Local Anesthetic Systemic Toxicity (LAST): Mini Review
- 1. Department of Anesthesiology and Intensive Care Unit, Baskent University, Turkey
Abstract
Local anesthetic systemic toxicity (LAST) significantly negatively affects life-threatening anesthesia practice. It arises after the increasing application of local anesthetic (LAs) drugs in clinical practice by various means and many anesthetic techniques. LAs exert different cellular effects in the central nervous (CNS) and cardiovascular systems (CVS); therefore, the underlying mechanisms of LAST are multifactorial. Although LAST, the most common neurological presentation, can occur atypically or less frequently with cardiovascular disease. There are various risk factors related to the drug used and the application. Ultrasound (US), while increasing the success of regional anesthesia (RA) techniques with its use, also increases the LAs dose and reduces the risk of anesthetic toxicity and complications. Critical points in LAST treatment are early lipid emulsion administration, rapid seizure management, and airway-respiratory and CVS-encouraging drug therapy. In preventing and treating LAST, knowing the underlying mechanisms, risk factors, pharmacokinetics, and pharmacodynamics of LAs drugs are vital in clinical practice for the practicing physician. Anesthesiologists can lead the education and training of those who practice LAs in the hospital setting in preventing and managing LAST. This review aims to emphasize LAST’s importance in our anesthesia practice.
Keywords
• Local anesthetics
• Toxicity
• Treatment
• Preventing
• Managing
• Knowing
CITATION
Çekmen N, Gökdemir BN (2023) Local Anesthetic Systemic Toxicity (LAST): Mini Review. Int J Clin Anesthesiol 11(1): 1124.
INTRODUCTION
Local anesthetic systemic toxicity (LAST) is uncommon but has a significantly lethal adverse effect. LAST can be recognized, prevented, managed, and treated with awareness and thorough knowledge [1,2]. Due to recent advances in RA and popular techniques, large volume increases the potential risk of LAST using LAs inside and outside the operating room [1-3]. With the widespread use of LAs techniques, the incidence of peripheral nerve blocks is estimated to be between 0.03% and 0.27%. Many conditions contribute to the risk of developing LAST, such as the widespread use of high-volume facial plane interventions [1,3], the increased use of continuous catheter techniques [4], the use of multiple LAs techniques in the same patient [5], and tumescent anesthesia [6]. While increasing the success of RA techniques with its use, the US also increases the LAs dose and reduces the risk of anesthetic toxicity and complications [1-4]. LAs in daily practice, especially by anesthesiologists, surgeons, perioperative nurses, and medical personnel, have become widespread among emergency room doctors and dentists. In preventing and treating LAST, knowing the underlying mechanisms, risk factors, pharmacokinetics, and pharmacodynamics of LAs drugs are vital in clinical practice for the practicing physician. Anesthesiologists can lead the education and development of those who practice LAs in the hospital setting in preventing and managing LAST and improving patient safety [1,2,7]. This review emphasizes LAST’s importance in our anesthesia practice.
Local Anesthet?cs
LAs are divided into two groups according to their chemical structure. The groups are also aromatic. Quaternary amine (secondary or tertiary amine) with a lipophilic part (benzene ring) determines the intermediate bond between the hydrophilic part in its structure. If this bond is amino-ester, the ester (-COO-) group, amino amide, forms the amide (-NHCO-) group [8,9].
LAs are weak bases in solution present in charged and uncharged forms. When the amine nitrogen is protonated (quaternary), the lipid solubility of LAs gradually decreases. The protonated, charged form has a greater affinity for Na+ channels because it is more soluble and bound in water. The intermediate amine form is uncharged, more soluble in fat, and easily crosses the nerve membrane [8,9].
Pharmacodynam?cs
The differences in LAs are due to inert properties such as pKa, lipophilicity, and protein binding.
- Lipophilicity: The potency of LAs
- pKa: The onset of action of LAs
- Protein binding: The duration of LAs
Pharmacok?net?cs
The systemic absorption rate of LAs determines the plasma concentration of LAs and the time it takes to reach these peak levels [8]. At the same time, serum concentration, injection site, and LAs dose are critical. Injection of the same amount of LAs depending on the vascular structure of the region, the highest blood peak concentrations in the water occur in order; Topical (mucosa)>intercostal block>epidural block>intrapleural block> plexus block>skin infiltration [8,9]. Ester-based LAs; are hydrolyzed by plasma esterases. They are stable and allergic to this group because their para amino benzoic acid breakdown products and reactions are more common. Amide-type LAs; in the liver metabolism by hepatic microsomal cytochrome P450 (CYP/CYP450) enzymes. Enzymes destroy them, are more stable, and allergic reactions are rarely observed in this group. Because of these differences, more amide-type LAs are used in the clinic [9].
