimized speed for specified time to form emulsion gel. Both solutions are mixed in an ultrasonic bath at specified frequency for required time under protection from light to form stable emulsion. Stable emulsion is used for further studies. Emulsions of turmeric extracts showed lower antioxidant capacities than turmeric extracts due to partial release of bioactive compounds during sample preparation. However, these antioxidant capacities are stable for 30 days due to the influence of structure of emulsions. These emulsions could be useful for food and pharmaceutical applications.
Curcuminoids Analytical studies
Separation and determination of trace amounts of curcuminoids from foods and herbs (turmeric drug, turmeric powder, and turmeric root herbal tea) is reported using vortex assisted DES [choline chloride - phenol (1:4)] emulsification liquid-liquid microextraction [VA-DES ELLME]. Optimized parameters are pH, molar ratio of DES composition, volume of DES, volume of tetrahydrofuran (THF), sample volume, vortex time, centrifugation speed and time (Table 1, No.1). DES is selected based on its properties of emulsification, self-aggregation and extraction efficiency towards curcuminoids. Known quantities of sample (containing curcumin) solution and buffer (pH 4) solution are mixed. Requisite quantity of DES is injected rapidly into the above solution. Homogenous solution is formed. Required quantity of emulsifier is added to the homogenous solution to form cloudy solution. Cloudy solution is subjected to vortex mixing for the extraction of curcuminoids into DES droplets. DES phase with curcuminoids is separated from aqueous phase by centrifugation at optimized conditions. Aqueous phase is removed. DES phase with curcuminoids is diluted to known volume with alcohol and analyzed by HPLC-UV [41]. Curcumin concentration augmented in DES phase and used for estimation. Recovery rate (96 to102%) and pre-concentration factors (12.5) are reported in this technique. LOD and LOQ are found to be 2.86 and 9.44 μg/L respectively. Curcumin trace quantities in real samples are determined using this VA-DES-ELLME method [Liquid samples (µg/L): Turmeric liquid-380; Herbal tea-Swedish syrup (turmeric)-54; Herbal tea-ginger-Lemon-50, Solid samples (mg/g): Drug-0.37 to 0.40; Powder-8.25 to 8.36; Root herbal tea-7.45 to 7.60]. Determinations of curcumin using UV-Vis and HPLC techniques are comparable.
Vortex assisted natural deep eutectic solvent [betaine hydrochloride - glycerol (1:3)] microextraction (VA-DES-ME) is reported for the separation of curcumin from food samples followed by high performance liquid chromatography (HPLC) analysis for estimation [42]. Optimized experimental variables are type, volume and composition of NADES, pH, aprotic solvent and its volume, vortex speed and time, sample volume and matrix effect (Table 1, No.3). NADES is selected basing on the strong H-bonding, ion-dipol and dipol-dipol interactions, π-π stacking and weak hydrophobic interactions with the target curcumin. Further Betaine hydrochloride can spontaneously self-assemble to form micelle-like assemblies depending on the solvent environment, act as hydrophilic and hydrophobic nanocontainers in apolar and polar solvents at isoelectric point of about 6.5, and undergo pH-dependent surface charge and size variations, as it is a zwitterionic amphiphilic surfactant. Identified quantity of ground and homogenized samples are extracted with known quantities of methanol successively, until a clear solution is achieved. Combined total extract is cooled and centrifuged. Supernatant is filtered and diluted to known volume. Requisite quantity of food sample solution (pH 6) is taken and known volume of NADES is injected rapidly to form microspheres. Specified volume of aprotic solvent is added to the mixture to obtain more microspheres. Total solution is subjected to vortex mixing for extraction of curcumin into NADES. Entire solution is centrifuged to separate aqueous phase and NADES phase with curcumin. Upper aqueous phase is removed. NADES phase is diluted suitably with ethanol and is analyzed by spectrophotometer. Maximum separation of curcumin even at ppb levels is achieved. Vortex-mixing increases the number of NADES microspheres and dispenses evenly in sample solution, which improves the mass transfer of the target compounds to the NADES phase. Centrifugation allows the separation of NADES phase with bioactive compounds. Recovery rate (90.9 to108.3%) and pre concentration factors (100) are reported. LOD and LOQ are found to be 1.5 and 5.0 μg/L respectively. The curcumin in selected food and beverage samples is reported in the range of 13.9 to 154.7 micro grams/liter. It can be applied to wide range (5 to 300 μg/L) of quantities. Time taken for analysis is shortest. Instrument used is spectrophotometer, which is cheapest, Estimation of curcuminoids in Curcuma Longa Rhizomes and turmeric tea using Deep eutectic solvent [tetra butyl ammonium chloride and decanoic acid (1:1) - selected among other hydrophobic and low density DES] dispersive liquid-liquid microextraction based on solidification of floating organic drop (DES-DLLME SFO) is reported [43]. Optimized variables of extraction are DES type and composition, sample pH, stirring rate,extraction time and temperature (Table 1, No.4). DES is selected based on its properties such as low aqueous solubility, high dispersion and extraction capacity, low density and melting point near to ambient temperature. Known quantities of sample and methanol are taken in suitable flask and soaked for overnight. Total solution is subjected to ultrasonication for required time. Solution is cooled and weight loss is made up with the addition of methanol. Total solution is filtered and filtrate is sample solution. Required quantities of sample solution and water are taken and hydrochloric acid is added to bring down the solution pH to 3. Total solution and known quantity of DES are mixed and stirred at optimized conditions. DES is dispersed as fine droplets to improve the contact area with target analytes for effective extraction to achieve concentrated curcuminoids into DES droplets. DES droplets (curcuminoids) are floating. These droplets are accumulated as aggregates on gentle rotation and cooled for solidification at low temperatures. Combined and solidified floating droplets are collected. Droplets are brought to room temperature for melting. Melted droplets solution is diluted with methanol for HPLC analysis to obtain best analytical results for three curcuminoids. Recovery rates (84.6 to 114.8%) and enrichment factors (608 to 848) are reported. LODs and LOQs for three curcuminoids are found to be in the range of 7 to 9×10-5 and 20 to 30×10-5 mg/L respectively. Real samples of turmeric rhizomes and turmeric tea are analyzed for curcuminoids. Small quantity of DES is enough. Dispenser solvent and centrifugation step are not required. Solvent terminated micro-extraction (STME) procedure for the estimation of curcuminoids using DES [L-menthol and lactic acid (1:2)] is developed [44]. Variable parameters optimized are sample volume, aqueous phase pH, salt concentration and volume, DES volume, shaking time, type of demulsifier, demulsifier volume and time (Table 1, No.21). DES is based its high extraction efficiency. Known quantities of sample and methanol are taken in suitable flask and soaked for overnight. Total solution is subjected to ultrasonication for required time. Solution is cooled and weight loss is made up with the addition of methanol. Total solution is filtered and filtrate is sample solution. Known quantities of sample solution, salt solution and DES are taken in a tube and total mixture is shaken under optimized conditions. Requisite quantity of demulsifier (methanol) is injected slowly to break the turbid solution. Emulsion is separated into two phases, after standing for required time. Upper phase DES with analytes is collected and diluted with methanol to analyze on HPLC. Curcuminoids forms hydrogen bonds with DES and gets extracted. Under optimized conditions, enrichment factors (279 to 350), recovery rates (85.3 to 108.9%), Limits of Detection (LODs - 0.1 to 0.4 ng/mL) and Limits of Quantification (LOQs - 0.4 to 1.2 ng/mL) are reported for curcuminoids. Dispersion liquid and centrifugation step are not required. Curcuminoids are determined in turmeric rhizomes and turmeric tea samples using the developed method. Turmeric rhizomes contained bisdemethoxy curcumin, demethoxy curcumin and Curcumin - 5.18, 2.40 and 6.19 mg/g respectively, whereas turmeric tea showed the presence of bisdemethoxy curcumin, demethoxy curcumin and Curcumin – 2.24, 1.43 and 4.70 mg/g respectively, This technique found to be efficient, when compared to other methods. An analytical method for estimation of curcumin in food samples (e.g., herbal tea, turmeric powder, turmeric drug) using a reusable and switchable deep eutectic solvent (octyl amine: succinic acid: water molar ratios 1:2:5) dispersive liquid-liquid microextraction [RS-DES-DLLME] is developed [45]. DES is selected basing on its switchable property from hydrophilic to hydrophobic and reverse using the addition of base or acid. Optimized experimental variables are type, volume and composition of DES, heating time, shaking time, sample volume, volumes and concentration of sodium hydroxide and hydrochloric acid, vortex time, ultrasonication time, centrifugation speed and time (Table 1, No.27). Known quantities of sample and methanol are taken and subjected to extraction using ultrasonication under optimized conditions. Extracted solution is centrifuged under specified conditions and supernatant is separated and taken as sample solution. Requisite quantities of sample solution and hydrophilic DES are taken in a tube and shaken manually to obtain a homogenous solution. Known quantity of alkali is added to homogenous solution and subjected to vortex for certain period. Hydrophobic DES (switched) is separated and extracted the target compounds into DES. DES layer is separated as upper phase with target compounds and is separated. Acid with known concentration and quantity is added to DES phase and converted to hydrophilic by manual shaking. Supernatant analyte phase is separated, diluted with methanol and subjected to analysis. Lower phase DES is regenerated and reused. Maximum separation of curcumin even at µg/Kg levels is achieved. Sodium hydroxide (NaOH-3M) and hydrochloric acid (HCl-3M) are used for DES phase transition. Sodium hydroxide is added to change the DES to hydrophobic for extraction of analytes. DES layer with analytes is treated with hydrochloric acid for the release and separation of DES and analytes. Recovery rates (92 to 100 %) and Enrichment factors (38.68) are reported. LOD and LOQ are found to be 10 and 35 μg/L respectively. Curcumin content in real samples of herbal teas, turmeric drug and powder is estimated (42 to 159 µg/Kg).
