Salicylic Acid Induced Salt Stress Tolerance in Plants
- 1. Department of Biotechnology, Kongunadu Arts and Science College, India
Abstract
Salicylic acid (SA) is a phenolic phytohormone acting as signaling molecule and tolerance against abiotic stresses. It plays a vital role within the plant response to adverse environmental conditions similar to salinity. Soil salinity is a major problem of food production because it limits crop yield and restricts use of land previously uncultivated. It plays vital role in plant growth, ion uptake and transport, preventing oxidative damage in plant by detoxifying super oxide radicals, produced as a result of salinity. This review provides the evidence that supports the role of SA during plant growth and development is reviewed by comparing various experiments performed by application of SA under salt stress conditions.
Keywords
• Abiotic stress
• Crops
• Phytohormone
• Salicylic acid
• Salinity tolerance
Citation
Rajeshwari V, Bhuvaneshwari V (2017) Salicylic Acid Induced Salt Stress Tolerance in Plants. Int J Plant Biol Res 5(3): 1067.
INTRODUCTION
Salt stress can affect physiological processes from seed germination to plant development, leading to reduced growth and yield. The complexity of the plant responses to salt stress can be partially explained by the fact that salinity imposes both ionic and osmotic stress as well as nutritional imbalance [1]. When plants are subjected to saline conditions, such biochemical changes occur as production of reactive oxygen species (ROS) like superoxide, hydrogen peroxide and hydroxyl radicals [2]. In order to avoid the harmful effects of these ROS, plants evolved an effective scavenging system composed of non enzymatic antioxidants (carotenoids, ascorbate, tocopherol), and enzymatic antioxidants, such as catalase (CAT) and ascorbate peroxidase (APX). The results of most studies suggest that the resistance to salt stress is usually correlated with a more efficient antioxidant system [3]. The enzymatic action of APX reduces H2 O2 using the ascorbate as an electron donor. CAT is additionally concerned in removal of H2 O2 . Salinity induced production of ROS disturbs the cellular oxidation- reduction system in favor of oxidized forms, and thereby creating an oxidative stress which will harm DNA, inactivate enzymes and cause lipid peroxidation [4].
Salicylic acid plays an important role in the regulation of plant growth and development, flowering, fruit yield, seed germination [5]. Ion uptake and transport [6], photosynthetic rate, stomatal conductance and transpiration [7] could also be affected by SA application. The role of the SA in defense mechanisms under both biotic and abiotic stresses suggests that it also alleviates the salt stress in plants [8,9] and also SA multiplies the ROS generation under stress [10]. A direct physiological impact of SA is the alteration of antioxidant enzyme activities in vivo. Exogenous application of SA enhances the activities of antioxidant enzymes and increase plant tolerance to the abiotic stress [11]. SA has received much attention due to its role in plant responses to abiotic stresses such as ozone [12], UV-B [13], heat stress [14-16], drought [8,17,18], oxidative stress [19], salt and osmotic stress [10,20,21]. The review summarized the role of exogenously applied and /or endogenous SA in physiological and biochemical changes that occur in plants under salt stress conditions.
Wheat (Triticum aestivum)
Wheat grain is a stable food in world wide. Its production is greatly affected by salinity. An enhanced tolerance aganist salinity stress was observed in wheat seedlings raised from the grains soaked in SA [22]. Exogenous application of SA increases the proline contentent in wheat seedlings under salinity stress, thereby alleviating the deleterious effects of salinity. Further, the treatment also lowered the level of active oxigen species and therefore the activities of super oxide dismutase (SOD) and peroxidase (POX) were also lowered in the roots of young wheat seedlings [23]. These findings indicate that the activities of these antioxidant enzymes are directly or indirectly regulated by SA, thereby providing protection aganist salinity stress [24]. SA treatment caused accumulation of both ABA and IAA in T. aestivum seedlings under salinity. However, the SA treatment did not influence on cytokinin content. Thus, protective SA action includes the development of anti stress programs and acceleration of normalization of growth processes after removal of stress factors [24].
Pretreatment of wheat seedling with 0.5mM SA one day before salt stress and different concentrations of NaCl were used used (80 and 120mM). Plants treated with SA increased the fresh and dry weight of wheat seedling and also increased the level of catalase and peroxidase activity in leaf and root samples. SA treatment decreases the H2 O2 content. So, treatment with SA induces salt tolerance may be related to induction of antioxidant enzymes [25].
