Vapor Liquid Equilibria Calculations for the Hydrofluorocarbons with the Patel-Teja EOS Coupled the Different Alpha Functions
- 1. Department of Chemical and Chemical Engineering, Hefei Normal University, Hefei, 230061, PR China
- 2. Department of Physics and Materials Engineering, Hefei Normal University, Hefei, 230061, PR China
ABSTRACT
A comparative study was made to represent the saturated vapor pressure of difluoromethane(R32),1,1,1,2-tetrafluoroethane(R134a), trans-1,3,3,3- tetrafluoropropene(R1234ze(E)), fluoroethane(R161) and 2,3,3,3-tetrafluoroprop-1-ene(R1234yf) with the Patel-Teja EOS coupled the five different alpha functions. Results show that the Patel-Teja coupled the Melhem function gets the adequate representation of the saturated vapor pressure. It also has been demonstrated that the Patel-Teja coupled the Joshipura and the Melhem alpha functions with the VDW mixing rules can represent the isothermal vapor liquid equilibrium for R32/R134a, R32/R1234ze(E),R32/R1234yf, R161/R1234yf,R32/R161 and R161/R1234ze(E) systems well if the EOSs has the right binary interaction parameters.
KEYWORDS
• Alpha functions
• Patel-Teja EOS
• VDW mixing rules
CITATION
Pia Z, Wang L (2020) Vapor Liquid Equilibria Calculations for the Hydrofluorocarbons with the Patel-Teja EOS Coupled the Different Alpha Functions. Chem Eng Process Tech 5(1): 1060.
INTRODUCTION
Numerous modifications to the Van der Waals cubic Equation of State (EOS) have been proposed to improve the predictions of volumetric,thermodynamic and phase equilibrium properties. Among the multiple cubic equations of state, the models of Peng- Robinson(PR) [1] and the Soave-Redlich-Kwong (SRK) EOS [2] are widely used to simulate chemical processes [3]. But the PR and SRK are two-parameter cubic EOSs, this kind of cubic equations of state can predict the same critical compressibility factor independent on the pure substances. This characteristic can predict the liquid density with considerable deviations. To calculate the liquid phase densities, the three-parameter EOS is a better choice [4].
The three-parameter cubic equations of state appear in the early 1980s and they are a step in the development of the cubic equations of state [3]. The Patel-Teja(PT) equation is one of the most recognized three-parameter cubic equations. In a recent study, the PT EoS is a member of the group which provides the most accurate predictions for 102 pure substances among 23 generalized equations of state [5,6]. Moreover, the PT EOS has been used to correlate complex systems with the advanced mixing rule [7].
The Patel-Teja model is a three-parameter (a,b,c) Van der Waals type EOS [8]. In this equation, the pure component parameter is a temperature-dependent. To calculate the parameter “a” in the PT EOS ,an expression to estimate the cohesion function α(Tr) is necessary .In the original PT EOS, the Soave alpha function has been incorporated in the attractive term ,this alpha function always predict a Boyle temperature without physical sense [9]. The expression F in the Soave alpha function and the critical compressibility factor(ξc) are generalized in terms of the acentric factor for nonpolar compounds, and there few values reported for the polar substances [10]. For solving these problems, the Soave alpha functions are always replaced with some exponential alpha functions in the attractive term of the PT EOS and the parameters have to be recalculated. In Velásquez’s work [11], the thermodynamic properties of the saturated pure fluids are evaluated for the Patel-Teja equation couple the five different alpha functions. The generalized expressions to estimate the parameters and the alpha functions in the PT EOSs are listed in Table 3 in Velásquez’s work [11].
In this work, the Patel-Teja equation coupled the five alpha functions which were generalized in Velásquez’s work [11]. Are selected to evaluate the influence of the alpha functions in the saturated vapor pressure calculation of hydrofluorocarbons. Finally, vapor liquid equilibrium calculations for some binary systems have been made with the PT-Joshipura and the PTMelhem EOSs, the Van der Waals one fluid mixing rules with the interaction parameters are used to show the influence of the alpha functions and the interaction parameters in mixtures calculations.
