The Bioelectric Activity of Trees: Very rapid signal transmission from leaf to root via xylem hydraulic pressure
- 1. Mori Laboratory of Plant Physiology, 443-5 Enden, Mori-machi, Shizuoka, Nagoya University, Japan
INTRODUCTION
There is an excellent guidebook for citizens who love trees, written by J. Edward Milner. The Tree Book: The indispensable guide to tree facts, crafts and lore” [1].
The second author received the book as a gift when organizing a workshop, “Plant Membrane Transport”, as a session of the International Botanical Congress (Yokohama, Japan 1993). This book covers the history of native trees of Britain and Ireland, managing, in relation to ecosystem, folklore and inspiration to the soul of artists and thinkers.
We value this book very much and only miss the lack of description on the physiology of trees as an individual and hope the present communication will be useful for the extension of the interest of people who love trees. (In Japan, there exists a social system of “Tree Doctor Society”, to which our first author belongs).
Tree root exhibits the pulse of xylem pressure, like our blood pulse, but very slowly (the period is diurnal).
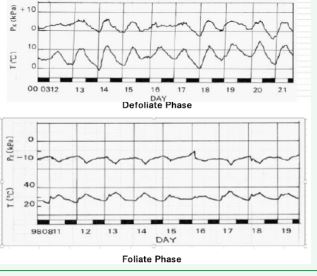
Figure 1 The diurnal change in xylem pressure (kaki tree root). Both defoliate and foliate season.
Figure 1 shows the periodic change in the xylem pressure of a kaki tree in our laboratory garden, in the defoliate and foliate season respectively [2].
These records are obtained with a sensor (specially designed injection needle) and a transducer which converts hydraulic pressure to the electric signal of mV order.
The xylem hydraulic pressure is positive in the defoliate phase and changes in the same phase with the change in atmospheric temperature. It is negative in the foliate phase and changes in the opposite direction as the temperature.
These results suggest; the water absorbed by the root is pushed up in the defoliate phase by the activity of the root and is passively pulled up in the foliate phase from the leaves by transpiration.
The bio-electric activity of root
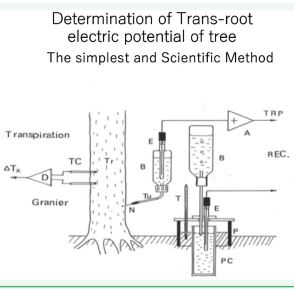
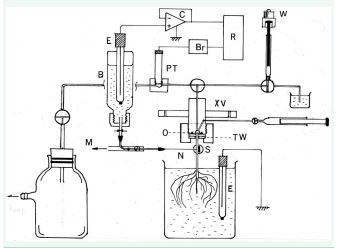
Has a tree root “bio-electric activity”, like our heart observed as an electro-cardiograph? Yes, we can obtain “electroradicograph” by means of a rather simple device illustrated in.
Figure 2 The apparatus to obtain the electro-radicograph and measure the transpiration rate of a tree. N: injection needle filled with 10 mM KCl aqueous solution. B: bottles ontaining the same solution. A: operational amplifier of high input impedance whose amplification factor is regulated as 1. PC: unglazed pottery E: Ag/AgCl unpolarizable electrodes (the same as the reference electrode of pH-meter). TC:Granier’s thermo-couples to measure ascending sap velocity.
Figure 2. Of course, the periodic change of the trans-root electric potential (TRP) thus observed is very slow as well as that of the hydraulic pressure, i.e. diurnal (Figure 3).
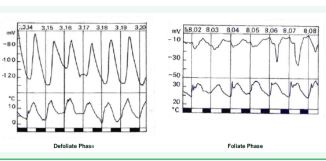
Figure 3 Diurnal rhythmic change in trans-root electric potential (TRP) in a kaki tree root. Both defoliate and foliate phase.
The amplitude of TRP change is rather larger in the defoliate phase when the water is pushed up by the root, while smaller in the foliate phase when it is drawn up by transpiration through leaves.
(The above diurnal change in TRP occurs as the periodic change in the resting potential, not as the action potential observed in the electro-cardiograph in an animal. It is the result of the antagonistic changes in two proton pumps activity sited in the root surface cell membrane and the xylem-side cell membrane; see the “appendix” for a more precise explanation.)
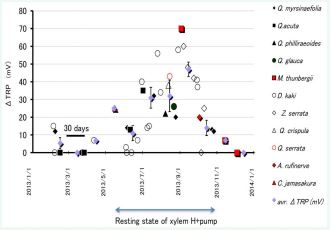
The following two determinative results were obtained through the long-term continuous observation of TRP over more than 15 years in a kaki tree in our garden.
