Comprehensive Dental Rehabilitation for a Paediatric Patient with Succinic Semialdehyde Dehydrogenase Deficiency Requiring General Anaesthesia
- 1. Department of Dentistry, Shenoy Hospital, India
- 2. Paediatric Dentist, Clinical Director, Tooth Buddies Dental, India
- 3. HOD Anesthesia, Shenoy hospital, India
- 4. MD Anesthesia, Consultant Anesthetist, India
- 5. Senior Consultant ENT Surgeon, Sahyadri Hospital, India
Citation
Bindra S, Moghe G, Kumar R, Kumar A, Shaikh S (2025) Comprehensive Dental Rehabilitation for a Paediatric Patient with Succinic Semial dehyde Dehydrogenase Deficiency Requiring General Anaesthesia. Int J Rare Dis Orph Drugs 7(1): 1018
INTRODUCTION
A male child, who had completed his first decade of life, presented to the dental department with a one-week history of pain and difficulty in chewing. The patient was non-verbal and exhibited altered and hyperkinetic behavior, demonstrating challenges with comprehension and attention maintenance. Due to the patient’s uncooperative behavior, the dental examination was inconclusive, and X-ray procedures were hindered. Despite extensive counseling and minimal mouth opening, only a few decayed teeth were identified. Upon reviewing the medical history, the child was found to be a known case of Succinic Semialdehyde Dehydrogenase Deficiency, a rare autosomal recessive disorder that impairs the metabolism of gamma-aminobutyric acid, leading to the accumulation of succinic semialdehyde and gamma-hydroxy-butyric acid. Clinically, affected individuals exhibit psychomotor delays, hypotonia, seizures, and intellectual disability [I,2].
Considering the patient’s medical condition, uncooperative behavior, and the need for comprehensive dental rehabilitation, the decision was made to provide the necessary treatment under general anesthesia.
CASE PRESENTATION
The patient was the second child born to the family, with no known family history of the condition. The firstborn was a female. No abnormalities were detected during the antenatal and postnatal periods, and the mother did not have any gestational comorbidities. Both deliveries were normal vaginal [3].
In early development, the parents noted challenges with the child’s neck control and inability to roll over during the initial six months. At 8 months, the child underwent neurological examination, which revealed alert responsiveness, normal vision and hearing, and no seizure activity. However, the child exhibited an inability to hold the neck, hypotonia, hyperreflexia, head lag, and arching.
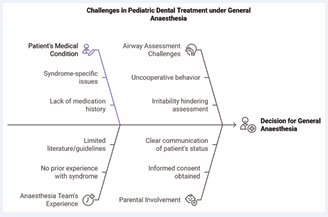
Newborn screening tests at 8 months, including Acylcarnitine, amino acid, and other profiles, were within normal limits. Brain MRI showed bilateral symmetrical hyperintensity in the globus pallidus, with small foci of restricted diffusion, and subtle T2 hyperintensity in the brainstem [4,5] (Figure 1).
Figure 1 Challenges in Pediatric Dental Treatment under General Anaesthesia.
The patient was prescribed 2.5ml of Carnitine syrup twice daily, along with methylcobalamin and vitamin D supplements. Physiotherapy was recommended, which revealed a developmental delay. The child underwent neck flexor activation exercises, side neck flexion exercises, weight-bearing on bilateral lower limbs, pull-to-sit training, and Swiss ball training. The patient started sitting at 15 months and walking at 24 months. At 2 years of age, further assessment by a medical geneticist revealed a head circumference of 49.5 cm, brachycephaly with subtle facial dysmorphism, mild limb dysmorphism, and normal genitalia. Physical examination also noted upper and lower limb brachydactyly, a broad-based gait, and foot aversion [6] (Table 1).