To assess the results of the correlations, the average absolute deviation, AAD, for the pressure and the vapor phase mole fraction are defined as follows:
(1)
(2)
EVALUATION OF THE ALPHA FUNCTIONS OF PT EOS FOR THE HYDROFLUOROCARBONS
Hydrofluorocarbons play an important role in industry because of their noticeable applications, including the development of alternative environmentally-friendly refrigerants. Before finding out the most appropriate hydrofluorocarbons for a given application, it is necessary to predict the fluid-phase behaviors in both sub- and super-critical regions.
Pure substances
The saturated vapor pressure of R32, R134a, R1234ze (E),R161 and R1234yf were measured at the temperatures from 283.15K to 323.15K with 5K interval in Hu’s work, and the experimental data were correlated by Peng-Robinson-StryjekVera(PRSV) EOS(Hu et al.,2017a,2017b) . In this paper, Patel-Teja EOS with the five different alpha functions was used to correlated the experimental data. The saturated vapor pressure for the five substances are totally predictive because they were not used to obtain the alpha functions in Velásquez’s work [11].
In Table 1,
Table 1: Comparison of the calculated vapor pressure data of pure components with the literature data [12,13].
|
|
T (k)
|
pexp
|
Soave |
Joshipura |
pcal Heyen |
Melhem |
Gasem
|
||||||
|
p |
|
p |
|
p |
|
p |
|
||||||
|
p
|
|
||||||||||||
|
R32 |
283.152 |
1.1073 |
1.1118 |
-0.406 |
1.0819 |
2.290 |
1.1247 |
-1.571 |
1.1146 |
-0.659 |
1.0947 |
1.138 |
|
|
288.154 |
1.2806 |
1.2880 |
-0.578 |
1.2553 |
1.976 |
1.3027 |
-1.726 |
1.2914 |
-0.843 |
1.2699 |
0.836 |
||
|
293.147 |
1.4740 |
1.4842 |
-0.692 |
1.4488 |
1.710 |
1.5004 |
-1.791 |
1.4881 |
-0.957 |
1.4650 |
0.611 |
||
|
298.165 |
1.6895 |
1.7030 |
-0.799 |
1.6653 |
1.432 |
1.7209 |
-1.859 |
1.7075 |
-1.065 |
1.6830 |
0.385 |
||
|
303.143 |
1.9258 |
1.9432 |
-0.904 |
1.9036 |
1.153 |
1.9624 |
-1.901 |
1.9481 |
-1.158 |
1.9225 |
0.171 |
||
|
308.150 |
2.1882 |
2.2095 |
-0.973 |
2.1686 |
0.896 |
2.2298 |
-1.901 |
2.2147 |
-1.211 |
2.1884 |
-0.009 |
||
|
313.146 |
2.4762 |
2.5015 |
-1.022 |
2.4600 |
0.654 |
2.5226 |
-1.874 |
2.5070 |
-1.244 |
2.4803 |
-0.166 |
||
|
318.143 |
2.7916 |
2.8215 |
-1.071 |
2.7802 |
0.408 |
2.8429 |
-1.838 |
2.8271 |
-1.272 |
2.8006 |
-0.322 |
||
|
323.145 |
3.1380 |
3.1715 |
-1.068 |
3.1316 |
0.204 |
3.1926 |
-1.740 |
3.1770 |
-1.243 |
3.1514 |
-0.427 |
||
|
R134a |
283.151 |
0.4138 |
0.4105 |
0.798 |
0.3980 |
3.818 |
0.4167 |
-0.701 |
0.4122 |
0.387 |
0.4016 |
2.948 |
|
|
288.165 |
0.4884 |
0.4840 |
0.901 |
0.4697 |
3.829 |
0.4913 |
-0.594 |
0.4862 |
0.451 |
0.4742 |
2.908 |
||
|
293.151 |
0.5712 |
0.5671 |
0.718 |
0.5510 |
3.536 |
0.5758 |
-0.805 |