The pre-soaking treatment of seeds with SA positively affected the osmotic potential, shoot and root dry mass, K+ /Na+ ratio and contents of photosynthetic pigments in wheat seedlings, under both saline and non - saline conditions [26]. The effect of SA on salt stress tolerance almost needed during the seed germination of wheat. But the SA reduced the damaning effect of salinity on seedling growth and accelerated the growth processes [27,28]. The alleviating effect of SA to abiotic stresses was studied through the SA application either by seed soaking of wheat genotypes [29] or through rooting medium of wheat [30]. Exogenously sourced SA (0.5mM) was reported to improve salt tolerance in wheat due to an enhanced transcript level of antioxidant genes; GPX1, GPX2, DHAR, GR, GST1, GST2, MDHAR, and GS, and an increased activity of ascorbate (AsA)-GSH pathway enzymes [31].
Kang et al. 2012 [32], reported that SA application increases the plant height, shoot length, fresh and dry weight of shoot and root, increased the photosynthetic pigments and decreased the MDA content. In proteomic analysis, 38 proteins were identified by 2D PAGE and MALDI-TOF/TOF MS after treatment with salt and SA. These protein are involved in signal transduction, stress defence, energy related function, metabolism and photosynthesis that are regulated by SA treatment. While, many studies have been reported that SA induced increase in the resistance of wheat to salinity, antioxidant and oxidative stress respectively [33,34].
Tomato (Lycopersicon esculuntum Mill.)
The tomato is the edible fruits, numerous varieties of tomato are widely grown in temperate climates across the world. Soil salinity affects plants many part of the world, particularly on irrigated land [35]. Taken together these two effects were found that SA pre-treatment improved the acclimation of tomato to high salinity [36-38].
An enhanced tolerance against salinity stress was observed in tomato plants raised from the seeds soaked in SA and was presumed to be due to the enhanced activation of some enzymes viz. aldose reductase and ascorbate peroxidase and to the accumulation of certain osmolytes such as proline [39]. Gulutathione transferases (GSTs) are important participants in adaptation to changes in environmental signals tomato plants (Solanum lycopersicum) [40].
Exogenous application of SA (0.5 mM) minimizes the negative effects of salt stress with evidence of increasing the growth and productivity of tomato plants which were in parallel line with enhancing the photosynthetic pigments, soluble carbohydrate, protein content, total proline and phenol, electrolyte leakage percentage and leaf relative water content of plants [41]. Sequence the DNA of treated and non-treated plants. The sequence analysis revealed that the amplified genes were proline protein gene and amino-transfer as a (aat) gene. Moreover, the DNA nucleotide sequence obtained from the non treated tomato plants showed the same sequence, no mutant was observed. So, the plant stressed by SA induced the membrane defense genes which play an important role to resist the effect of salinity on the treated plants [41].
The application of SA via root drenching protected Lens esculentum against NaCl stress and increased photosynthetic rates under salt stress [42,43] Gemes et al. (2011) [44], suggested that, the cross-talk of signaling pathways induced by SA and high salinity may occur at the level of ROS and NO production. They discovered that SA-induced generation of H2 O2 and NO are considered to be useful links of cross-tolerance to numerous stressors. SA-stimulated pre-adaptation state was useful in the acclimation to subsequent salt stress in Solanum lycopersicum. At the whole plant level, SA-induced huge H2 O2 accumulation only at high concentrations (1–10 mM), that later ends up in the death of the plant.
Maize (Zea mays L.)
Maize has become a staple food in many parts of the world, with total production surpassing that of wheat or rice. Maize considered as a moderately salt-sensitive plant. The mitigating effects of SA to abiotic stresses were investigated through SA application by foliar spray of maize [21]. Many studies supported that SA induced increases in the resistance of maize to salinity and osmotic stress, respectively [45].
Tufail et al. (2013) [46], reported that SA treatment induce physiological and biochemical changes in two genotypes of maize (Sahiwal-2002 and EV-20) in the presence and absence of salt. Salicylic acid at 0, 0.25 and 0.50 mM along with 120 mM NaCl and Hogland’s nutrient solution were applied as rooting medium to 25 days old plants. Application of SA reduce Na+ but increased K+ and Ca2 + concentration, shoot biomass under salt stress. Exogenous application of various concentrations of SA upgrade photosynthetic rate, transpiration rate, stomatal conductance, sub-stomatal CO2 concentration, chlorophyll b contents and carotenoids in both genotypes of maize under salt stress regulated by mineral nutrients.
Zea mays treated with SA exhibited increased growth, decreased lipid peroxidation and membrane permeability, which were increased by salt stress [47]. Studies have been employed the effects of SA on some physiological and biochemical characteristics of maize seedlings under salt stress were derived [48-50].
Medicago sataiva
Torabian (2010) [51] reported that pre-treatment with SA induced adaptive responses in Medicago sativa plant under salinity stress and consequently, encouraged protective reactions in biotic membranes which improved the growth of seedlings. SA pre-treatment improved growth and resulted in higher resistance of plants to salinity, so it increased germination percentage, seed vigor index and growth parameters of the seedlings. Also, salinity intensified electrolyte leakage, whereas, SA diminished it and this decrease was stronger at SA concentration.