Curcuminoids solubility and stability studies
High solubility and increased stability of curcumin in novel water-in-oil micro emulsions formed using a non ionic surfactant Triton X-100 (0.3M) and a hydrophobic deep eutectic solvent [HDES - tetra-n-butyl ammonium chloride and n-decanoic acid (1:2 mole ratio) is reported [46]. DES is selected based on its hydrophobic nature, as curcuminoids are hydrophobic. Known quantities of DES and surfactant solution of optimal concentration are taken and subjected to shaking. Pre-calculated optimum quantity of water is added to the DES surfactant solution. Surplus quantity of curcumin is added to DES-surfactant water microemulsion system and stirred at optimized conditions. Total solution is kept for equilibrium at ambient temperature and is centrifuged. Supernatant containing curcuminoids is analyzed. Water addition to the DES-surfactant system, formed the micro emulsion and increased the solubility of curcumin due to solvation at interfacial region. Surfactant based water-in-DES micro emulsion system as a potential solvent system for solubility [up to 51 mg/mL under ambient conditions-indicated by Ultra-Violet (UV) and Dynamic Light Scattering (DLS) study] and stability of curcumin is produced. Under the optimized conditions (Table 1, No. 7), excellent uptake of curcumin is observed in the system. This micro emulsion system also exhibited improved stability of curcumin towards sunlight (4h – 25% degradation) and long-term storage (15 days in dark at room temperature – 25% degradation). These micro emulsions could be useful in pharmaceutical industry. Enhanced solubility of curcumin in low viscosity hydrophobic DES [camphor and menthol (1:1) - selected among others] is reported [47]. Optimized conditions are temperature, stirring time and speed, centrifugation speed and time, buffer pH (Table 1, No. 6). DES is selected based on its hydrophobic nature, as curcuminoids are hydrophobic. Excess quantity of curcumin is added to known volume of DES and mixed using magnetic stirrer at optimized speed and temperature for required period. Saturated solution is kept overnight and subjected to centrifugation. Supernatant diluted with methanol and buffer is added before ultra violet –visible (UV-VIS) analysis. Curcumin solubility is doubled when temperature is increased to 35-40oC, when compared to room temperature with in very short time of stirring. Under optimized conditions curcumin solubility increased to 22.07 mg/mL. Curcumin can exist in keto-enol forms. Presence of keto and alcoholic groups in curcumin favours solubility in the camphor menthol DES. Increased curcumin concentrations in DES may improve the use of curcumin pharmaceutical and food industries as per authors’ claim. Solubility of curcumin is increased in aqueous media with deep eutectic solvents [NADES: choline chloride - ethylene glycol (1:2) – selected among other choline chloride based DESs] as co-solvent [48]. Co solvent DES is selected based on its activity coefficient, aqueous miscibility and curcumin solubility. Surplus quantity of curcumin is added to water and co-solvent NADES mixture. Mixture stirred at optimized speed and temperature to obtain saturated solutions of curcumin. Solution is allowed to reach equilibrium. Saturated curcumin solution is taken and determined the quantity of curcumin. Correlation between solubility of curcumin observed with concentration of co-solvent (NADES) and temperature (33000 fold@40oC). Observations are confirmed using thermodynamic functions and activity coefficients [electrolytes - Non-random two-liquid model (9.85%) > Universal quasi-chemical model (10.86%)]. Further, curcumin dissolution in aqueous NADES media is found to be endothermic (Table 1, No.8). Solubility of curcumin in ethanol, ethanolic hydrogen bond acceptors (betaine, L-carnitine, choline chloride, tetra propyl ammonium bromide), ethanolic hydrogen bond donors (lactic acid, levulinic acid, pyruvic acid, 2-oxobutanoid acid, 2–oxovaleric acid, acetyl butyric acid, L-mandelic acid, hexanoic acid) and ethanolic DESs is evaluated. One gram each of HBAs, HBDs, and NADESs in ethanol are taken separately and stirred to obtain homogenous solutions. HBAs and HBDs are dissolved upto their solubility limits in ethanol. NADES are mixed upto a maximum of 70 % in ethanol. Excess curcumin is added to these ethanolic solutions and saturated curcumin solutions are prepared by stirring at room temperature for one hour. Saturated solutions are filtered and curcumin quantity is determined in filtrates. Solubility of curcumin is increased in ethanolic HBAs and HBDs. However solubility of curcumin is increased (twenty-fold) enormously in ethanolic solutions of natural deep eutectic solvents [NADES: HBAs -levulinic acid - selected among other acids]. Correlation between solubility of curcumin, with concentration of NADES is observed. These observations are confirmed using conductor-like screening model for realistic solvent / solvation (COSMO-RS) and nuclear magnetic resonance spectroscopy (NMR) [49]. It is reported that the highest observed solubility of curcumin in ethanol is due to target-specific intermolecular interactions of quaternary ammonium compounds and levulinic acid (Table 1, No.13). Highest solubility is observed with ethanolic DESs containing levulinic acid is due to formation of cyclic confirmation between curcumin and levulinic acid through target specific interactions with functional groups as well as synergistic effect of DES. Structural and thermodynamic features of NADES (based on choline chloride and nicotinamide) and curcumin using COSMO-RS technique is characterised. Initially COSMO-RS technique studied the formation of hydrogen bondings between the components of NADES as well as water molecules. Later, COSMOS-RS used to determine the activity coefficients of curcumin in NADES. Activity coefficients of curcumin in all studied NADES and individual components of NADES are higher than water. It provided the information related to solubility and extractability of curcumin in NADES to develop the best method for processing. This approach is useful to screen NADES and solutes. It is concluded that solubility increase is due to intermolecular hydrogen bonding and Van der Waals interactions between curcuminoids and DESs [50]. Curcumin is more soluble in choline chloride - glycerol NADES than the other NADES examined, as per the activity coefficient. Authors claim COSMO-RS method may screen for the best solvent for any other solutes also. This technique could be used to screen a broad range of solvents used for solubility or extraction. It may reduce experimental effort and save time in the industry. It can create the data base of novel high-performance solvents in the pharmaceutical biological and chemical fields. Enhancement of solubility (1700 times) of curcuminoids (Table 1, No.23) in aqueous DES [choline chloride - 1, 2-propanediol (1:4) + water – selected, NADES mass fraction-0.8] is described [51]. Known measures of DES and water are mixed and excess quantity of curcumin is added. Total mixture is stirred for five days in a thermostat water bath to obtain saturated solution and to reach equilibrium. Saturated solution is subjected to centrifugation and filtration to remove undissolved curcumin. Curcumin is determined in the saturated transparent solution using HPLC. Electrostatic potential (ESP) and density functional theory (DFT) analyses are applied for the evaluation of interaction energies of NADES and curcumin systems. Aqueous solubility of curcumin increased due to the stability of curcumin in NADESs with the presence of Van der Waals interactions and hydrogen bonding between NADESs structure and curcumin molecule as per theoretical and experimental calculations. These calculations indicated lowest interaction energies in NADES and curcumin system, which provide more stability to the system. Hence, the NADES-Curcumin with lowest interaction energy, respective NADES may become as co-solvent to improve the solubility in aqueous medium. Therefore, the above NADES [i.e., choline chloride - 1,2-propanediol (1:4) + water] - curcumin system could be useful for dissolution and utilization of curcumin drug in medicinal and pharmaceutical industry as per the authors. Authors provided quantum chemistry calculations for the enhancement of solubility. Solubility of curcuminoids extracted from turmeric using the NADES [choline chloride - malonic acid (1:1) + 50% water; reasons for NADES selection are provided in earlier paragraphs] is upto 590,000 times (Table 1, No.17) higher than water. Hansen solubility parameter are used to select the NADES and its composition, which considered the interactions between solute and solvent [33].