0.5699 |
0.228 |
0.5565 |
2.574 |
||
|
298.161 |
0.6651 |
0.6604 |
0.707 |
0.6424 |
3.413 |
0.6703 |
-0.782 |
0.6637 |
0.211 |
0.6490 |
2.421 |
||
|
303.156 |
0.7698 |
0.7653 |
0.585 |
0.7454 |
3.170 |
0.7765 |
-0.870 |
0.7692 |
0.078 |
0.7531 |
2.170 |
||
|
308.150 |
0.8859 |
0.8827 |
0.361 |
0.8611 |
2.799 |
0.8953 |
-1.061 |
0.8872 |
-0.147 |
0.8698 |
1.817 |
||
|
313.147 |
1.0153 |
1.0129 |
0.236 |
0.9896 |
2.531 |
1.0268 |
-1.133 |
1.0180 |
-0.266 |
0.9992 |
1.586 |
||
|
318.141 |
1.1584 |
1.1570 |
0.121 |
1.1322 |
2.262 |
1.1722 |
-1.191 |
1.1626 |
-0.363 |
1.1428 |
1.347 |
||
|
323.146 |
1.3161 |
1.3157 |
0.030 |
1.2897 |
2.006 |
1.3302 |
-1.071 |
1.3218 |
-0.433 |
1.3010 |
1.147 |
||
|
R1234ze(E) |
283.145 |
0.3086 |
0.3056 |
0.972 |
0.2960 |
4.083 |
0.3098 |
-0.389 |
0.3064 |
0.713 |
0.2984 |
3.305 |
|
|
288.149 |
0.3647 |
0.3613 |
0.932 |
0.3502 |
3.976 |
0.3664 |
-0.466 |
0.3625 |
0.603 |
0.3534 |
3.098 |
||
|
293.155 |
0.4280 |
0.4241 |
0.911 |
0.4113 |
3.902 |
0.4302 |
-0.514 |
0.4257 |
0.537 |
0.4154 |
2.944 |
||
|
298.139 |
0.4990 |
0.4953 |
0.742 |
0.4810 |
3.607 |
0.5024 |
-0.681 |
0.4973 |
0.341 |
0.4859 |
2.625 |
||
|
303.142 |
0.5789 |
0.5750 |
0.674 |
0.5590 |
3.438 |
0.5832 |
-0.743 |
0.5774 |
0.259 |
0.5649 |
2.418 |
||
|
308.159 |
0.6684 |
0.6641 |
0.643 |
0.6465 |
3.277 |
0.6734 |
-0.748 |
0.6670 |
0.210 |
0.6533 |
2.259 |
||
|
313.159 |
0.7674 |
0.7634 |
0.521 |
0.7441 |
3.036 |
0.7738 |
-0.834 |
0.7667 |
0.091 |
0.7519 |
2.020 |
||
|
318.156 |
0.8771 |
0.8734 |
0.422 |
0.8526 |
2.793 |
0.8849 |
-0.889 |
0.8772 |
-0.011 |
0.8613 |
1.801 |
||
|
323.141 |
0.9978 |
0.9953 |
0.251 |
0.9732 |
2.465 |
1.0079 |
-1.012 |
0.9995 |
-0.170 |
0.9827 |
1.513 |
||
|
R161 |
283.148 |
0.6014 |
0.6026 |
-0.200 |
0.5833 |
3.010 |
0.6069 |
-0.915 |
0.6018 |
-0.067 |
0.5927 |
1.447 |
|
|
288.157 |
0.6982 |
0.7005 |
-0.329 |
0.6788 |
2.779 |
0.7057 |
-1.074 |
0.6999 |
-0.244 |
0.6898 |
1.203 |
||
|
293.147 |
0.8056 |
0.8095 |
-0.484 |
0.7852 |
2.532 |
0.8156 |
-1.241 |
0.8090 |
-0.422 |
0.7980 |
0.943 |
||
|
298.14 |
0.9248 |
0.9308 |
-0.649 |
0.9041 |
2.238 |
0.9379 |
-1.417 |
0.9305 |
-0.616 |
0.9185 |
0.681 |
||
|
303.152 |
1.0574 |
1.0658 |
-0.794 |
1.0366 |
1.967 |
1.0739 |
-1.560 |
1.0656 |
-0.776 |
1.0527 |
0.445 |
||
|
308.155 |
1.2034 |
1.2146 |
-0.931 |
1.1830 |
1.695 |
1.2236 |
-1.679 |
1.2146 |
-0.931 |
1.2007 |
0.224 |
||
|
313.158 |
1.3636 |
1.3783 |
-1.078 |
1.3446 |
1.393 |
1.3883 |
-1.811 |
1.3784 |
-1.085 |
1.3638 |
-0.015 |
||
|
318.152 |
1.5390 |
1.5575 |
-1.202 |
1.5219 |
1.111 |
1.5684 |
-1.910 |
1.5578 |
-1.222 |
1.5424 |
-0.221 |
||
|
323.147 |
1.7306 |
1.7534 |
-1.312 |
1.7163 |
0.826 |
1.7652 |
-1.999 |
1.7539 |
-1.346 |
1.7379 |