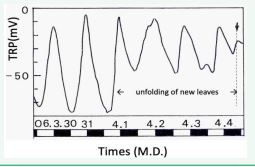
The first is the conspicuous increase of the TRP amplitude just before the opening of the leaf buds when they need water, but the transpiration activity is still poor and its very rapid decrease after the bud opening with the start of transpiration from the leaves (Figure 4), [3].
Figure 4 Conspicuous increase of TRP amplitude just before leaf unfolding and its rapid decrease after leaf opening.
The second discovery is illustrated in Figure 5.
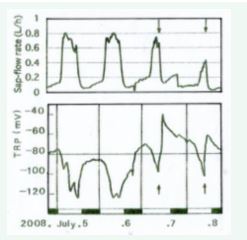
Figure 5 Effect on TRP of the inhibition of transpiration by the heavy rains.
When the transpiration is very active and the TRP shows a very negative level in summer, the thunder-storm came on two consecutive days (at July 7 and 8, 2008, in the afternoon,). The very rapid positive shifts of TRP levels took place almost at the same time with the fall in transpiration rate by the heavy rain.
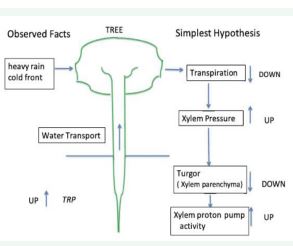
The latter observation suggested a very rapid signal transfer from leaves to root system. We established a hypothesis shown in Figure 6.
Figure 6 Our hypothesis - See text.
Inhibition of transpiration reduces the pulling up force for water. Then the xylem hydraulic pressure increases instantaneously and the turgor across the root xylem parenchyma membrane is transiently reduced.
Further progress of our investigation hereafter needed the adoption of our theory of “Electro-physiological Structure” of the plant axial organ which has been constructed in collaboration with Nagoya University for the term 1968~1978, called “Nagoya Model” shown in Figure 7.
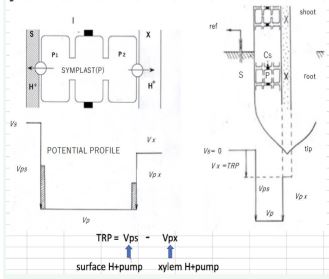
Figure 7 “Nagoya Model”: the theory of the electro-physiological structure of axial organ of vascular plants. S: the outide solution of a root, X: xylem apoplast, P1: the surface side H+pump, P2: the xylem side H+pump, I: Casparian strip: I, as the insulator). Electric potentials: Vs: root surface, Vx: xylem. Potential differences: Vps: between root surface and symplast, Vpx: between symplast and xylem apoplast. The relation with TRP is illustrated.
[4] From the following theoretical consideration by that model, the hydraulic signal transmitted through xylem vessels must activates the ”xylem proton pump”, bringing about the positive shift of the TRP.
Activation of xylem proton pump and the positive shift of TRP [4]
TRP has the following structure: it is the difference of the two electric membrane potentials, Vps (surface side) and Vpx (xylem side).
i.e. TRP= Vps – Vpx
Where Vps is the electric cell membrane potential, over half of which is generated by the activity of the surface side electrogenic proton pump Ps.
Vpx is the electric membrane potential, over half of which is generated by the activity of the xylem side electrogenic proton pump Px.
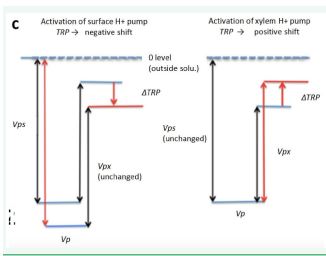
If the pump Ps alone is activated, the absolute value of Vps increases, then TRP must shift in a negative direction,
If the pump Px alone is activated, the absolute value of Vpx increases, then TRP shifts in a positive direction (Figure 8).
Figure 8 Schematical relationship between the TRP shift and the changes in Vps.
Experimental examination of the Hypothesis [5]
For the first step of the experimental proof of the hypothesis, we constructed an apparatus which enabled us to test if an artificially applied hydraulic xylem pressure really brought about the +shift of TRP (Figure 8-right).
Figure 9 The apparatus to examine if the artificially applied hydraulic xylem pressure really produce the +shift of TRP of tree root. E: Ag/AgCl unpolarizable electrodes, S: The seedling of trees. N: needle C: operational amplifier.
As shown in Figure 9, TRP is measured between two silver/ silver chloride electrodes. Excess xylem pressure is applied as shown by the mechanics in the figure and converted to voltage signal by a pressure-transducer and recorded.