Bioavailability of curcuminoids
Isolation of extract containing curcuminoids using NADES [choline chloride - propylene glycol (1:1) + 30% water] from turmeric is described [52]. Optimized parameters are type and composition of NADES, water quantity in NADES, solid to liquid ratio, ultrasonication temperature and time, centrifugation speed and time (Table 1, No.19). NADES is selected based on its high extraction efficiency towards total curcuminoids as well as individual curcuminoids. Further hydrogen bonding donating ability of propylene glycol is also one of the reasons. Curcuma longa rhizomes are dried and powdered. Known quantities of sample and DES are taken in a tube and subjected to extraction using ultrasonication under optimized conditions for required time at necessary temperature. Total mixture is subjected to centrifugation under selected parameters. Supernatant is taken and purified by solid phase extraction using chosen steps. Purified extract is subjected to in vivo bioavailability studies as well as analysis on HPTLC and HPLC. It is reported that bioavailability of curcuminoids from DES extract is enhanced more than 450% times, when compared to organic solvent extracts from pharmacokinetic observations. Curcumin concentration in rat plasma is increased by 6.93 times. Thirty-one curcumin metabolites are identified using UPLC-MS-MS in rat plasma/serum. Reductive metabolites (Phase 1; absorption-689%), glucuronide and sulphate conjugates (Phase 2; absorption-321%) of the three curcuminoids are identified and absorption improvement is reported. Natural deep eutectic solvents-water-in-oil high internal phase emulsion (NADES-HIPEW/O) is prepared using natural deep eutectic solvents (glucose-sucrose at different molar ratios) to enhance the loading and stability of nutraceuticals (e.g., curcumin) [53]. Optimized parameters are type and composition of natural DES, water quantity in NADES, solid to liquid ratio, heating
Table S1: Classification of deep eutectic solvents and Natural deep eutectic solvents used in the processing of turmeric for bioactive phytochemicals [61-63].
|
Bioactive phytochemicals |
Type 3 |
Type 5 |
|
Curcuminoids-extraction |
choline chloride + lactic acid (1:1), [29]; fructose-choline chloride-water (2:5:5), [30]; sucrose-choline chloride-water (1:4:4), [30]; lactic acid-choline chloride-water (1:1:2), [30]; choline chloride-lactic acid (1:1), [32]; choline chloride-malonic acid (1:1), [33]; choline chloride-propylene glycol (1:2), [34]; choline chloride-1,4 butane diol (1:6), [36]; choline chloride-citric acid (1:1), [37]; citric acid-propanediol-betaine (2:2:1), [38]; (Ternary) |
citric acid – glucose (1:1) [28]; fructose-lactic acid-water (1:5:5), [30]; sucrose-lactic acid-water (1:5:7), [30]; octanoic acid-menthol (1:3.6), [35]; [octanoic acid - menthol (20:80, mass ratio)] /Tween 80: propylene glycol (1: 1)/water, 25/70/5], [39]; Menthol-Lactic acid (1:2), [40]; Menthol-Acetic acid (1:1), [40]; |
|
curcuminoids - estimation |
choline chloride - phenol (1:4), [41]; betaine hydrochloride - glycerol (1:3), [42]; tetra butyl ammonium chloride-decanoic acid (1:1), [43]; |
L-menthol and lactic acid (1:2), [44]; octyl amine-succinic acid-water molar ratios (1:2:5), [45] |
|
curcuminoids solubility and stability |
tetra-n-butyl ammonium chloride-n-decanoic acid (1:2), [46]; Choline chloride - Ethylene Glycol (1:2), [48]; betaine-lactic acid (1:2), [49]; betaine-levulinic acid (1:2), [49]; betaine- pyruvic acid (1:2), [49]; L-carnitine-lactic acid (1:1), [49]; Lcarnitine-levulinic acid (1:2), [49]; Lcarnitine-pyruvic acid (1:2), [49]; choline chloride-lactic acid (1:1), [49]; choline chloride-levulinic acid (1:1), [49]; choline chloride-pyruvic acid (1:2), [49]; choline chloride and nicotinamide, [50]; Choline chloride-1, 2-Propanediol (1:4), [51]; choline chloride-malonic acid (1:1), [33]; |
camphor-menthol (1:1), [47]; |
|
Curcuminoids - bioavailability |
choline chloride-propylene glycol (1:1), [52]; |
Glucose-Sucrose [53]; |
|
Curcuminoids-drug carrier |
choline chloride and glycerol (1:1), [54]; |
|
|
curcuminoids-antimicrobial activity |
|
Malic acid-Sucrose-Water (1:1:18), [56]; |
|
Curcuminoids starch nanoparticles- industry |
Choline chloride and lactic acid (1:1), [57]; |
|
|
curcumin nanifibres-drug adsorption |
|
Thymol: menthol (1:1), [58]; |
|
Textile dyeing |
|
glycerol-urea (1:1), [59]; |
|
Methylglyoxal trapping |
betaine-glycerol (1:2), [60]; |
|
|
Antioxidant compounds - extraction |
|
menthol + lactic acid (2:1), [31]; menthol + acetic acid (1:1), [31]; |
|
turmeric essential oil |
choline chloride-oxalic acid (1:1), [55]; |
|
temperature and time as well as cooling time (Table 1, No.26). NADES is comprised of both hydrogen bond donors (i.e., sugars). Known quantities of curcumin and NADES are taken and kept for stirring at optimized temperature for required period to obtain curcumin containing NADES solution. Oil phase is prepared by mixing known quantities of coconut oil and polyglycerol polyricinoleate. NADES HIPEs are prepared by mixing required quantities of NADES curcumin solution and oil phase using a dispersing device at optimized temperature, speed for required time. Total mixture is cooled in ice water to form semi solid curcumin loaded NADES-HIPEs. NADES-HIPEW/O emulsions exhibited centrifugal, thermal, freeze-thaw, and storage stability. Curcumin in NADES-HIPEW/O emulsions exhibited higher UV stability up to 72 h, when compared to coconut oil (Table 1, No.26). Degradation of curcumin is reduced, as curcumin is less exposed to UV light due to the presence of sugar crystals and water droplets. NADES-HIPEW/O emulsions also improved the curcumin gastrointestinal digestible stability as well as the curcumin concentration in the micelles during in vitro digestion. This is due to encapsulation of curcumin in NADES-HIPEW/O emulsions. These are useful in food and drug applications.
Turmeric extract from DES as drug carriers Extract containing curcumin is isolated from turmeric using natural deep eutectic solvent [NADES- choline chloride and glycerol (molar ratio 1:1)]. NADES is selected based on the high solubility of curcumin. Known measure of NADES is taken and excess amount of curcumin is added into it. Mixture is stirred and allowed to reach equilibrium at ambient temperature for required period. Total solution is centrifuged and filtered. Filtrate / supernatant is subjected to spectrophotometric analysis to determine the ‘curcumin solubility’ in the NADES. Extraction of turmeric powder is described. Known quantities of turmeric powder and NADES are taken in a sealed vessel and subjected to extraction using mechanical stirrer for required time. Curcumin concentration is determined in the extract as described above. Higher extractability, higher solubility, stability for longer periods using the NADES is reported. Curcumin in the extracted product is stable (even under the exposure to sunlight for 72h) without degradation. It is owing to dominant intermolecular interactions and lowest Gibbs free energy with NADES-curcumin complex. DES extracted product is directly used in simulated gastrointestinal fluids (i.e., fasted state simulated intestinal fluid and fasted state simulated gastric fluid) for curcumin delivery as drug [54]. Solubility of curcumin (7.25mg/g) in NADES is 12,000 times higher than in aqueous solution. Quantum chemistry computations showed the reason (hetero-molecular pairs with choline chloride and glycerol are formed) for the improved solubility of curcumin in NADES in the presence of gastrointestinal fluids.