-0.422 |
||
|
R1234yf |
283.153 |
0.4374 |
0.4359 |
0.343 |
0.4228 |
3.338 |
0.4410 |
-0.823 |
0.4367 |
0.160 |
0.4277 |
2.218 |
|
|
288.147 |
0.5099 |
0.5087 |
0.235 |
0.4939 |
3.138 |
0.5147 |
-0.941 |
0.5098 |
0.020 |
0.4998 |
1.981 |
||
|
293.157 |
0.5917 |
0.5907 |
0.169 |
0.5742 |
2.958 |
0.5975 |
-0.980 |
0.5920 |
-0.051 |
0.5811 |
1.791 |
||
|
298.151 |
0.6819 |
0.6819 |
0.000 |
0.6638 |
2.654 |
0.6897 |
-1.144 |
0.6836 |
-0.249 |
0.6717 |
1.496 |
||
|
303.153 |
0.7825 |
0.7835 |
-0.128 |
0.7639 |
2.377 |
0.7923 |
-1.252 |
0.7856 |
-0.396 |
0.7727 |
1.252 |
||
|
308.157 |
0.8936 |
0.8962 |
-0.291 |
0.8752 |
2.059 |
0.9059 |
-1.377 |
0.8986 |
-0.560 |
0.8849 |
0.974 |
||
|
313.157 |
1.0178 |
1.0206 |
-0.275 |
0.9983 |
1.916 |
1.0311 |
-1.307 |
1.0232 |
-0.531 |
1.0088 |
0.884 |
||
|
318.158 |
1.1532 |
1.1574 |
-0.364 |
1.1341 |
1.656 |
1.1687 |
-1.344 |
1.1602 |
-0.607 |
1.1453 |
0.685 |
||
|
323.152 |
1.3014 |
1.3073 |
-0.453 |
1.2833 |
1.391 |
1.3192 |
-1.368 |
1.3104 |
-0.692 |
1.2950 |
0.492 |
||
|
a |
|||||||||||||
the relative deviations of the saturated vapor pressure are calculated for R32, R134a, R1234ze(E),R1234yf and R161 with the PT EOS coupled the five different alpha functions. The Joshipura function presents the highest average absolute relative deviation (AAD(p)) and it has a value of 2.39%. The lowest average absolute deviation for the vapor pressure reported in Table 1 is 0.56% and it corresponds to the PTMelhem EOS. The other average absolute deviations are 0.61% for the PT-Soave EOS, 1.22% for the PT-Heyen EOS,1.39% for the PT-Gasem EOS. For R32, the deviations are negative for the PTSoave, PT- Heyen and PT-Melhem EOSs. The absolute deviations of the PT-Joshipura and the PT-Gasem EOSs show a decrease trend with the temperature, on the contrary, the absolute deviations of the PT-Soave, PT-Heyen and the PT-Melhem EOSs all show an increase with the temperature, the deviations scatter around the baseline for the PT-Melhem and PT-Gasem EOSs. For R1234ze(E),The average absolute deviations for the vapor pressure are 0.67%, 3.40%,0.70%,0.33% and 2.44% for PTSoave, PT-Joshipura, PT-Heyen ,PT-Melhem and PT-Gaesm EOSs, respectively. The PT-Joshipura and PT-Gasem EOSs show the big deviations, especially at the low temperature zones. For 134a and R1234yf, the PT-Melhem EOS gets the lowest average absolute deviations, the deviations are all within 0.7% around the baseline. For R161, an interesting fact that can be noticed is that the PTGasem EOS improves the performance ,the average absolute deviation for the vapor pressure is 0.62%,while deviations with the Soave,Joshipura, Heyen,
Melhem and Gasem functions are 0.78%, 1.95%,1.51%,0.75%,respectively.