The high serum concentration of LAs is due to systemic absorption or accidental intravascular injection. Both minor and significant may develop in LAST due to elevated serum concentration. Minor symptoms of LAST include numbness around the mouth and tongue, metallic taste, and visual and cognitive changes; Major symptoms are usually seizures, arrhythmias, and respiratory and cardiac arrest. LAST can be seen in the standard and very high doses without side effects [1,2,10]. Nevertheless, it is necessary to achieve the desired effect. The total amount of LAs should be the lowest [10].
Mechan?sm
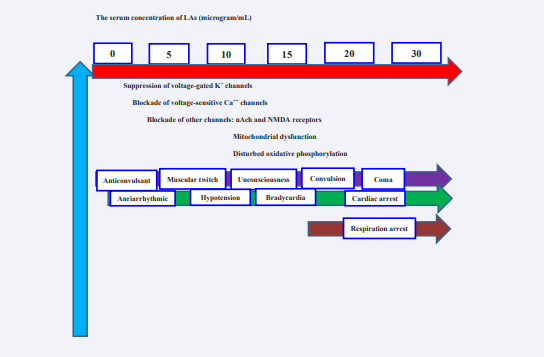
LAs act in the interior of the voltage-gated sodium (VGNa+ ) channel. The VGNa+ channel consists of alpha and beta subunits. For LAs, the Alpha subunit functions as the functional ion channel, and the beta subunits modulate the kinetics and voltage dependence for activation/inactivation. LAs inhibit conduction in neuronal tissues by reversibly inhibiting ion transfer and neuronal depolarization [8-10]. LAs can interfere with cell membrane signaling, affecting multiple cellular cAMP processes and protein kinases [8,9]. LAs can also block Ca++, K+ , Na+ -K+ ATPase channels and other channels nAch (nicotinic acetylcholine, and NMDA (N-methyl-D-aspartate). The LAs also impair mitochondrial metabolism and disturb oxidative phosphorylation, ATP production, and ryanodine receptor at the sarcoplasmic reticulum (Figure 1) [8,10].
Figure 1: Mechanisms and symptoms of LAST. Las: Local anesthetics; LAST: Local anesthetic systemic toxicity; nAch: nicotinic acetylcholine; NMDA: N-methyl-D-aspartate.
LAs reduce or stop potential action conduction by blocking Na+ channels in nerves. As a result, it first increases excitability in the CNS, leading to widespread depression. On the other hand, it reduces excitability in the heart and prolongs the refractory period. In addition, vasodilation occurs when vascular smooth muscle is affected and can lead to critical hemodynamic changes. LAST’s mechanisms and symptoms, as shown in Figure 1 [1,2,8-10]. LAST has a complex clinical picture because of the many sites affected by the LAs. A comparison of LAs according to potencies, duration of action, and maximum doses is given in Table 1 [8-10].
Table 1: A comparison of LAs according to potencies, duration of action, and maximum doses
|
Local anesthetic (LAs) |
Potency |
Duration of action |
Maximum doses |
|
Procaine (Esters) |
1 |
Short |
7-10 mg/kg |
|
Chloroprocaine(Esters) |
2-4 |
Middle |
10-12 mg/kg |
|
Articaine (Amides) |
5 |
Middle |
5 mg/kg |
|
Lidocaine (Amides) |
4 |
Middle |
5 mg/kg |
|
Mepivacaine (Amides) |
3-4 |
Middle |
4.5-5 mg/kg |
|
Prilocaine (Amides) |
3-4 |
Middle |
6 mg/kg |
|
Bupivacaine (Amides) |
16 |
Long |
2-2.5 mg/kg |
|
Ropivacaine (Amides) |
14-16 |
Long |
2-3 mg/kg |
|
Levobupivacaine(Amides) |
16 |
Long |
2 mg/kg |
|
Tetracaine (Esters) |
16 |
Long |
1-3 mg/kg |
Las: Local anesthetics
Presentat?on
LAST, in both SSS and CVS, occurs with findings that can be seen first in the stimulation period and then in the depression period, and this is presented in Table 2 [1,2].
Table 2: The effects of LAs on CNS and CVS
|
CNS |
CVS |
||
|
Stimulation |
Depression |
Stimulation |
Depression |
|
|
|
|
Las: Local anesthetics; CNS: central nervous system; CVS: cardiovascular system.