Extraction of essential oils from turmeric
A method to obtain essential oil using NADES [choline chloride-oxalic acid (molar ratio 1:1)] microwave assisted hydro distillation (MAHD) is developed [55]. Optimized conditions are type and composition of NADES, solid to liquid ratio, microwave power, heating temperature and time for pretreatment as well as MAHD time to obtain maximum quantity of essential oil (Table 1, No 12). NADES is selected based on its high extraction efficiency of essential oil through acid-base catalysis mechanism, which can cleave ether linkages and separate from biomass. Sample is pretreated with NADES and MAHD is used for isolation of essential oil. Known quantities of sample and NADES are taken in three necked flask and solution is pre-treated under optimized microwave power and temperature for necessary time. Required volume of water is added to the pre-treated solution. Clevenger hydro distillation system is fixed to the flask to collect essential oil. Solution is brought to boiling point under selected microwave power, temperature and time. Hydro distillation is carried out under optimized microwave power and temperature for necessary time to collect essential oil. Essential is separated from aqueous phase and dried over anhydrous sodium sulphate. Essential oil is preserved at 4 oC for further analysis. Higher temperature during pre-treatment controlled the DES viscosity, which facilitated the penetration of DES into material and release of essential oils efficiently. Yield of essential oil using NADES based MAHD is very high (0.85%), when compared to other methods. Forty-nine compounds are identified using GC-MS. Oxygenated compounds in NADES based MAHD essential oil are more, when compared to other methods. In general, oxygenated compounds contribute more towards the flavor of the essential oil. Hence essential oil obtained in the developed technique (NADES-MAHD) is high valued for food, flavor and fragrance industries.
Miscellaneous applications of deep eutectic solvents in curcumin / turmeric processing.
Curcuminoids dissolved in designated volume of NADES [MAS: malic acid-sucrose-water (1:1:18)] under sonication assistance. Curcuminoids concentration (3.00 mM) is optimized. Curcuminoids-NADES (MAS-H2O) showed effective antimicrobial activity against Escherichia coli and Staphylococcus aureus, when compared to the other NADES [56]. The antimicrobial activity of curcuminoids in MAS-H2 O to both organisms may be due to the low pH condition of NADES itself since malic acid has high acidity (pH <3). NADES weaken microbes by extracting water soluble and insoluble components from the membrane. Authors are expecting the curcumin in NADES will be useful for antimicrobial photodynamic therapy (aPDT).
Starch nanoparticles are filled with Curcumin (7.99%) using deep eutectic solvent [(choline chloride and lactic acid (1:1)]. NADES is selected based on its extraction efficiency towards curcuminoids. Known quantities of debranched starch (1.5g) and distilled water (10mL) are taken and starch is gelatinized using autoclave at optimized conditions (temperature-120oC, time-30 minutes). Required amount of curcumin (12g) in dissolved in NADES (8 to 14g). Dissolved curcumin is added to gelatinized starch solution and vortexed using necessary conditions (speed-3200 rpm, time-3minutes). Mixed solution is kept at 4oC for 24 h. Solution is washed with water and centrifuged under required parameters (speed - 4800 rpm, time - 20 minutes). Supernatant is removed and precipitate is washed with ethanol to remove free curcumin. Product is lyophilized to acquire self-assembly curcumin-loaded starch nanoparticles and yield is calculated [57]. Curcumin starch nanoparticles are analysed by scanning electron microscopy and confocal laser scanning microscopy and indicated the formation of non-inclusion complex. Curcumin starch nanoparticles provided pH stability (even under alkaline conditions) and photo stability to curcumin as indicated by NMR and FT-IR. Authors expected that curcumin filled starch nano particles may find application in functional foods and edible packaging.Cur-HNADES- PVA nanofibers are prepared using hydrophobic natural deep eutectic solvent [HNADES: thymol-menthol (1:1)], curcumin and PVA mat [58]. Known quantity of curcumin (4.8mg) is added gently to HNADES (1.0mL), while stirring to obtain orange solution of Cur HNADES. Circular piece of PVA nanofibers mat (dia.1.3cm) is soaked for 24 hours in Cur-HNADES (~2.0 mL) solution in a sealed petri dish. Mat colour changed from white to orange. Cur-HNADES/PVA nanofibers sorbent mat dried in vacuum oven at 60 oC for 2 h. Cross-linking occurs between the functional groups of Cur-HNADES and the PVA nanofibers’ mat due to dipole-dipole interactions, hydrogen-bonding and π-π stacking. Curcumin immobilized into the non-toxic and biodegradable polyvinyl alcohol (PVA) electro-spun nanofibers’ mat. Curcumin is added to enhance the hydrophobicity and functionality of the sorbent mat to analyse five antidiabetic drugs from serum and plasma, which showed higher efficiency in the analysis. Cur-HNADES-PVA sorbent mat can adsorb specified anti diabetic drugs in the range of 72.52 to 77.77 mg /g. Sorbent mat can be reused (upto 115 cycles).
Circular and sustainable cotton dyeing process using NADES [glycerol-urea (1:1) + 20% water-selected] and curcumin is reported [59]. Optimized conditions are type and composition of NADES, molar ratio of HBA and HBD and water content, fibre to liquid ratio, time of dyeing. NADES is selected based on its uptake of curcumin, colorimetric parameters of the dyed fibers and cost-effective parent compounds (i.e., HBA and HBD). Cotton fibre (0.1 g), is dyed in 3mL of NADES (contains 250 ppm curcumin) in a dyeing bath for 60 min in dark without air bubbles. Fibers are removed from bath and squeezed to remove excess solution. Then fibers are rinsed in water and excess water removed. Fibers dyed with NADES exhibited superior color fastness compared to controls (i.e., water, glycerol, glycerol with 20% water). Consumption of 30 % curcumin and color differences (ΔE) of 24.0 in one dyeing cycle is reported, when curcumin in NADES is used for dyeing. The enhanced solubility of curcumin in the dye bath is enabled by the hydrogen bond network intrinsic to NADES. Owing to these linkage curcumin interactions with the cotton fibers reinforced and achieved to more effective curcumin uptake. Waste water pollution can be decreased, as the developed technique can avoid the usage of inorganic salts during the dyeing of fibres. Methylglyoxal (MGO) is a precursor for advanced glycation end products (AGEs), which are root cause of several diseases. Curcuminoids (diarylheptanoids) can trap methylglyoxal. However unstable and poor solubility nature of curcuminoids could not be used for this purpose. Solubilised and stabilised curcuminoids are isolated from turmeric using NADES [betaine-glycerol (1:2) + 10 % water – selected] to trap the methylglyoxal [60]. Optimized parameters for the isolation of solubilised and stabilised curcuminoids are molar ratio of HBA and HBD and water content, ultrasonication frequency and time. NADES is selected based on its viscosity, pH, fluidity and curcumin solubility. Selected NADES components are available in human body. Turmeric (1 g) was extracted at room temperature using 20 mL of NADES through ultrasonic extraction for 30 min at a frequency of 45 kHz to obtain NADES turmeric extract. NADES turmeric extract (1 mL, 0.08 mM curcuminoids) is mixed with NADES-MGO solution (4 mL, 8mM methylglyoxal) and incubated at 37oC for 12 h. NADES is eluted using solid phase extraction C18 column. The sample is filtered (0.22 µm) and analysed (LC MS/MS) for complexes of diarylheptanoid-methylglyoxal. Reaction rate constants of curcuminoids are reported. Analysis showed the presence of twenty-one stable and soluble diarylheptanoids in NADES extract of turmeric, out of which ten can trap the methylglyoxal molecules. This study exhibits the role of turmeric in the inhibition of formation of AGEs by scavenging MGO.
SIGNIFICANT OBSERVATIONS DURING THE USE OF DES / NADES AS ALTERNATE GREEN SOLVENTS FOR PROCESSING OF BIOACTIVE PHYTOCHEMICALS FROM TURMERIC.
Important understandings during extraction
Effects on sample / substrate structure: Plant cell walls consist of cellulose, hemicellulose and pectin, which is a barrier for the release of intracellular substances (e.g., bioactive phytochemicals). The extraction yields of bioactive compounds are improved and extraction times are reduced with the use of DES / NADES as extraction solvent. Scanning electron microscopic (SEM) analysis revealed disruption of raw material matrix structure owing to dissolution of cellulose / the reduction in particle size along with the rupture and / or formation of porous structure during the use of DES / NADES [16,17,32,36,37]. DESs can penetrate deep into the matrix, mass transfer increases and releases the metabolites. Low pH can promote their hydrolysis and increase the penetration ability of DESs. The changes in the microstructures of the DES / NADES treated samples are observed and indicate that the DES / NADES broke the cells and cell walls, and also dissolve the weak bonds in samples during extraction as indicated by SEM. Polarity, viscosity, solubility and extractability are the important properties to consider in the selection of DES / NADES as an extraction solvent, and these could be adjusted based on the consideration of the molecular structure of target compounds, with the selection of suitable HBA and HBD [15]. DES extraction phenomenon is explained through conductor-like screening model for realistic solvents computational analysis. The higher extraction efficiency of NADES is owing to solvent interactions with the cell walls of the sample and not due to solubility and polarity profiles of the solvent [36].