Binary vapor equilibrium calculations
The isothermal vapor liquid equilibrium data of R32/ R134a,R32/R1234ze(E),R32/R1234yf,R161/R1234yf,R32/ R161 and R161/R1234ze(E) were reported. The vapor liquid equilibrium data were correlated by PRSV equation of state combined with the Wong and Sandler(WS) mixing rule and the NRTL activity coefficient model [12-14]. In this work, the isothermal vapor liquid equilibrium calculations of the above binary systems are performed with the Van der Waals one fluid mixing rules with the PT-Joshipura and the PT-Melhem models because the Melhem alpha function is the best to represent the vapor liquid equilibrium of the pure substances, and the Joshipura has the biggest average absolute deviations to calculate the saturated vapor pressure of the pure substances. The Van der Waals one mixing rules to estimate parameters with the PT EOSs are
(3)
(4)
(5)
Here, xi are the mole fraction of component “i” in a mixture, kij is the binary interaction parameter characterizing the molecular interactions between the molecules “i” and “j”. When i equals j, kij is zero. In this paper, the values for kij are those that minimize the following objective function:
In Equation.(4),N is the number of experimental data, the subscripts “exp” and “cal” represent the experimental and the calculated results, respectively.
The binary interaction parameters obtained at each temperature and the average absolute deviations of the pressure and the vapor phase mole fraction between the measured and the calculated values are listed in Table 2.
Table 2: Interaction parameter (kij) and average absolute percent deviation (AAD) of p and y.
|
|
|
|
PT-Joshipura |
|
PT-Melhem |
PT- Joshipura |
|
PT-Melhem |
|
|||||||||||||||||||||||||
|
T(K) |
kij |
|
|
|
AAD |
AAD |
kij |
AAD |
AAD |
kij |
AAD |
AAD |
kij |
AAD |
AAD |
|
||||||||||||||||||
|
|
|
|
|
(pa)/% |
(y1b) |
|
(pa)/% |
(y1b) |
|
(pa)/%(y1b) |
|
(pa)/% |
(y1b) |
|
||||||||||||||||||||
|
R32/R134 |
|
|
|
|
|
|
|
|
|
|
|
R32/R1234ze(E) |
|
|
|
|
|
|||||||||||||||||
|
283.15 |
0.004 |
0.99 |
|
0.009 |
0.000 |
0.37 |
0.009 |
0.009 |
1.93 |
0.011 |
0.005 |
1.44 |
0.010 |
|||||||||||||||||||||
|
293.15 |
|
|
|
|
|
|
|
|
|
|
|
0.009 |
1.88 |
0.010 |
0.005 |
1.48 |
0.010 |
|||||||||||||||||
|
303.15 |
0.003 |
0.76 |
|
0.009 |
-0.001 |
0.39 |
0.009 |
0.009 |
1.73 |
0.010 |
0.005 |
1.39 |
0.010 |
|||||||||||||||||||||
|
313.15 |
|
|
|
|
|
|
|
|
|
|
|
0.009 |
1.60 |
0.008 |
0.005 |
1.33 |
0.008 |
|||||||||||||||||
|
323.15 |
0.002 |
0.53 |
|
0.006 |
-0.001 |
0.37 |
0.005 |