Table 1: Table explains the Patient diagnosis and the treatment given to them.
|
Aspect |
Details |
|
Patient Diagnosis |
Succinic Semialdehyde Dehydrogenase Deficiency |
|
Dental Treatment Approach |
Performed under General Anesthesia |
|
Reason for Anesthesia |
Patient's inability to cooperate and follow instructions |
|
Decision Making Process |
Collaboration between dental, medical, and anesthesia teams |
|
Challenges Faced |
Lack of established guidelines for managing syndromic patients under general anesthesia |
|
Strategies for Future Patients |
Individualized treatment plans, early intervention, and interdisciplinary care |
|
Genetic Finding |
Genetic analysis detected pathogenic mutations in the ALDH5A1 gene |
|
Potential Quality of Life Improvement |
Successful dental treatment can alleviate pain, improve chewing, and enhance quality of life |
|
Anesthesia Management |
Tailored drug selection and vigilant monitoring for optimal outcomes |
|
Outcome |
Successful dental treatment without complications, with potential for improved quality of life |
The patient presented with hypotonia, global developmental delay with motor delay surpassing mental delay, speech delay, behavioral concerns, inattention, inconsistent auditory-visual responses, social challenges, and features suggestive of Autism Spectrum Disorder. Investigations, including Thyroid-stimulating hormone, BERA, CPK test, calcium level, newborn screening test, Electroencephalogram, fundoscopy, Serum B12, and vitamin D3, were within normal ranges. The Auditory Brainstem Response test indicated normal bilateral hearing sensitivity, and the CPK test report was also normal [7-9].
In addition to the previously mentioned investigations, further diagnostic tests were performed, including Tandem Mass Spectrometry, amino acid analysis, and measurements of pyruvate, lactate, zinc, copper, and ceruloplasmin levels. The lactate level was elevated, indicating a markedly increased lactate-to-pyruvate ratio of 27.67. Gas Chromatography Mass Spectrometry analysis revealed the presence of 4-hydroxybutyric acid, a pathognomonic metabolite whose elevated excretion is characteristic of 4-hydroxybutyric aciduria, also known as Succinic Semialdehyde Dehydrogenase deficiency. Furthermore, next-generation sequencing through a targeted gene panel test identified a mutation in the ALDH5A1 gene, specifically involving exons 3 and 5. Integrating the patient’s medical history, clinical examination, blood investigations, MRI findings, and genetic analysis, a definitive diagnosis of SSADH deficiency was established [10,11].
TREATMENT PLAN
Given the child’s medical and psychological condition, as well as the impracticality of providing the necessary dental treatment under local anesthesia, the decision was made to proceed with the procedure under general anesthesia. Informed consent was obtained from the parents, and the anesthesiologist was involved in the decision-making process. This case posed a significant challenge for the anesthesia team, as they had not previously encountered any patient with this syndrome, and there was a lack of supporting literature or guidelines available for reference [12-15].
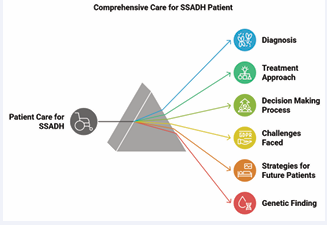
The patient, a moderately built child with a height of 137 cm and weight of 31 kg, presented with normal pre-operative vital signs. While the respiratory and cardiovascular parameters were within normal limits, the airway examination was hindered by the patient’s uncooperative and irritable behavior. The surgical profile results, including ECG and chest X-ray, were normal, suggesting no significant cardiac or respiratory issues. However, the patient’s behavior impeded a comprehensive airway assessment. The parents explicitly communicated that the patient had not been prescribed any anti-epileptic or other medications (Figure 2).
Figure 2 Comprehensive Care for SSADH Patient
Dental Procedure Done Under General Anaesthesia (G.A)
The patient underwent comprehensive dental rehabilitation under general anesthesia. Prior to induction, the following medications were administered intravenously: Glycopyrrolate 0.02mg/kg, midazolam 0.3mg/kg, ondansetron 0.15mg/kg, and levetiracetam 10mg/kg. Anesthesia was induced using intravenous propofol 10mg/kg and fentanyl 2μg/kg. Tracheal intubation via the nasal route was facilitated with a loading dose of cisatracurium 0.5mg/kg, followed by incremental doses of 0.1mg/kg. Maintenance included inspired sevoflurane 2%, with oxygen and nitrous oxide. Ventilation was conducted in a volume-controlled mode with tidal volumes of 6-8 mL/kg, a ventilatory frequency of 14 breaths per minute, and a positive end-expiratory pressure of 5 cm H2O. The patient was monitored throughout the surgery using a multiparameter monitor, including heart rate, blood pressure, oxygen saturation, end-tidal carbon dioxide, body temperature, and a bispectral index monitor to regulate the depth of anesthesia. Neuromuscular monitoring using train-of-four was utilized to assess recovery [16-18].