Palma et al. (2013) [52], reported that the SA alleviated the negative effect of salt stress in the shoot dry weight, root dry weight and photosynthetic yield of the Medicago sativa in symbiosis with Sinorhizobium meliloti. Pretreatment with SA prevented the negative effect of NaCl on nitrogenase activity and nodule dry weight of the plant. SA may act as protector against salinity by increasing the level of the antioxidant metabolism due to the higher activities that exhibited POX, SOD, APX and dihidroascorbate reductase with a low (0.1 mM and 0.5 mM) SA dose.
Sunflower (Helianthus annuus L.)
The foliar applied SA -induced increase the growth and photosynthetic rate of sunflower under salt stress condition, particularly at 200mg/L SA level [53]. Two varieties of sunflower seed were grown under greenhouse and different concentration (100, 200, 300 mg/L) of SA were applied as a foliar spray at vegetative state. SA treatment increased the growth rate (shoot dry weight) and antioxidant capacity of sunflower plant in salt stress conditions (SOD, CAT, POX), but this was mainly regulated by peroxidase activity [54].
Torreya grandis
The genus Torreya is an endangered and primitive member of the yew family (Taxaceae) and consists only of six species with a restricted worldwide distribution [55]. The pot experiment of Torreya sprayed with SA over the leaves three days before NaCl treatment and also continued during the NaCl application. SA treatment significantly reduces the decrease in plant shoot and root dry weight and relative water content, but increase the proline content, photosynthetic rate, carbon dioxide concentration, transpiration rate and stomatal conductance under salt stress conditions. SA efficiently ameliorated the negative effects of salt stress over the growth and photosynthesis of T. grandis by increasing the chlorophyll content and also enhancing the plants antioxidant enzyme mechanisms, thus alleviate the membrane oxidative damage from salinity [56].
Arabidopsis thaliana
Arabidopsis seeds were grown in hydroponic and soil cultures after three weeks old plants were supplemented with different concentration (10, 50, 100, 500μM) of SA and exposed to salt stress. Pretreatment of SA in both soil and hydroponic culture under salt stress condition increase the shoot development, relative water content and biomass of the plant. In 10, 50, 100μM SA treatment reduced the salt induced K+ efflux and H+ influx from the matured root zones and also enhanced the K+ retention in the cytosol. SA treatment increase the H+ -ATPase activity, lower the membrane depolarization and decreased the K+ leakage through depolarization activated GORK channels under salt stress. SA concentrations 10, 50, 100μM SA showed the more beneficial activity under salt stress conditions [57].
Lee et al. 2010 [58], found that high concentration of SA affects the seed germination in salt induced conditions. In contrast, lower level of SA concentration increased the seed germination rate, decrease H2 O2 level by reduce oxidative damage under salt stress condition. So, salt induced negative effects were significantly diminished by SA pretreatment of the plants.
Chickpea (Cicer arietinum L.)
Chickpea seeds were soaked in 1.5 and 3mM concentration of SA for 24 hours after that seed sown in plastic pots filled with soil and watered with 100mM NaCl and normal water. Normal water treated plants served as a control. Salt stress decrease the germination rate, chlorophyll content, dry weight of shoot and root and increased proline and MDA content of the plants. When combination of SA and NaCl increase the germination, chlorophylls, dry weight of shoot and root, decrease proline and MDA content [59]. Boukraa et al. 2013 [60], also found that SA treatment alleviating the negative results of chickpea under salt stress by increasing antioxidant enzymes.
White bean (Phaseulus vulgaris)
Bean is considered as a sensitive plant to salinity and is adversely affected by salt stress in terms of growth and yield. Hadi et al. 2014 [61], used three types of SA application methods (soil, foliar and priming) and four SA concentrations (0, 0.1, 0.5 and 1.0 mM) of salt stressed white bean. In his findings 0.1 mM soil applied SA was the most effective method on chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, proline, protein and soluble sugars of NaCl stressed white bean.
Strawberry (Fragaria ananassa)
Strawberry is considered as salinity sensitive species [62] and it has been shown to reduce leaf number, leaf area, shoot dry weight and number of crowns and low yield under salt stress [63]. Salt stress affected the growth, chlorophyll content and mineral uptake of strawberry plants. Strawberry plants treated with SA often had greater shoot fresh weight, shoot dry weight, root fresh weight and root dry weight as well as higher chlorophyll content under salt stress [64].