0.009 |
1.48 |
0.007 |
0.005 |
1.30 |
0.007 |
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
R32/R1234yf |
|
|
|
|
|
|
|
|
|
|
|
R161/R1234yf |
|
|
|
|
|
|||||||||||||||||
|
283.15 |
0.015 |
|
2.36 |
|
|
0.021 |
0.011 |
2.34 |
0.021 |
0.003 |
0.72 |
0.002 |
-0.001 |
0.26 |
0.002 |
|||||||||||||||||||
|
293.15 |
0.015 |
|
2.18 |
|
|
0.020 |
0.011 |
2.11 |
0.020 |
0.003 |
0.56 |
0.001 |
-0.001 |
0.21 |
0.001 |
|||||||||||||||||||
|
303.15 |
0.014 |
|
2.02 |
|
|
0.019 |
0.010 |
1.91 |
0.018 |
0.003 |
0.65 |
0.001 |
-0.001 |
0.30 |
0.001 |
|||||||||||||||||||
|
313.15 |
0.013 |
|
1.87 |
|
|
0.017 |
0.010 |
1.75 |
0.018 |
0.002 |
0.37 |
0.001 |
-0.002 |
0.41 |
0.001 |
|||||||||||||||||||
|
323.15 |
0.012 |
|
1.71 |
|
|
0.016 |
0.010 |
1.66 |
0.017 |
0.001 |
0.46 |
0.001 |
-0.002 |
0.31 |
0.001 |
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
R32/R161 |
|
|
|
|
|
|
|
|
|
|
|
R161/R1234ze(E) |
|
|
|
|
|
|||||||||||||||||
|
283.15 |
0.005 |
|
1.13 |
|
|
0.004 |
0.001 |
0.56 |
0.003 |
-0.001 |
1.62 |
0.011 |
-0.005 |
1.19 |
0.011 |
|||||||||||||||||||
|
293.15 |
0.005 |
|
1.01 |
|
|
0.003 |
0.001 |
0.60 |
0.003 |
-0.001 |
1.41 |
0.010 |
-0.005 |
1.06 |
0.010 |
|||||||||||||||||||
|
303.15 |
0.005 |
|
0.86 |
|
|
0.003 |
0.001 |
0.62 |
0.003 |
-0.001 |
1.24 |
0.008 |
-0.005 |
0.98 |
0.008 |
|||||||||||||||||||
|
313.15 |
0.004 |
|
0.77 |
|
|
0.003 |
0.001 |
0.72 |
0.003 |
-0.001 |
1.09 |
0.007 |
-0.005 |
0.92 |
0.007 |
|||||||||||||||||||
|
323.15 |
0.003 |
|
0.58 |
|
|
0.003 |
0.000 |
0.66 |
0.003 |
-0.001 |
0.92 |
0.006 |
-0.005 |
0.88 |
0.006 |
|||||||||||||||||||
|
a b |
||||||||||||||||||||||||||||||||||
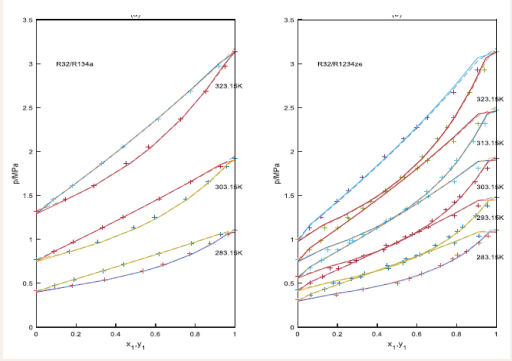
The vapor liquid equilibrium data for the R32/R134a, R32/R1234ze(E),R32/ R1234yf,R161/R1234yf,R32/R161 and R161/R1234ze (E) of the experiments [12-14]. and the results of the calculation with the PT EOSs are compared in Figure 1.
Figure 1: The prediction of isothermal curves for the binary systems using the PT- Joshipura and the PT-Melhem models.
+: experimental data, solid lines: the curves predicted with the PT- Joshipura model, the dashed line: the curves predicted with the PT-Melhem model.
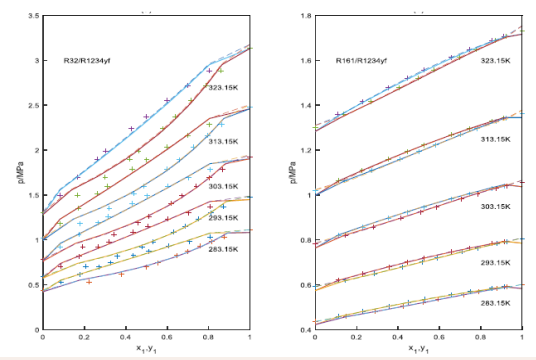
Figure 2.
Figure 2: The prediction of isothermal curves for the binary systems using the PT- Joshipura and the PT-Melhem models.
+: experimental data, solid lines: the curves predicted with the PT- Joshipura model, the dashed line: the curves predicted with the PT-Melhem model.
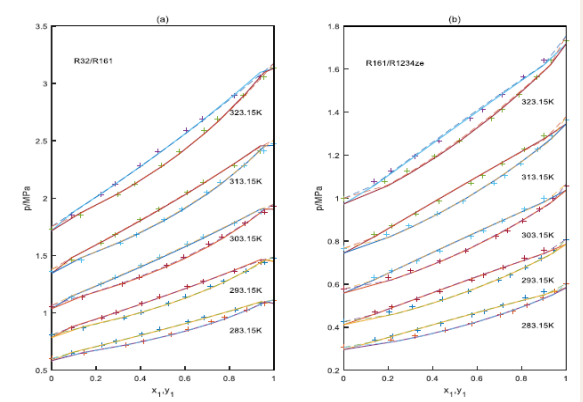
Figure 3.