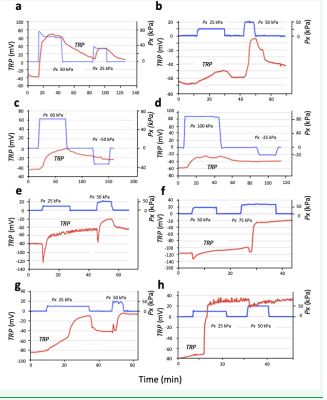
Figure 10 Eight examples of the tree root responses as the effect of the applied hydraulic xylem pressure. See text.
In Figure 10, eight examples of the TRP reaction to the applied xylem pressure (from 25KPa to 100 kPa) is shown by the blue line. a. An example of kaki tree. Reaction is repeatable. The other experiments, too, show that positive pressure always induces a positive shift of TRP c. and d. Negative pressure produces no reaction.
Figure 11 The season dependency of the effect of applied excess xylem pressure to the TRP of 11 species of tree seedlings. See text
Figure 11 illustrates the season dependency of the pressure induced positive TRP reaction in 5 evergreens and 6 deciduous tree seedlings. Big reaction is observed only during the hot season, when the transpiration is vigorous and the xylem H+pump Px is in the resting state.
There remained an argument from an expert of electrophysiology in Europe. “The positive shift of TRP could occur not only by the “activation” of Px, but also by the “inhibition” of Ps (see Figure 8-left).
Although it is difficult to imagine, the inhibition of Ps which is working to absorb water and nutrients from the outside while the acropetal part of the tree needs water, in reply to this question we determined Vps and Vpx simultaneously by means of intracellular micro-electrode technique.
Appendix
Understanding of further reports of the development of our study might require too much professional knowledge. Therefore we present further results as an Appendix.
Results of the experiments based on the intracellular micro-electrode technique [5]
The primary objective of this study was to clarify whether the positive shift in TRP triggered by a hydraulic signal is due to hyper-polarization of Vpx promoted by the activation of an electrogenic xylem proton pump Px, or to depolarization of Vps induced by the inhibition of the surface pump Ps, as has been previously questioned from a critical specialist.
For the experiment, the main root of burdock was used: having the same bioelectric property as tree roots. In this regard, it is necessary to consider the fact that the effect of applied xylem pressure on the TRP is entirely dependent upon the “season”. Hereafter, we report the results obtained from the application of excess xylem hydraulic pressure in different seasons and its effect on both membrane potentials Vps and Vpx. The patterns of the season-dependent response can be classified into the following three categories: Winter (November~March), Summer (July~September), and Intermediate type in between.
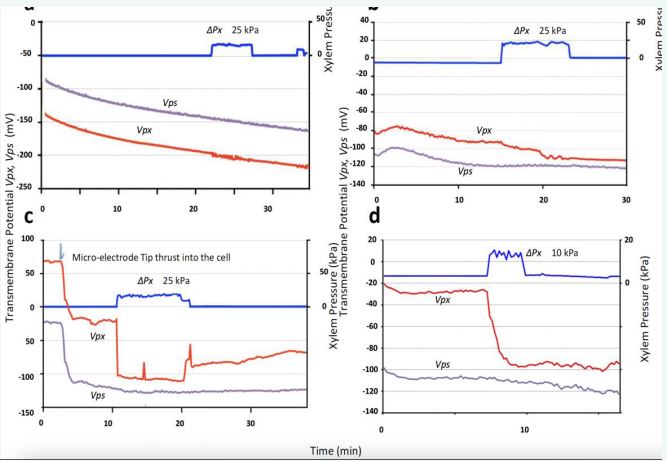
Figure 12- a shows the winter type behavior. Application of hydraulic pressure to xylem evoked almost no significant reaction in both potentials, whereas their steady levels are -150~-200 mV (very deep). During this period, both electrogenic pumps are in full active states, showing no more response to the hydraulic signal.
Figure 12 a: Winter type reaction. Both Ps and Px are in fully active state, and both Vps and Vpx show no more reaction to pressure signal. -b: Intermediate type reaction. Vps shows no reaction. Vpx showes a little hyper-polarization; Px begins to slow down a little because of active transpiration. -c: Summer type reaction: Vps shows no reaction, but Vpx fully hyperpolarized. Px in the full resting state is highly activated (25kPa).