DES / NADES characteristics and water addition – effects on substrate: Dilution with appropriate quantity of water could increase the diffusion through penetration, as the viscosity of DES / NADES decreases in the system as well as swelling of plant cells happens. However, excess water may hinder the release owing to disturbance in DES / NADES molecular structure. Polarity of the DES / NADES also changes with the quantity of water and affects the extraction of target compounds. Water content in DES / NADES needs optimization [24,28,30,32,33,34,36]. Hansen solubility computational parameters are used to determine water content required, which considered the interaction between solute and solvent [33].
Significant points owing to sample particle size: Smaller the particle size of the sample, DES / NADES can enter the cell easily, as the diffusion path required by solvent to reach cell matrix is decreased. Contact surface between sample cells and solvent is increased. Cell exposure to solvent is improved. More quantity of target bioactive phytochemicals are extracted. If the particle size is decreased too much (< 0.15 mm), agglomeration of sample is reported owing to high surface energy. Extraction efficiency is reduced, as the penetration of DES / NADES into sample matrix is decreased [32,34].
Effects with microwave assistance: Curcuminoids are extracted using DES under MAE from turmeric roots. Temperature is found to be critical parameter, as solubility of the curcuminoids escalates with temperature up to certain level as well as surface tension and viscosity of DES decline with increase in temperature. Further sufficient quantity of DES in the material facilitates easy mass transfer. Longer periods of microwave irradiation may degrade the bioactive compounds [30,34]. Critical factors in NADES-MAE are sufficient DES volume for greater exposure, small particle size for high penetration of DES, soaking in water for sufficient time for easy extraction of target molecules [37].
Important observations with ultrasonication assistance: Bioactive molecules from turmeric are isolated using UAE-DES. Sample to solvent ratio and particle size are critical for proper creation of cavitation with breakage of raw material by ultrasonication, which provides suitable mass transfer of target molecules to DES. Kinetics is described with the help of Peleg’s model for curcuminoids extraction from turmeric [32]. UAE-Hydrophobic DES extracts are prepared from turmeric. Extracts exhibited high antioxidant and antibacterial activities as well as enzyme inhibition activities. Further, these hydrophobic DES extracts are found to be non-toxic to Allium cepa cells and indicated safe for human consumption [31].
Significant points during extraction by the formation of microemulsions: Hydrophobic DES and its micro emulsions are used to extract bioactive compounds from turmeric and found to be more efficient than hydrophilic DESs and routine organic solvents. Hydrophobic DES based micro emulsions are used for the extraction of turmeric with high efficiency. Hydrophobic DES microemulsions can extract both volatile and non volatiles compounds. These extracts improved the anti inflammatory efficacy of bioactive compounds owing to direct delivery into macrophages. The micro emulsion worked as carriers of bioactive compounds [35,39]. Curcumin solubility and stability are increased in DES / NADES based microemulsions [29,40,46,53].
Important explanations during bioactivity studies DES based bioactive-extracts can be used directly for health and nutrition applications [31,38]. Homogenous selenium sulphide nanoparticles with DES extract of turmeric in NADES inhibited the growth of pathogenic fungi and bacteria at minimal biocidal concentrations due to the improved dispersion of nanoparticles in NADES extract. Nanoparticles in NADES extract suppressed (99%) influenza viruses and B-coronavirus, as these nano particle damaged the viral surface proteins and inhibited the viral replication [38]. Hydrophobic DES based micro emulsions produced the turmeric extracts, which enhanced the anti-inflammatory efficacy of bioactive compounds for inhibition of NO production due to lowered inflammatory cytokines tumour necrosis factor-α, interleukin (IL)-6, and IL-1β production in lipopolysaccharide-activated murine macrophages. These micro emulsions delivered bioactive compounds directly into macrophages and improved the anti-inflammatory efficacy [39]. Three curcuminoids are isolated using DES with solid-liquid extraction method. DPPH radical scavenging activity of DES extract is comparable to that of routine solvent extracts, which is higher than butylated hydroxyl toluene (BHT). Curcuminoids are more stable in DES extract at lower temperatures in dark (30 days) owing to interaction between curcuminoids and DES [28]. Antioxidant capacities of polymer emulsions of turmeric DES extracts are stable for 30 days, as influenced by the structure of emulsions. Hydrogen bond interactions between emulsion components and solvent, controlled the mobility of bioactive compounds and protecting from degradation [40].
Noteworthy points during estimations Reduction of analysis time and estimation at ppb levels is possible, without interference from matrix, if DES is designed properly. DES based microextraction methods are reported with good sensitivity, higher enrichment factors and lower detection limits. DES based emulsification / dispersive microextraction methods for estimation of bioactive compounds are reported. VA DES-ELLME, VA-DES-ME, DES-DLLME-SFO, STLLME / STME and RS-DES-DLLME are used for the estimation of curcumin from turmeric rhizomes, turmeric powder, turmeric root herbal tea, food, beverage, water and herbal tea samples with high recovery values [41-45]. Vortex mixing escalates the number of DES microspheres and distributes throughout sample solution, which increases the mass transfer of the target compounds to the DES phase. Centrifugation allows the separation of DES phase with bioactive compounds [41,42]. DES-DLLME-SFO method for estimation of curcuminoids from turmeric rhizomes and turmeric tea using low density and melting point near to room temperature DES is reported. Higher pH disturbed the structure of low density DES and analytes anionic forms may form. Lower pH (1-2) analytes’ cations are formed. Ionic forms of analytes are not favorable for extraction. Moderate pH in sample solution is favorable. At lower temperatures (80oC) is observed. Further, enrichment factors for analytes are higher, when the extraction temperatures are between 30-45oC. Dispersion solvent and centrifugation step are not required [43]. STME-NADES technique for estimation of curcuminoids from turmeric rhizomes and turmeric tea is reported. Dispersive liquid and centrifugation step are not required. Insufficient amount of demulsifier improves emulsification level, whereas higher amounts of demulsifier is not required for liquid-liquid microextraction. If demulsification time is short, phase separation is not complete. While long demulsification time may provide re-emulsification, which is not required. Hence, both parameters are optimized [44]. In RS-DES-DLLME method for estimation of curcumin, sodium hydroxide (NaOH) and hydrochloric acid (HCl) are used for phase transition. Concentration and volume of NaOH and HCl are critical. NaOH is added to convert the DES to hydrophobic DES for extraction of bioactive compounds. DES layer with bioactive compounds is treated with HCl for the release and separation of neutral hydrophilic DES and analytes [45].
Major factors during solubility studies
Curcumin solubility in aqueous media is improved by water-in-oil emulsion using hydrophobic DES-ME. Surfactant concentration and water loading capacity of DES surfactant microemulsion are the critical factors for the improvement of solubility of curcumin at the interfacial region [46]. Presence of keto and alcoholic groups in curcumin favours solubility in the low-viscosity hydrophobic DES (camphor: menthol-1:1), however it is less when compared to above microemulsions [47]. Curcuminoids extracted using DES as adjuvant in water and surfactant free microemulsion method. Solubility is increased by two-fold due to intermolecular interaction (π-interactions) between DES solvent and curcuminoids. NADES (as adjuvant) is used successfully in cyclic extractions. The curcuminoids are enriched up to seven cycles and solvent is saved [29]. Curcumin solubility in aqueous media is increased multiple fold, when DES used as cosolvent. Solubility improved by 33,000 fold with increase in temperature up to 40oC as well as viscosity and structure of DESs also played a role [48]. Curcumin solubility in aqueous media is reported as endothermic from thermodynamic calculations and quantum chemistry calculations [48,51]. COSMO-RS is used to estimate the activity coefficients of curcumin in NADES. This technique provides the information to select best DES / NADES for solubility and extraction. Solubility increase is owing to intermolecular hydrogen bonding and Van der Waals interactions between curcuminoids and solvent as per the above approach. It is useful to create the data base of novel high-performance solvents for use in pharmaceutical biological and chemical industries [50]. Lowest interaction energy of curcumin and DES / NADES system (as per DFT and ESP analysis) provides more stable solvent-curcumin system favourable for curcumin solubility. Hence, the particular DES / NADES target molecule with lowest interaction energy, respective DES / NADES may become best cosolvent to improve the solubility of target molecule in aqueous medium [51]. Curcumin solubility in DES is up to 12,000 fold higher, when compared to water. Higher solubility is due to the formation of hetero molecular complexes of curcumin with DES components (in particular with choline chloride) as per quantum chemistry calculations [54]. Curcuminoids solubility in NADES [choline chloride- malonic acid (1:1) + 50 % Water] is 5,92,667 times higher than water owing to the strong interaction between the solute and solvent. This interaction is supported using Hansen solubility computational parameter [33]. Curcumin solubility in ethanol is increased in presence of DES. Curcumin solubility in ethanol as well as ethanolic HBAs, ethanolic HBDs as well as ethanolic DESs is evaluated. Solubility of curcumin in ethanolic HBAs is increased owing to diffusion and non-point hydrogen bonding interactions. Curcumin solubility in ethanolic HBDs is improved owing to hydrogen bonding with different functional groups. It is reported solubility is higher in ethanolic levulinic acid due to formation of cyclic conformation between levulinic acid and curcumin, as revealed through COSMOS-RS calculations. Ethanolic DESs showed higher solubility of curcumin, when compared to ethanolic HBAs and HBDs. Highest solubility of curcumin is observed in ethanolic DESs containing levulinic acid [49].