Figure 3: The prediction of isothermal curves for the binary systems using the PT- Joshipura and the PT-Melhem models.
+: experimental data, solid lines: the curves predicted with the PT- Joshipura model, the dashed line: the curves predicted with the PT-Melhem model.
Results in Table 2 and Figures 1-3 show using the PTJoshipura and the PT-Melhem models, the good results can be obtained for the systems evaluated. However, the results also show that the average absolute deviations of the bubble pressure with the two equations, the PT-Melhem equation is lower than those predictions employing the PT-Joshipura equation, the average absolute deviations for the bubble pressure are 1.23%, 0.98%,respectively. But the PT- Joshipura and the PT-Melhem models give the same average absolute deviation in the vapor molar fractions, they are all 0.008.Comparison between the PTJoshipura and the PT-Melhem models show that the predictions are very similar for the systems in this study. In the other hand, a more detailed analysis is made for the results reported in Table 2 and Figures 1-3.
For the R32/R134a systems, the binary interaction parameters are around 0.000 at the temperatures with the PTMelhem EOS. The average absolute deviation of the bubble pressure is 0.38%, while the average absolute deviation in the vapor molar fraction (AAD(y1)) is 0.008. For the PT- Joshipura EOS, the binary parameters are dependent on the temperatures, the average absolute deviation of the vapor pressure is bigger than the PT-Melhem EOS. In Figure 1(b), five predicted isothermal phase diagrams of the R32/R1234ze(E) are plotted with the experimental data published by Hu et al, [12] The optimized binary interaction parameters kij are independent of the temperature, the values of the binary interaction parameters of the PTJoshipura and PT-Melhem are 0.009 and 0.005, respectively. Again, a satisfactory agreement is observed between the experimental data and the calculated curves, although the bubble pressure of the R32/R1234ze(E) system is slightly overestimated when the mole fraction of R32 tends to one with both models. The average absolute deviation of pressure (AAD(p) ) and the average absolute deviation of vapor phase mole fraction (AAD(y1)) are 1.72%,1.39% and 0.009,0.009 for the PT-Joshipura and the PTMelhem EOSs. Figure 2(a) shows the isothermal phase diagrams for the R32/R1234yf systems, the optimized binary interaction parameters kij are almost independent of temperature. The binary interaction parameters in the two equations are relative bigger, this may be due to the polarity of R1234yf .The average absolute deviations of the bubble pressure and the average absolute deviations of the vapor phase mole fractions are the same for the two EOSs, because the bubble pressure of the R32/ R1234yf system is overestimated when the mole fraction of R32 tends to one, the absolute deviations of bubble pressure are all around 2.0%,the absolute deviations of vapor phase mole fractions are all around 0.02.In Figure 2(b) p-x-y data and the predicted curves are presented for the R161/R1234yf binary systems. The optimized binary interaction parameters kij are also independent on the temperature, an accurate predictions are obtained with the PT- Joshipura and PT-Melhem models. The average absolute deviation of pressure (AAD(p) ) and the average absolute deviations of the vapor phase mole fraction(AAD(y1)) are 0.55%,0.30% and 0.001,0.001 for these systems. In Figure 3 (a), the results of the vapor liquid equilibrium of the R32/R161 system are shown. The average absolute deviation of pressure (AAD(p) ) and the average absolute deviation of vapor phase mole fraction(AAD(y1)) are 0.87%,0.63% and 0.003,0.003. In Figure.3(b), for the R161/R1234ze binary system, the binary interaction parameters are all around 0.005 for the PT- Joshipura model at the five temperatures, the average absolute deviation of pressure and the average absolute deviation of vapor phase mole fraction are 0.87% and 0.005,but for the PT-Melhem EOS, the binary interaction parameters are all around 0.001,the average absolute deviation of pressure and the average absolute deviation of vapor phase mole fraction are 0.63% and 0.003,and the binary interactions parameters are also independent on the temperatures.
CONCLUSION
The Patel-Teja coupled the five different alpha functions are used to calculate the saturated vapor pressure of R32,R134 , R1234ze(E),R1234yf ,R161.The PT-Melhem model shows the lowest average absolute deviation of pressure(AAD(p) ),the one-parameter exponential alpha function, as the Joshipura expression, gives the worst results for the saturated vapor pressure. To our surprise, two or three parameter exponential alpha functions does not produce better results than those estimated using the Soave function except for the Melhem function.