Oral examination revealed normal oral mucosa and gingiva. Decayed teeth included bilateral maxillary and mandibular deciduous first and second molars, as well as all four permanent first molars. Local anesthesia was infiltrated in the labial mucosa around the affected teeth, and pre-operative intraoral periapical radiographs were obtained for a comprehensive assessment of pulpal involvement.
Carious dentin was excavated, and stainless steel crowns were placed on the right maxillary permanent first molar and left mandibular permanent first molar. Glass ionomer cement restorations were performed for the maxillary left and mandibular right permanent first molars. Pulp therapy and a stainless steel crown were provided for tooth 85. Additionally, extractions were carried out for teeth maxillary right deciduous first molar, bilateral mandibular deciduous first molars and mandibular left second deciduous molar, as they were severely decayed and had poor prognosis.
Upon completion of the 2-hour dental procedure, sevoflurane inhalation was discontinued, and the patient was transitioned to 100% oxygen. Observing the patient’s response to the weaning of drugs and noting a neuromuscular monitor TOF reading >1.0, the reversal agent Sugammadex 5mg was administered intravenously, followed by extubation [17-20].
In the post-operative recovery phase, the patient was continuously monitored using a multi-parameter monitor to assess vital signs. To mitigate anxiety and irritability, the patient received intravenous midazolam 1mg. After 3 hours of uneventful observation in the recovery area, with an Aldrete score of 8/10, the patient was transferred to the room [21-27].
DISCUSSION
SSADH deficiency is a rare genetic autosomal recessive disorder that impacts the degradation of the major inhibitory neurotransmitter, gamma-aminobutyric acid. It has been documented in over 450 cases and is also known as aldehyde dehydrogenase 5a1, located on chromosome 6p22.3. Individuals with SSADH deficiency typically exhibit 4-hydroxybutyric aciduria in urine organic acid analysis. The clinical spectrum ranges from mild intellectual disability to severe developmental delay, hypotonia, ataxia, language disorders, and seizures. However, the literature on the dental management of these patients is limited.
The first case of SSADH was identified in 1981, and the deficiency of SSADH activity was demonstrated as the underlying cause two years later. The incidence of SSADH deficiency is unknown, but recent estimates suggest a prevalence of approximately 1:106. In our case, the presence of 4-hydroxybutyric acid was confirmed using gas chromatography and mass spectrometry
According to studies, only a few cases of SSADH deficiency have been reported from India, while a larger study revealed 182 patients from 40 countries. The prevalence and carrier frequency suggest a worldwide prevalence of around 1/460,000, with the highest carrier frequencies observed in East Asian and South Asian populations. There is no documented gender predilection for this disorder.
Our patient, born to non-consanguineous parents, presented with delayed head holding and was evaluated by a pediatric neurologist at 8 months of age, which aligns with the typical age range for the initial manifestation of symptoms in similar cases. However, the patient was diagnosed at the age of 2 years, which is not in accordance with the literature findings.
This metabolic disorder is characterized by cognitive impairment, language deficits, intellectual disability, hallucinations, autistic behaviors, and mild ataxia. Many affected individuals exhibit psychiatric symptoms like hyperactivity, aggression, inattention, self-injury, and sleep disturbances. Anxiety and obsessive-compulsive disorder can also occur. Our patient exhibited most of these features.
Epilepsy affects about half of those with this condition, but was not present in our case. However, an anti-epileptic drug was administered to prevent potential seizures during the procedure.
Imaging reveals characteristic changes in the globus pallidus region of the brain. Genetic testing identified mutations in the ALDH5A1 gene, which encodes the enzyme crucial for breaking down the neurotransmitter GABA. Disruption of this pathway leads to the accumulation of GABA and related molecules, contributing to the neurological manifestations.
In conclusion, this case highlights the importance of an interdisciplinary approach in managing the dental care of patients with SSADH deficiency.