Vital points during stability and bioavailability studies
Curcumin is stable for 30 days in hydrophobic DES based microemulsion of turmeric extracts. Bioavailability of curcumin in DES based microemulsion is higher when compared to curcumin suspensions [39]. Bioavailability of curcuminoids from DES extract is enhanced and observed in the form of Phase 1 and 2 metabolites of three curcuminoids, when compared to organic solvent extracts in in vivo studies from pharmacokinetic observations on plasma / serum [52]. Curcumin in NADES-HIPEW/O emulsions exhibited centrifugal, thermal, freeze-thaw, storage stability and UV stability up to 72 h. Degradation of curcumin is reduced, as curcumin is less exposed to UV light due to the presence of sugar crystals and water droplets. These properties of NADES-HIPEW/O emulsions improved the curcumin gastrointestinal digestible stability during in vitro digestion due to encapsulation of curcumin in NADES-HIPEW/O emulsions [53]. NADES (choline chloride-glycerol)-curcumin system showed stability up to 72 h without degradation even under sunlight owing to dominant intermolecular interactions and lowest Gibbs free energy [54]. Researchers are using DES / NADES as alternate solvents as carriers of bioactive compounds in organisms / in vivo studies to achieve maximum activity and to improve bioavailability of bioactive components [54].
Main factors during isolation of essential oil
Yield of essential oil is more using NADES based MAHD technique from DES pre-treated turmeric, when compared to other methods. More oxygenated compounds are observed in essential oil collected from NADES based MAHD method. More oxygenated compounds in essential oil provide high flavor value in the industry [55].
Key points during applications
Curcuminoids dissolved in acidic NADES showed antimicrobial activity due to low pH and these could be useful in antimicrobial photodynamic therapy [56]. Starch nano particles filled with curcumin (with basic pH stability and photo stability) are prepared using NADES could find application in functional foods as food additive as well as in edible packaging [57]. Biodegradable polyvinyl alcohol electro-spun nanofibers’ mat with incorporation of curcumin through hydrophobic NADES is used to analyse five antidiabetic drugs from plasma and serum. It showed higher efficiency in the analysis [58]. Sustainable cotton dyeing process using curcumin dissolved in NADES is reported. Fibres dyed with curcumin in NADES exhibited superior colour fastness compared to controls. The enhanced solubility of curcumin in the dye bath is enabled by the hydrogen bond network intrinsic to NADES. Owing to these linkage curcumin interactions with the cotton fibres reinforced. Waste water with inorganic salts can be avoided during dyeing [59]. NADES solubilised and stabilised curcuminoids from turmeric can trap the methylglyoxal molecules (It is precursor of advanced glycation end products, which are the root cause of several diseases) efficiently. Advantage is selected NADES constituents (i.e., betaine, glycerol) are available in human body. Role of curcuminoids in human body is disclosed [60].
Most of the compounds used to produce DES are approved under safety regulations. NADES had a significant impact on the extraction of target compounds with higher yield, premium quality, and more quantity from turmeric, with minimum extraction time, when used along with other suitable techniques or independently. The application of DES and NADES in the food industry is a promising area.
CLASSIFICATION OF DES / NADES USED DURING THE SEPARATION AND PURIFICATION OF BIOACTIVE PHYTOCHEMICALS FROM TURMERIC
DES / NADES used in the processing of turmeric are type 3 and type 5 (supplementary information Table S1) [61-63]. Most of the DES / NADES belong to type 3 (i.e., the combination of a quaternary ammonium salt with a HBD). HBAs are quaternary ammonium salts such as choline, betaine, cartinine and tetra butyl ammonium chlorides. HBDs are small organic molecules such as amides, carboxylic acids (e.g., mono- or dicarboxylic acids, urea, citric acid or amino acids) polyols (e.g., glycerol, ethylene glycol or carbohydrates) and phenol. One ternary eutectic mixture [i.e., citric acid-propanediol-betaine (2:2:1)] is also reported [38]. However, some of the DES / NADES are type 5 (i.e. non-ionic). Both HBA and HBD are small organic molecules (i.e., carboxylic acids, amino acids, sugars, mono terpenes, amines).
MAJOR ADVANTAGES OF DES / NADES DURING THE SEPARATION AND PURIFICATION OF BIOACTIVE PHYTOCHEMICALS FROM TURMERIC.
Preparation procedures of these homogenous liquids are simple and economical. These procedures are viable with complete atom economy and does not generate waste. DESs are designer solvents and several combinations are possible. Physico-chemical characteristics are regulated for the specific purpose. A broad polar range, with reasonable solubilisation strength for various target compounds is possible. Significant features of these solvents are low harmfulness, decomposable, recyclable, non-volatility and non-flammability. DES / NADES provide higher extractions yields, solubilities, bioavailability and bioactivities, when compared to traditional solvents and techniques [28-37,39,40,44,45,48,49,51-53,55]. The economics of DES/NADESs is comparable to conventional solvents [15,23,64,65]. DES/NADESs have almost zero vapour pressure and recovery of target compound could be difficult in selected cases. However, recovery of curcuminoids with high purity from DES extracts is reported using solid phase extraction columns [28] and anti-solvent (e.g., water) [30,32,34,36,37]. Selected DES hydrophobic extracts are found to be non-toxic to Allium cepa cells and indicated safe for human consumption [31]. Extracts from hydrophobic DES and hydrophobic DES based micro emulsions with target compounds are well-suited with water based food and beverage applications and provide additional health benefits [35]. NADES-HIPEW/O emulsions improved the UV stability of curcumin [53]. In selected methods (e.g., DES-DLLME-SFO, STME-NADES), small quantity of DES is enough for extraction and estimation of analytes [43,44]. Dispersion liquid and centrifugation step are not required in some techniques like DES-DLLME-SFO, STME-NADES, RS-DES-DLLME [43-45]. DES can be reused in RS-DES DLLME technique [45]. Application of DES / NADESs on industrial scale is improving continuously, as the extracts are being used directly (many of the ingredients of the DESs are GRAS category) without expensive downstream purification steps. DES extracts of curcuminoids with low pH exhibited more stability [35,43]. Bioavailability of curcuminoids from DES extracts is more, when compared to routine organic solvent extracts, as observed in in vivo studies [52]. Curcumin in NDES-HIPEW/O emulsions improved the gastrointestinal digestible stability [53]. NADES-curcumin complexes showed higher solubility and stability due to dominant intermolecular interactions [54]. Higher yields of essential oils are isolated using pre treatment of turmeric with DES [55]. Scale-up studies on using DES are initiated and parameters are being optimized. Energy requirements and time of extraction are least in MAE-DES method [37].
LIMITATIONS OF DES / NADES DURING THE PROCESSING OF BIOACTIVE PHYTOCHEMICALS FROM TURMERIC.
Large number of combinations of HBAs and HBDs are possible. Hence, characterisation of DESs as well as generalisations of the properties is not an easy task. High viscosity makes handling difficult in selected cases (isolation and purification of bioactive molecules). Separation of impurities (due to reactants) / target products /compounds is also difficult task. More toxicity / biodegradability studies on DESs are required to avoid environmental pollution [64]. In vitro toxicity studies are reported, however, more in vivo toxicity studies are required in case of several DESs for further applications in food / health as well as pharmaceutical products. In select cases curcuminoids are not separated from NADES extracts and could be used directly [29]. Disadvantages are the high viscosities of DES/NADESs in selected cases could be a restrictive; however, the researchers overcome this problem by the addition of water [24,28,30,32,33]. Starting materials of DESs can indicate the biodegradability of final DESs to some extent. However, it is not comprehensive. Computational predictive methods needs to be developed for the above purposes.