Minimizing the objective function, the adjusted parameters in the model are regressed from the experimental data. It has been demonstrated that the PT-Joshipura and the PT-Melhem EOS with the VDW mixing rule can correlate the bubble pressure and the vapor phase mole fractions well for the R32/R134a, R32/ R1234ze(E),R32/R1234yf, R161/R1234yf, R32/R161 and R161/ R1234ze(E) systems. Compared with the PRSV-WS-NRTL model, whereas the PT-Joshipura and the PT-Melhem with the VDW mixing rule is relatively simple with only one fitted parameter.
ACKNOWLEDGMENT
This work was supported by the Educational Commission Key Program of Natural Science Foundation of Anhui Province of China (KJ2016A579),the Natural Science Research Project of Anhui Province(1908085MB50).
REFERENCES
- Peng DY, Robinson DB. “A New Two constant Equation of State”. Ind Eng Chem Fundam. 1976; 15: 59.
- Soave G. “Equilibrium Constants from a Modified Redlich-Kwong Equation of State”. Chem Eng Sci. 1972; 27: 1917.
- Valderrama JO. “The state of the cubic equations of state”. Ind Eng Chem Res. 2003; 42: 1603-1618.
- Valderrama JF, Alfaro OM. “Liquid Volumes from Generalized Cubic Equations of State: Take It with Care”. Oil Gas Sci Technol. 2000; 55: 523.
- Abdollahi-Demneh F, Moosavian MA, Montazer-Rahmati MM, Omidkhah MR, Bahmaniar H. “Comparison of the prediction power of 23 generalized equations of state: Part I. Saturated thermodynamic properties of 102 pure substances” Fluid Phase Equilibria. 2010a; 288: 67.
- Abdollahi-Demneh F, Moosavian MA, Montazer-Rahmati MM, Omidkhah MR, Bahmaniar H. “Comparison of the prediction power of 23 generalized equations of state: Part II-Parametric evaluation” Fluid Phase Equilibria. 2010b; 291: 48.
- Yang T, Chen GJ, Yan W, Guo TM. “Extension of the Wong-Sandler mixing rule to the three-parameter Patel-Teja equation of state: “Application up to the near-critical region ”. Chem Eng J. 1997; 67: 27.
- Patel NC, Teja AS. “A new cubic equation of state for pure fluids and mixtures” Chem Eng Sci. 1981; 37: 463.
- Segura H, Kraska T, Mejía A, Winniak J, Polishuk I. “Unnoticed Pitfalls of Soave -Type Alpha Functions in Cubic Equations of State”. Ind Eng Chem Res. 2003; 42: 56-62.
- Forero LA, Velásquez jA. “A Method to Estimate the Patel-Teja Equation of State Constants”. J Chem Eng Data. 2010; 55: 50-94.
- Forero LA, Velásquez jA. “The Patel-Teja and the Peng-Robinson EOSs performance when Soave alpha function is replaced by an exponential function”. Fluid Phase Equilibria. 2012; 332: 55-76.
- Xiaozhen Hu, Xianyang Meng, Jiangtao Wu. “Isothermal vapor liquid equilibrium measurements for difluoromethane(R32)+trans-1,3,3,3-tetrafluoropropene(R1234ze(E))”. Fluid Phase euilibria. 2017a; 431: 58-65.
- Xiaozhen Hu,Tao yang, Xianyang Meng, Shengshan Bi, Jiangtao Wu. “Vapor liquid equilibrium measurements for difluoromethane (R32)+2,3,3,3-tetrafluoroprop-1-ene(R1234yf) and fluoroethane(R161)+2,3,3,3-tetrafluoroprop-1-ene(R1234yf)”. Fluid Phase Equilibria. 2017b; 438: 10-17.
- Xianyang Meng, Xiaoz hen Hu, Tao yang, Jiangtao Wu. “Vapor liquid equilibria for binary mixtures of difluoromethane(R32)+fluoroethane(R161)andfluoroethane(R161)+trans-1,3,3,3-tetrafluoropropene(R1234ze(E))”. J Chem Thermodyn. 2018; 118: 43.