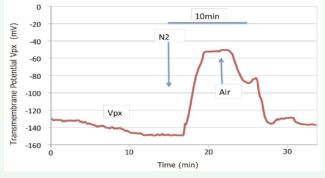
Figure 12-b presents an example of the intermediate type. Small hyper-polarization was observed in Vpx, whereas Vps showed almost no reaction. Figure 12-c and -d, show the two examples of the summer-type response. During this hot season, transpiration activity is particularly high and under such circumstances the Px appears to maintain in a resting state, with Vpx being notably shallow, almost at the diffusion potential level shown afterwards in Figure 12-b. In contrast, in response to the application of excess xylem pressure, full hyper-polarization reaction of Vpx took place (-75 mV and -80 mV, Figure 12-c and -d). In contrast, Vps showed virtually no response (12-c) or only a small hyper-polarization (12-d) induced by the pressure signal. The applied xylem hydraulic pressure was 25 kPa in Figure 12-c, the same as in the other experiments in this section, whereas a pressure of 10 kPa was applied in the example shown in Figure 12-d. The reason of this exception is the impression of the very rapid hyperpolarization process in the first case (-c). We applied less pressure to find the slower speed of the hyperpolarization (-d).
Figure 13 a: The hyper-polarization of Vpx in response to hydraulic signal is extinguished by anoxia, but reversibly. -b: The hyperpolarization effect of pressure signal is not utterly observed under anoxic condition.
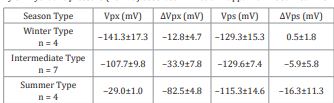
Such hyperpolarization of Vpx generated by hydraulic pressure is almost completely extinguished by anoxic condition, but reversibly (Figure 13-a). As illustrated in Figure 13-b, the respiration-dependent potentials across both cell membranes Vps and Vpx in this material occupy a very large part of the total membrane potential. In the figure, the respiration-dependent components of the Vps and Vpx is ca. 126 mV and 80 mV respectively. In contrast, the passive diffusion potentials are very small: ca. 32 mV and 38 mV. Under anoxic conditions, application of excess xylem pressure has almost no effect on either of the two membrane potentials (Figure 13-b). This fact indicates the hydraulic response of Vpx is a pump phenomenon but not a channel phenomenon. All the results are summarized in Table 1. In these 15 experiments, it is clearly shown the hydraulic signal did not depolarize Vps, rather brought about very slight hyper-polarization. Hyper-polarization of Vpx, in other words activation of the Px, always occurred by the hydraulic signal, except winter, prominently in the hot season when Px was in a resting state.
Table 1 Season dependency of the double electric resting potential Vpx, Vps and those of the hyper-polarization reaction (ΔV px, ΔVps) evoked by the change in xylem hydraulic pressure (25 kPa) observed in Arctium lappa main root. Mean ± SD
Evolution of the terrestrial vascular plants and still unknown mechanism of active water transport
All the vascular plants are said to evolute from Charophyta, whose membrane potential is kept unchanged against change in the environmental conditions such as temperature, light, so on, whose membrane H+pump always working in active state to absorb water and nutrients from the outside.
In the root of vascular plants, Vps, maintained by Ps, just corresponds to this Chara membrane potential. Vpx, maintained by Px, must have evolved with the occurrence of the vascular plants. The root xylem pump Px changes its activity responding to the change in the environmental conditions, through the hydraulic signal transmission from the leaves.
The remaining hot problems in this research field are the molecular mechanism of the active acropetal transport of water driven by Px as the energy converter and that of the hydraulic signal converter in the root that activates Px.
The velocity of the hydraulic signal in plants is very high [6], corresponding to the sonic speed in water: 1500m/sec, while the transmission velocity of action potential in vertebrate nerve threads is 1000m/sec.
Gigantic trees several multiples of ten meters in height, must be able to transmit hydraulic signal demanding water from its top leaves to their root system within a moment.
REFERENCES
- J.E Milner (1992) The Tree Book, A major 4 TV series, A Channel four Book ACACIA production Ltd, Collins & Brown.
- Masaki N, Okamoto H (2007) Correlation between the seasonal changes in electrogenic activity across root xylem/symplast interface, sap flow rate and xylem pressure in field trees (Diospyros Kaki). Trees 21:443-442
- Masaki N, Okamoto H (2009) Demonstration of the root surface electrogenic ion pump activity revealed from the seasonal inversion in the phase relation between electro-radicogram and the diurnal oscillation of air temperature in a field tree (Diospyros Kaki). Trees 23:473-478
- Okamoto H, Ichino K, Katou K (1978) Radial electrogenic activity in the stem of Vigna sesquipedalis: involvement of spatially separate pumps. Plant Cell Environ 1:279-284
- Okamoto H, Kitamura S, Masaki N, 2022. Activation of the root xylem proton pump by hydraulic signals from leaves under suppressed transpiration. J. Plant Res. 135:311-322
- Malone M (1993) Hydraulic signals. Trans Royal Soc B 341:33-39