CONCLUSIONS
Turmeric is one of the major root spice consumed for several purposes throughout the globe, due to its colour, flavour, therapeutic and nutritional properties. Recently, several research studies are reported on the use of DES/NADES for the processing of turmeric. Most of the studies are on the extraction of bioactive molecules. Selected analytical methods for the estimation of bioactive molecules (viz., curcuminoids) are reported. Studies on solubilisation and stabilisation of bioactive molecules are also reported. Most of these studies indicate the use of DES/NADES is efficient in the respective method for turmeric processing. Extraction times are reduced. Time for estimation of target compounds is decreased. Activity coefficients of curcumin in DESs are one of the important factors to determine the extractability and solubility in respective DES / NADES. Solubility of bioactivity molecules in the aqueous DES extract is increased. Selected extracts with target molecules are used directly as drugs, as DES/ NADES are safe, when compared to organic solvents. Further, it is indicated that volatile organic solvents can be replaced in several examples, as the derived products (in particular extracts from NADES) can be directly used in the food, pharmaceutical as well as cosmetic products. Most of the constituents of the NADES are safe as reported in international safety regulations. NADES are in general non volatile and harmless. The stability of phenolic bioactive compounds (i.e., curcuminoids) is increased due to DES intervention. Addition of water is possible to selected NADES since they are fully water soluble / miscible. DES surfactant-water micro emulsion improved the solubility and stability of curcumin and are useful in pharmaceutical industry. Several NADES are safe in high doses and have received generally regarded as safe (GRAS) certification (e.g., Fructose, glucose). With the high extraction capacity and the low toxicity of their components, NADES are very suitable for extraction of bioactive compounds, flavors and fragrances. Derived extracts could be used as additives in foods, as drug carriers in pharmaceuticals and as ingredients in cosmetics safely. Use of DES/NADES is expected to increase in future from the above advantages. UAE-DES method yielded highest yield of curcuminoids (164.51 mg/g) from turmeric, when compared to earlier reports [33]. Pilot scale extraction using deep eutectic solvents and evaluation of economic feasibility studies are commenced. Researchers are using DES / NADES as alternate solvents during turmeric processing for various purposes (viz., extraction, estimation of target compounds in complex matrices, enhancement of solubility of bioactive compounds in aqueous media, carriers of bioactive compounds in organisms / in vivo studies to achieve maximum activity and to improve bioavailability of bioactive components), in spite of toxicological and environmental problems. Main reason is as these are safe when compared to general organic solvents. Additionally, NADES extracts of turmeric find applications in antimicrobial photodynamic therapy, edible food packaging, antidiabetic drugs adsorption, textile dyeing, and trapping of methylglyoxal. However, methods for complete recovery of curcuminoids from DES extracts need to be improved.
REFERENCES
- Khalandar SD, Adithya TN, Basha SJ, Koshma M, Reddy UVS, Reddy VJS. A current review on curcuma longa linn, plant. Int J Pharm Chem Biol Sci. 2018; 8: 68-73.
- Tomeh MA, Hadianamrei R, Zhao X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int J Mol Sci. 2019; 20: 1033.
- Jayaprakasha GK, Jaganmohanrao L, Sakariah KK. Chemistry and biological activities of C. longa. Trends Food Sci Technol. 2005; 16: 533-548.
- Raundal DE, Shinde AD, Sonawane KS, Deore NP, Deore AR, Deshmukh YH. A Review on Turmeric: Curcuma longa. Int J Pharm Res Appl. 2023; 8: 1577-1582.
- Kumari A, Kaur M, Sharma S. A review on curcumin and its medicinal properties. Acta Sci Pharm Sci. 2021; 5: 31-37.
- Ahsan R, Arshad M, Khushtar M, Ahmad MA, Muazzam M, Akhter MS, et al. A Comprehensive Review on Physiological Effects of Curcumin. Drug Res (Stuttg). 2020; 70: 441-447.
- Oglah MK, Mustafa YF, Bashir MK, Jasim MH. Curcumin and its derivatives: A review of their biological activities. Sys Rev Pharm. 2020; 11: 472-481.
- Rathore S, Mukim M, Sharma P, Devi S, Nagar JC, Khalid M. Curcumin: A review for health benefits. Int J Res Rev. 2020; 7: 273-290.
- Jyotirmayee B, Mahalik G. A review on selected pharmacologicalactivities of Curcuma longa L. Int J food prop. 2022; 25: 1377-1398.
- Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front Pharmacol. 2022; 13: 820806.
- Matthewman C, Krishnakumar IM, Swick AG. Review: bioavailability and efficacy of 'free' curcuminoids from curcumagalactomannoside (CGM) curcumin formulation. Nutr Res Rev. 2024; 37: 14-31.
- Wang T, Wang Q, Guo Q, Li P, Yang H. A hydrophobic deep eutectic solvents-based integrated method for efficient and green extraction and recovery of natural products from Rosmarinus officinalis leaves, Ginkgo biloba leaves and Salvia miltiorrhiza roots. Food Chem. 2021; 363: 130282.
- Sekharan TR, Chandira RM, Tamilvanan S, Rajesh SC, Venkateswarlu BS. Deep Eutectic Solvents as an Alternate to Other Harmful Solvents. Biointerface Res Appl Chem. 2022; 12: 847-860.
- Torres-Ortiz D, García-Alcocer G, Berumen-Segura LC, Estevez M. Green extraction of secondary metabolites from plants: Obstacles, current status, and trends. Sustain Chem Environ. 2024; 8: 100157.
- Fernández MLÁ, Boiteux J, Espino M, Gomez FJV, Silva MF. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal Chim Acta. 2018; 1038: 1-10.
- Zainal-Abidin MH, Hayyan M, Hayyan A, Jayakumar NS. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal Chim Acta. 2017; 979: 1-23.
- Jablonský M, Škulcová A, Malvis A, Šima J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J Biotechnol. 2018; 282: 46-66.
- Lee J, Jung D, Park K. Hydrophobic deep eutectic solvents for the extraction of organic and inorganic analytes from aqueous environments. Trends Anal Chem. 2019; 118: 853-868.
- Kalyniukova A, Holusa J, Musiolek D, Kadukova JS, Wasylka JP, Andruch V. Application of deep eutectic solvents for separation and determination of bioactive compounds in medicinal plants. Ind Crops Prod. 2021; 172: 114047.
- Wen Q, Chen JX, Tang YL, Wang J, Yang Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere. 2015; 132: 63-69.
- Vanda H, Dai Y, Wilson EG, Verpoorte R, Choi YH. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C R Chim. 2018; 21: 628-638.
- Ferreira IJ, Oliveira F, Jesus AR, Paiva A, Duarte ARC. Current methodologies for the assessment of deep eutectic systems toxicology: Challenges and perspectives. J Mol Liq. 2022; 362: 119675.
- Benvenutti L, Zielinski AAF, Ferreira SRS. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci Technol. 2019; 90: 133-146.
- Fuad FM, Nadzir MM, Kamaruddin AH. Hydrophilic natural deep eutectic solvent: A review on physicochemical properties and extractability of bioactive compounds. J Mol Liq. 2021; 339:116923.
- Gonzalez-Campos JB, Perez-Nava A, Valle-Sanchez M, Delgado-Rangel LH. Deep eutectic solvents applications aligned to 2030 United Nations Agenda for Sustainable Development. Chem Eng Process: Process Intensif. 2024; 199: 109751.
- Jiang T, Ghosh R, Charcosset C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review. Trends Food Sci Technol. 2021; 112: 419-430.
- Devi TG, Devi TJ, Singh PS, Willingson L. Vibrational and molecular properties of curcumin-natural deep eutectic solvent mixture using experimental and theoretical methods. J Mol Liq. 2024; 397: 124055.
- Liu Y, Li J, Fu R, Zhang L, Wang D, Wang S. Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind Crops Prod. 2019; 140: 111620.
- Huber V, Muller L, Degot P, Touraud D, Kunz W. NADES-based surfactant-free microemulsions for solubilization and extraction of curcumin from Curcuma Longa. Food Chem. 2021; 355: 129624.
- Doldolova K, Bener M, Laliko?lu M, A?ç? YS, Arat R, Apak R. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 2021; 353: 129337.