Genetic analysis of our patient detected two heterozygous pathogenic variants in the ALDH5A1 gene, specifically in Exon 3 and Exon 5. These variants are predicted to cause a frameshift, leading to premature termination of the SSADH protein. The truncated protein, anticipated to be 164 amino acids instead of the normal 548, is likely to lack crucial functional regions, resulting in a loss of enzyme function. Additionally, the premature stop codon suggests the aberrant transcript may undergo nonsense-mediated mRNA decay. This novel variant has not been previously reported but is considered pathogenic due to its proximity to other known pathogenic variants.
Dental treatment under general anesthesia was necessary due to the child’s non-cooperation and inability to follow instructions. There were no established guidelines for managing syndromic patients undergoing general anesthesia, with only one case report involving a 23-year-old female with SSADH deficiency.
The anesthesia medications were carefully calculated based on the patient’s weight. Cisatracurium, a non depolarizing agent, was chosen for neuromuscular blockade due to limited literature on muscle relaxant use in this disorder. Cisatracurium undergoes Hofmann elimination, a temperature- and pH-dependent process that reduces the risk of accumulation in patients with liver or renal impairment.
The depth of anesthesia was monitored using the Bispectral Index, a validated measure of sedative and hypnotic drug effects. Intraoperatively, the patient’s BIS values were maintained in the appropriate range, indicating adequate anesthesia depth. Additionally, a peripheral nerve stimulator was used to assess neuromuscular transmission and ensure proper muscle relaxation.
The anesthesia reversal was uneventful, and to our knowledge, this is the first reported case of general anesthesia in a pediatric patient with Succinic Semialdehyde Dehydrogenase deficiency. The anesthesia management, including the use of antiepileptic drugs, followed standard protocols. The postoperative recovery was smooth.
REFERENCES
- Sergi C, Parayil Sankaran B. Succinic Semialdehyde Dehydrogenase Deficiency. 2023.
- Didiášová M, Banning A, Brennenstuhl H, Jung-Klawitter S, Cinquemani C, Tikkanen R, et al. Succinic Semialdehyde Dehydrogenase Deficiency. 2020; 9: 477.
- Pearl PL, Gibson KM, Quezado Z, Dustin I, Taylor J, Trzcinski S, et al. Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology. 2009; 73: 423-429.
- Pearl PL, Wiwattanadittakul N, Roullet JB, Margaret P, Jerry F, Ghayda M, et al. Succinic Semialdehyde Dehydrogenase Deficiency. 1993.
- Jakobs C, Bojasch M, Monch E, Rating D, Siemes H, Hanefeld F. Urinary excretion of gamma-hydroxybutyric acid in a patient with neurological abnormalities. The probability of a new inborn error of metabolism. Clin Chim Acta. 1981; 111: 169-178.
- Gibson KM, Sweetman L, Nyhan WL, Jakobs C, Rating D, Siemes H, et al. Succinic semialdehyde dehydrogenase deficiency: An inborn error of gamma-aminobutyric acid metabolism. Clin Chim Acta.1983; 133: 33-42.
- Malaspina P, Roullet JB, Pearl PL, Ainslie GR, Vogel KR, Gibson KM. Succinic semialdehyde dehydrogenase deficiency (SSADHD): Pathophysiological complexity and multifactorial trait associations in a rare monogenic disorder of GABA metabolism. Neurochem Int. 2016; 99: 72-84.
- Attri SV, Singhi P, Wiwattanadittakul N, Goswami JN, Sankhyan N, Salomons GS, et al. Incidence and Geographic Distribution of Succinic Semialdehyde Dehydrogenase (SSADH) Deficiency. JIMD Rep. 2017; 34: 111-115.
- Martin K, McConnell A, Elsea SH. Assessing Prevalence and Carrier Frequency of Succinic Semialdehyde Dehydrogenase Deficiency. J Child Neurol. 2021; 36: 1218-1222.
- Tay CG, Ariffin H, Yap S, Rahmat K, Sthaneshwar P, Ong LC. Succinic Semialdehyde Dehydrogenase Deficiency in a Chinese Boy: A Novel ALDH5A1 Mutation with Severe Phenotype. J Child Neurol. 2015; 30: 927-931.