The CNS is more sensitive than CVS for the risk of developing LAST. This means the serum concentration of LAs required to establish the clinical picture of LAST is lower in the CNS than in CVS. The most common LAST-related toxicity is CNS toxicity, with an incidence of 68-77% and often presenting first, whereas CVS toxicity is less frequent, although 40% occurs atypically [1,2,11,12].
R?sk Factors
LAST-related risk factors are patient, anesthetic, and practice setting factors. And these factors are presented in Table 3 [12].
Table 3: LAST-related risk factors are patient, LAs, and practice setting factors
|
Patient |
Local anesthetic |
Practice setting factors |
|
Elderly patients Pediatric patients Pregnancy Co-morbidities
|
Type Dose Potency Concentration Volume Duration of action |
Technique Site of block
Single/Continuous dose |
LAST: Local anesthetic systemic toxicity; LAs, Local anesthetics
Prevent?on
The basic principle in medicine is “Prevention is better than cure.” it should be treated accordingly. The main aim should be prevention, and RA applications should avoid LAST. It is essential to recognize LAST early, prevent it, and take the necessary safety measures to minimize its severity, reduce the risk factors, and monitor closely [12].
Dose Management
The first step in preventing LAST is administering the LAs dose individually according to the specified algorithms and clinical situation. Gradual bolus injections, slow injection over time, and light aspiration at intervals are recommended practices to avoid adverse effects. Making fractionated injections of LAs in fractional <5 mL is recommended, stopping for 30-45 seconds between injections [8-10]. Adding epinephrine to LAs drug at doses as low as 2.5 to 5 μg/mL increased heart rate, and systolic blood pressure acts as a marker for intravascular injection [11].
Ultrasound Gu?dance
US has been observed to decrease the LAST risk by 60%-65% compared to peripheral nerve stimulation alone. US guidance maximizes block success and minimizes complications in nerves, surrounding structures, needle tip, and simultaneous spread of LAs injection allows to be displayed. LAST was not wholly terminated with the use of the US, but a significant decrease was observed in its rate [12,13].
Factors to be considered to reduce the risk of toxicity when applying the block:
- US-guided application
- Frequent aspiration
- Intermittent injection
- Making a test dose
- Close follow-up [1,2,12,13].
Awareness, rigorous safety precautions, close monitoring, and proper dose and US guidance to prevent LAST are always required.
Management
Situations that should be done during the development of LAST:
- Recognition
- Emergency management
- Treatment
- Close follow-up [1,2,12].
Intravenous (IV) vascular access, oxygen, and standard monitoring should be applied to all patients receiving LAs injections against the possibility of LAST development. Postapplication monitoring should continue for at least 30 minutes. It should be kept in mind that it may develop hours after the completion of the LAST injection. Management of the clinical picture when LAST develops includes general supportive measures and specific therapeutic interventions. When LAST is suspected, LAs injection/infusion should be stopped first, and then cardiopulmonary resuscitation (CPR) should be started [1,2,12,14].
Support?ve Measures
- Close monitoring
- Basic supportive measures to ensure airway management, ventilation with 100% oxygen
- Stop injecting the LAs
- If necessary, advanced airway device and endotracheal entübation
- Immediate initiation of CPR and effective CPR (Reduce individual epinephrine boluses to ≤1 μg/kg)
- Avoiding hypoxia, hypercapnia, acidosis, and also hyperventilation
- Providing rapid coronary perfusion
- Reduce the concentration of LAs in the tissue
- Prompt initiation of IV lipid solution
- Avoiding vasopressin, Ca++ channel blockers and β-blockers, and other LAs
- Amiodarone should be preferred as an antiarrhythmic
- Lidocaine is contraindicated in these situations
- If CPR is insufficient, consider cardiopulmonary bypass
- In case of seizure, to ensure airway management, ventilation with 100% oxygen
- Benzodiazepines should be the first choice drug for seizure control. Propofol (1 mg/kg IV) can control seizures when needed in uncontrolled seizures. However, high doses should be avoided in hemodynamically unstable and elderly patients
- Seizures should be controlled, and hypoxemia, hypercarbia, and acidosis should be avoided as much as possible
- If seizures cannot be controlled with benzodiazepines and propofol, neuromuscular blockers can be considered
- If there is a CVS feature after LAST, patients should be followed up for at least 6 hours, and if there is a CNS picture, at least 2 hours, even if the improvement is achieved
- If necessary, consider staying in intensive care ünit [12,14].
Intravenous L?p?ds
Intravenous lipid therapy is an essential vital component in LAST therapy. In treating LAST, an intravenous dose of 20% lipid emulsion should be administered initially as a bolus and then continued with infusion, as shown in Table 4.