- Oliveira G, Marques C, Oliveira AD, Santos ADAD, Amaral WD, Ineu RP, et al. Extraction of bioactive compounds from Curcuma longa L. using deep eutectic solvents: In vitro and in vivo biological activities. Innov Food Sci EmergTechnol. 2021; 70: 102697.
- Patil SS, Pathak A, Rathod VK. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason Sonochem. 2021; 70: 105267.
- Shekaari H, Moattar MTZ, Mokhtarpour M. Effective ultrasonic- assisted extraction and solubilization of curcuminoids from turmeric by using natural deep eutectic solvents and imidazolium-based ionic liquids. J Mol Liq. 2022; 360: 119351.
- Le NT, Hoang NT, Van VTT, Nguyen TPD, Chau NHT, Le NTN. Extraction of curcumin from turmeric residue (Curcuma longa L.) using deep eutectic solvents and surfactant solvents. Anal Methods. 2022; 14: 850-858.
- Kongpol K, Sermkaew N, Makkliang F, Khongphan S, Chuaboon L, Sakdamas A, et al. Extraction of curcuminoids and ar-turmerone from turmeric (Curcuma longa L.) using hydrophobic deep eutectic solvents (HDESs) and application as HDES-based microemulsions. Food Chem. 2022; 396: 133728.
- Kisanthia R, Hunt AJ, Sherwood J, Somsakeesit L, Phaosiri C. Impact of Conventional and Sustainable Solvents on the Yield, Selectivity, and Recovery of Curcuminoids from Turmeric. ACS Sustain Chem Eng. 2022; 10: 104-114.
- Patil SS, Rathod VK. Extraction and purification of curcuminoids from Curcuma longa using microwave assisted deep eutectic solvent based system and cost estimation, Process Biochem. 2022; 126: 61-71.
- D?ugosz O, Ochnik M, Sochocka M, Franz D, Orzechowska B, Anna CK, et al. Antimicrobial and antiviral activity of selenium sulphide nanoparticles synthesised in extracts from spices in natural deep eutectic solvents (NDES). Sustainable Materials and Technologies. 2022; 32: e00433.
- Supaweera N, Chulrik W, Jansakun C, Bhoopong P, Yusakul G, ChunglokW. Therapeutic deep eutectic solvent-based microemulsion enhances anti-inflammatory efficacy of curcuminoids and aromatic-turmerone extracted from Curcuma longa L. RSC Adv. 2022; 12: 25912.
- Coelho ALK, Muniz VRGF, Alberti A, Freitas RAD, Kaspchak E, Mafra MR, et al. Chemical stability of Curcuma longa extract stored in hydrophobic deep eutectic solvent and polymers emulsion. Food Hydrocoll. 2024; 146: 109166.
- Aydin F, Yilmaz E, Soylak M. Vortex assisted deep eutectic solvent (DES)-emulsification liquid-liquid microextraction of trace curcumin in food and herbal tea samples. Food Chem. 2018; 243: 442-447.
- Altunay N, Elik A, Gürkan R. Preparation and application of alcohol based deep eutectic solvents for extraction of curcumin in food samples prior to its spectrophotometric determination. Food Chem. 2020; 310: 125933.
- Xie LY, Bai J, Zhang XX, Chen X, Bai XH, Hu S. Simultaneous determination of curcuminoids in Curcumae Longae Rhizoma and turmeric tea using liquid-phase microextraction based on solidification of floating deep eutectic solvent drop. Microchem J. 2020; 159: 105341.
- Yang L, Xie LY, Chen X, Bai XH, Hu S. Solvent terminated natural deep eutectic solvent microextraction for concentration of curcuminoids in Curcumae Longae Rhizoma and turmeric tea. J Sep Sci. 2022; 45: 2252-2261.
- Salamat Q, Soylak M. Novel reusable and switchable deep eutectic solvent for extraction and determination of curcumin in water and food samples. Talanta. 2024; 269: 125401.
- Dhingra D, Bisht M, Bhawna B, Pandey S. Enhanced solubility and improved stability of curcumin in novel water-in-deep eutectic solvent microemulsions. J Mol Liq. 2021; 339: 117037.
- Sekharan TR, Chandira RM, Rajesh SC, Tamilvanan SP, Vijayakumar CT, Venkateswarlu BS. pH, Viscosity of Hydrophobic Based Natural Deep Eutectic Solvents and the Effect of Curcumin Solubility in it. Biointerface Res Appl Chem. 2021; 11: 14620-14633.
- Shekaari H, Mokhtarpour M, Faraji S, Moattar MTZ. Enhancement of curcumin solubility by some choline chloride-based deep eutectic solvents at different temperatures. Fluid Ph Equilib. 2021; 532: 112917.
- Huber V, Hioe J, Touraud D, Kunz W. Uncovering the curcumin solubilization ability of selected natural deep eutectic solvents based on quaternary ammonium compounds. J Mol Liq. 2022; 361: 119661.
- Alioui O, Sobhi W, Tiecco M, Alnashef IM, Attoui A, Boudechicha A, et al. Theoretical and experimental evidence for the use of natural deep eutectic solvents to increase the solubility and extractability of curcumin. J Mol Liq. 2022; 359: 119149.
- Yu W, Bo Y, Luo Y, Huang X, Zhang R, Zhang J. Enhancing effect of choline chloride-based deep eutectic solvents with polyols on the aqueous solubility of curcumin–insight from experiment and theoretical calculation. Chin J Chem Eng. 2023; 59: 160-168.
- Abouheif SA, Sallam SM, Sohafy SME, Kassem FF, Shawky E. A green extraction approach using natural deep eutectic solvents enhances the in-vivo bioavailability of curcuminoids from turmeric extracts. Ind Crops Prod. 2022; 189: 115790.
- Miao J, Zuo X, McClements DJ, Zou L, Liang R, Zhang L, et al. Fabrication of natural deep eutectic solvent based water-in-oil high internal phase emulsion: Improving loading capacity and stability of curcumin. J Food Eng. 2024; 366: 111862.
- Jeli?ski T, Przyby?ek M, Cysewski P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm Res. 2019; 36: 116.
- Xu FX, Zhang JY, Jin J, Li ZG, She YB, Lee MR. Microwave-assisted Natural Deep Eutectic Solvents Pretreatment Followed by Hydrodistillation Coupled with GC-MS for Analysis of Essential Oil from Turmeric (Curcuma longa L.). J Oleo Sci. 2021; 70: 1481-1494.
- Rachmaniah O, Gama GRF, Pratama ZA, Rachimoellah M. Antimicrobialeffect of dissolved curcuminoid in natural deep eutectic solvents (NADES) to Escherichia coli and Staphylococcus aureus: A promising candidate for antimicrobial photodynamic therapy (aPDT). Mal J Fund Appl Sci. 2020; 16: 514-518.
- Zhong C, Luo S, Xiong R, Liu C, Ye V. A new green self-assembly strategy for preparing curcumin-loaded starch nanoparticles based on natural deep eutectic solvent: Development, characterization and stability. Food Hydrocoll. 2024; 151: 109878.
- Khodayari P, Ebrahimzadeh H. A green QuEChERS syringe filter based micro-solid phase extraction using hydrophobic natural deep eutectic solvent as immobilized sorbent for simultaneous analysis of five anti-diabetic drugs by HPLC-UV. Anal Chim Acta. 2023; 1279: 341765.
- Jorge AMS, Ribeiro HF, Pereira JFB. Natural and eco-friendly cotton dyeing process using eutectic solvents and curcumin. J Environ Chem Eng. 2025; 13: 115553.
- Zhang Q, Zhang X, Jiang Q, Li X, Xu J, Jiang M. Exploring the role of diarylheptanoids derived from turmeric in trapping methylglyoxal with natural deep eutectic solvents. Food Chem. 2025; 479: 143851.
- Mannu A, Blangetti M, Baldino S, Prandi C. Promising Technological and Industrial Applications of Deep Eutectic Systems. Materials (Basel). 2021; 14: 2494.
- Abranches DO, Coutinho JAP. Type V deep eutectic solvents: Design and applications. Curr Opin Green Sustain Chem. 2022; 35: 100612.
- Gao Y, Fan M, Cheng X, Liu X, Yang H, Ma W, et al. Deep eutectic solvent: Synthesis, classification, properties and application in macromolecular substances. Int J Biol Macromol. 2024; 278: 134593.
- Dheyab AS, Abu Bakar MF, AlOmar M, Sabran SF, Hanafi AFM, Mohamad A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations. 2021; 8: 176.
- Ferreira C, Sarraguça M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals (Basel). 2024; 17: 124.