Barley (Hordeum volgaris)
Barley is an important crop cultivated in Saudi Arabia. One of the approaches to improve growth and productivity of crop plants under water deficit and soil salinity is the hormonal and fertilizer treatments which are capable to withstand unfavorable environmental conditions. To resist or avoid stress conditions, plants have evolved complex mechanisms to counter NaCl toxicity and low water potential in soil caused by salinity [65].
The role of SA in regulating the salt and water stress response of barley, and suggest that SA acts as a potential growth enhancer to improve plant growth, photosynthetic pigment content and reduce Na+ uptake. SA provoked reduction in oxidative stress in plants subjected to salt and water deficit stresses. The soluble carbohydrate plays an important role in the osmotic adjustment in stressed plants. SA can help reduce the adverse effects of salt and drought and may increase the barley growth, enhance antioxidant activity and K+ content in stressed plants and thus protecting membrane against oxidative stress. This protective effect led to a decrease in the ratio of Na+ /K+ in barley leaves, which is a critical determinant under salt and water deficit stresses. These results indicate that barley could be possibly cultivated in moderate saline and drought stressed soils due to its capacity for osmotic adjustment [66].
El Tayeb (2005) [67] found that SA application to barley induced a pre-adaptive response to salt stress, enhanced the synthesis of Chl a, Chl b and Car, and maintained membrane integrity, leading to improvement of plant growth. SA-pretreated plants exhibited less Ca2+ and more accumulation of K+ , and soluble sugars in roots under saline condition.
Mungbean (Vigna radiata L.)
In mungbean plants SA alleviates salt-induced decrease in photosynthesis and minimizes the leaf Na+ , Cl− , and H2 O2 content [68]. This was accompanied by increased N and S assimilation through inducing the activity of NR and ATPs.
Khan et al. 2010 [69], found that foliar spraying of SA (0.1, 0.5, 1.0mM) under 50mM NaCl stressed mungbean decreased Na+ , Cl– , H2 O2 , and thiobarbituric acid reactive substances (TBARS), and electrolyte leakage. SA treatment exhibit increased N, P, K, and Ca content, activity of antioxidant enzymes, glutathione content, photosynthesis, and yield under control and saline condition. Application of 0.5mM SA alleviate the negative effects of NaCl on decreased the content of leaf Na+ , Cl– , H2 O2 , and TBARS, and electrolyte leakage, and increased leaf N, P, K, and Ca content, and activity of antioxidant enzymes and glutathione. This treatment resulted in reduced negative effects of salt stress on growth, photosynthesis, and yield while 1.0mM SA proved inhibitory or there was no additional benefits.
Foliar spraying of SA (0.5mM) on mung been under salt stress condition induces glycinebetaine accumulation through increased methionine and suppresses ethylene formation under salt stress and enhances antioxidant system resulting in alleviation of adverse effects of salt stress on photosynthesis and growth. These effects of SA were substantiated by the findings that application of SA-analogue, 2, 6, dichloro-isonicotinic acid (INA) and ethylene biosynthesis inhibitor, amino ethoxyvinylglycine (AVG) resulted in similar effects on Methionine, glycinebetaine, ethylene production, photosynthesis and growth under salt stress [70].
CONCLUSION
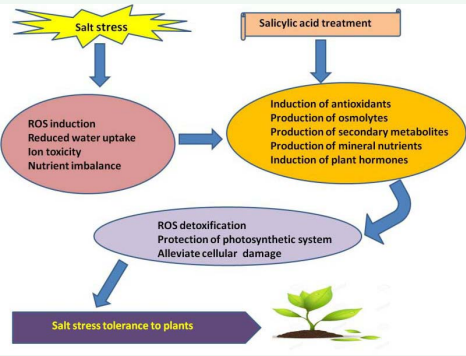
Hence, it may be resolved from the survey of literature cited above that salicylic acid plays diverse physiological role in plants and potentially alleviates the devastating effects generated by salt stress (Figure 1). Exogenous application of SA enhances the growth and productivity of plants under salt stress. The lower concentration of SA enhances the photosynthesis, growth and various physiological and biochemical characteristics of salt stressed plants. However, at high concentrations, SA itself may cause a high level of stress in plants. Application of SA protects and enhances the activities of antioxidant enzyme system and the enzymes of nitrate metabolism under stressful environments.
Figure 1 Mechanism of Salt stress tolerance induced by Salicylic acid in plants.
In future, the exogenous application of salicylic acid might act as a powerful tool in enhancing the growth, productivity and also protect the plants from abiotic stresses. The future application of this plant hormone holds great promise as a management tool for providing tolerance to our agricultural crops against the aforesaid constraints consequently aiding to accelerate potential crop yield in near future.
ACKNOWLEDGMENT
Authors are thankful to Secretary, Principal and Department of Biotechnology of Kongunadu Arts and Science College for providing facilities and encouragement to carry out this work.