- Gibson KM, Christensen E, Jakobs C, Fowler B, Clarke MA, Hammersen G, et al. Theclinical phenotype of succinic semialdehyde dehydrogenase deficiency (4-hydroxybutyric aciduria): case reports of 23 new patients. Pediatrics. 1997; 99: 567-574.
- Gibson KM, Doskey AE, Rabier D, Jakobs C, Morlat C. Differing clinical presentation of succinic semialdehyde dehydrogenase deficiency in adolescent siblings from Lifu Island, New Caledonia. J Inherit Metab Dis. 1997; 20: 370-374.
- Al-Essa MA, Bakheet SM, Patay ZJ, Powe JE, Ozand PT. Clinical, fluorine- 18labeled 2-fluoro-2-deoxyglucosepositron emission tomography (FDG PET), MRI of the brain and biochemical observations in a patient with 4-hydroxybutyric aciduria; a progressive neurometabolic disease. Brain Dev. 2000; 22: 127-131.
- Yalçinkaya C, Gibson KM, Gündüz E, Koçer N, Fiçicio?lu C, KüçükercanI. MRI findingsin succinic semialdehyde dehydrogenase deficiency. Neuropediatrics. 2000; 31: 45-46.
- Pearl PL, Shukla L, Theodore WH, Jakobs C, Michael Gibson K. Epilepsy in succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism. Brain Dev. 2011; 33: 796-805.
- Pearl PL, Gibson KM, Acosta MT, Vezina LG, Theodore WH, Rogawski MA, et al. Clinical spectrum of succinic semialdehyde dehydrogenase deficiency. Neurology. 2003; 13: 1413-1417.
- Pearl PL, Capp PK, Novotny EJ, Gibson KM. Inherited disorders of neurotransmitters in children and adults. Clin Biochem. 2005; 38: 1051-1058.
- Kim KJ, Pearl PL, Jensen K, Snead OC, Malaspina P, Jakobs C, et al. Succinic semialdehyde dehydrogenase: biochemical-molecular- clinical disease mechanisms, redox regulation, and functional significance. Antioxid Redox Signal. 2011;15: 691-718.
- Pearl PL, Gibson KM, Cortez MA, Wu Y, Knerr I, Forester K, et al. Succinic semialdehyde dehydrogenase deficiency: lessons from mice and men. J Inherit Metab Dis. 2009; 32: 343-352.
- Knerr I, Gibson KM, Jakobs C, Pearl PL. Neuropsychiatric morbidity in adolescent and adult succinic semialdehyde dehydrogenase deficiency patients. CNS Spectr. 2008; 13: 598-605.
- Gibson KM, Gupta M, Pearl PL, Tuchman M, Vezina LG, Snead OC 3rd, et al. Significant behavioral disturbances in succinic semialdehyde dehydrogenase (SSADH) deficiency (gamma-hydroxybutyric aciduria). Biol Psychiatry. 2003; 54: 763-768.
- Pearl PL, Gibson KM. Clinical aspects of the disorders of GABA metabolism in children. Curr Opin Neurol. 2004; 17: 107-113.
- Pearl PL, Gibson KM, Quezado Z, Dustin I, Taylor J, Trzcinski S, et al. Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology. 2009; 73: 423-429.
- Pearl PL, Gibson KM. Succinic Semialdehyde Dehydrogenase Deficiency. In: NORD Guide to Rare Disorders. Lippincott Williams & Wilkins. Philadelphia, PA. 2003; 499.
- Gibson KM, Hoffman GF, Hodson AK. 4-Hydroxybutyric acid and the clinical phenotype of succinic semialdehyde dehydrogenase deficiency, an inborn error of GABA metabolism. Neuropediatrics. 1998; 29: 14-22.
- Vogel KR, Ainslie GR, Walters DC, McConnell A, Dhamne SC, Rotenberg A, et al. Succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism: an update on pharmacological and enzyme- replacement therapeutic strategies. J Inherit Metab Dis. 2018; 41: 699-708.
- Flowers J, Veneziano G, Cambier G, Tobias JD. Anesthetic care of a patient with succinic semi-aldehyde dehydrogenase deficiency. Pediatric Anesthesia and Critical Care J. 2024; 12: 1-5