Table 4: Lipid dose management
|
20 % lipid emulsion IV dose management |
||
|
Patient weight (kg) |
Immediately |
After |
|
Bolus |
Infusion |
|
|
>70 kg (most adults) <70 kg |
100 mL over 2-3 min. 1.5 mL/kg over 2-3 min. |
200 to 250 mL over 15 to 20 min. 0.25 mL/kg/min. |
|
||
IV: intravenous
If hemodynamic stability is not achieved, the doses should be doubled. After obtaining hemodynamic stability, lipid infusion should be continued for approximately 10 minutes. The maximum recommended initial lipid dose should be 12 mL/kg [12,14,16].
The mechanism of action of IV lipid administration in treating LAST is explained in three ways:
1- Helps to redistribute LAs with the “scavenging effect,” where the lipids act as a shuttle
2- It provides an improvement in cardiac output and blood pressure with a direct effect
3-The cardioprotective effect reduces ischemic reperfusion injury and improves cardiac function [12,14-17]. Although there are few side effects related to lipid therapy, they have been reported in the literature and presented in Table 5 [16,17].
Table 5: Adverse effects of rapid lipid emulsion infusion
|
CONCLUSION
LAST is a significant adverse event in life-threatening anesthesia practice. LAs exert different cellular effects in the CNS and CVS; therefore, the underlying mechanisms of LAST are multifactorial. Awareness, rigorous safety precautions, close monitoring, and proper dose and US guidance to prevent LAST are always required. Critical points in LAST treatment are early lipid emulsion administration, rapid seizure management, and airwayrespiratory and CVS-encouraging drug therapy. In preventing and treating LAST, knowing the underlying mechanisms, risk factors, pharmacokinetics, and pharmacodynamics of LAs drugs are vital in clinical practice for the practicing physician. Anesthesiologists can lead the education and training of those who practice LAs in the hospital setting in preventing and managing LAST.
REFERENCES
- El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current Perspectives. Local Reg Anesth. 2018; 11: 35-44.
- Dillane D, Finucane BT. Local anesthetic systemic toxicity. Can J Anesth. 2010; 57: 368-380.
- El-Boghdadly K, Pawa A. The erector spine plane block: plane and simple. Anaesthesia. 2017; 72: 434-438.
- Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The Erector Spinae Plane Block. Reg Anesth Pain Med. 2016; 41: 621-627.
- Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011; 113: 904-925.
- Pawa A, Wight J, Onwochei DN, Vargulescu R, Reed I, Chrisman L, et al. Combined thoracic paravertebral and pectoral nerve blocks for breast surgery under sedation: a prospective observational case series. Anaesthesia. 2018;73: 438-443.
- Barrington MJ, Weinberg GL, Neal JM. A call to all readers: educating all surgeons on preventing and treatment of local anaesthetic systemic toxicity. ANZ J Surg. 2016; 86: 636-637.
- Butterworth JF, Strichartz GR. Molecular mechanisms of local anesthesia a review. Anesthesiology. 1990; 72: 711-734.
- Becker DE, Reed KL. Local anesthetics: a review of pharmacological considerations. Anesth Prog. 2012; 59: 90-102.
- Di Gregorio G, Neal JM, Rosenquist RW, Weinberg GLM. Clinical presentation of local anesthetic systemic toxicity: a review of published cases, 1979 to 2009. Reg Anesth Pain Med. 2010; 35: 181-187.
- Gitman M, Barrington MJ. Local anesthetic systemic toxicity: a review of recent case reports and registries. Reg Anesth Pain Med. 2018; 43: 124-130.
- Neal JM, Barrington MJ, Fettiplace MR, Gitman M, Memtsoudis SG, Mörwald EE, et al. The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on Local Anesthetic Systemic Toxicity: executive summary 2017. Reg Anesth Pain Med. 2018; 43: 113-123.
- Bainbridge D, McConnell B, Royse C. A review of diagnostic accuracy and clinical impact from the focused use of perioperative ultrasonography. Can J Anaesth. 2018; 65: 371-380.
- Wolfe JW, Butterworth JF. Local anesthetic systemic toxicity: update on mechanisms and treatment. Curr Opin Anesthesiol. 2011; 24: 561.
- Fettiplace MR, Weinberg G. The mechanisms underlying lipid resuscitation therapy. Reg Anesth Amp Pain Med. 2018; 43: 138.
- Weinberg GL. Lipid infusion therapy: translation to clinical practice. Anesth Analg. 2008; 106: 1340-1342. 17. FettiplaceMR, Ripper R, Lis K, Lin B, Lang J, Zider B, et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013; 41: e156-162.