Pulmonary Vascular Tangle: Unusual Cause of Hemoptysis
- 1. Department of Medicine, SUNY Upstate Medical University, Syracuse, New York, USA
About the Corresponding Author
Dr. Sravanthi Nandavaram
Summary of background:
Finished internal medicine residency, and currently pursuing Pulmonary Critical Care Fellowship.
Current research focus:
COPD
Permanent e-mail address: drsnandavaram@gmail.com
ABSTRACT
Pulmonary vascular malformation is an unusual cause of hemoptysis. These abnormal vascular communications between pulmonary circulations or between pulmonary and systemic circulations can result in significant dyspnea, hemoptysis and hypoxemia. Pulmonary vascular malformations can take various forms. Here we present a case of an abnormal collection of thrombosed arteries and veins, without the classic features of pulmonary arterio-venous malformation and presented as a focal opacity on imaging, that can be easily confused as air space process or lung consolidation. Identification and treatment of these abnormal vascular communications is vital as they might result in life threatening hemoptysis.
KEYWORDS
• Hemoptysis
• Dyspnea
• Consolidation
• Vascular Anomaly
CITATION
Nandavaram S, Alam B, Amzuta I (2017) Pulmonary Vascular Tangle: Unusual Cause of Hemoptysis. Int J Rare Dis Orphan Drugs 2(1): 1003
INTRODUCTION
Pulmonary vascular malformation is an unusual cause of hemoptysis. Pulmonary vascular malformations are abnormal vascular communications between pulmonary circulations or between pulmonary and systemic circulations and can result in significant dyspnea, hemoptysis and hypoxemia [1-4]. Here we present an unusual pulmonary vascular anomaly with abnormal collection of arteries and veins, however, lacking the classic features of pulmonary arterio- venous malformation, presenting with chronic hemoptysis and as a focal opacity on radiographic imaging.
CASE PRESENTATION
A 47-year-old female with medical history significant for hypertension, type 2 diabetes mellitus presented to our emergency room with complaints of chronic intermittent hemoptysis for the last 23 years. She moved from Somalia to United States 1 month prior to the presentation. She did not report any fever, chills, and shortness of breath or chest pain or epistaxis. She has been a lifelong non-smoker. At the time of presentation, she was afebrile, blood pressure was 115/54, and pulse was 88, respiratory rate was 20, oxygen saturation 96% on room air. Physical exam was benign without any evidence of any muco-cutaneous lesions or telangiectasias.
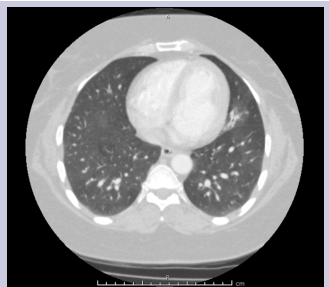
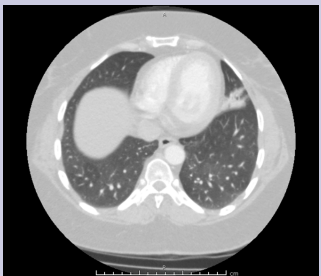
Diagnostic work up showed white blood cell count 7.3, hemoglobin 11.8, platelets 210, and erythrocyte sedimentation rate 30. AFB sputum was negative; legionella urine antigen was negative, blood cultures were negative, INR was 0.9. Non-contrast transthoracic echocardiogram was normal. There was no evidence of shunt on the two-dimensional transthoracic echocardiogram bubble study. Computed tomography of the chest revealed focal opacity in the inferior segment of the lingula [Figure 1 & 2].
Figure 1: CT Thorax showing a focal opacity involving the left lingula
Figure 2: CT Thorax showing a focal opacity involving the left lingula
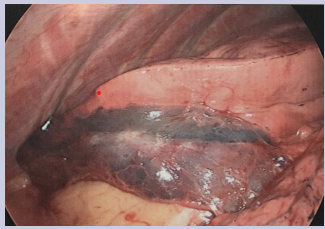
Fiberoptic bronchoscopy revealed streaks of blood in the left main bronchus. Fresh blood and blood clot was found in the inferior lingular segment of the left upper lobe. Broncho-alveolar lavage was performed in the left upper lobe inferior lingular segment and the return was bloody. Broncho-alveolar lavage specimens were sent for microbiology and cytology and were negative for infection and malignancy. Given the findings of active persistent bleed, 3-D and MIP reconstructions were done from the prior CT Chest which did not show any bronchial artery branches or parasitized intercostal artery branches supplying the lingular consolidation. There were only normal sized pulmonary arteries and veins supply the inferior segmental lingular consolidation. It was deemed by Interventional Radiology that the vessels are not amenable for embolization, given the lack of classic features of pulmonary arterio-venous malformation. Thoracic Surgery was consulted for resection of the lingula. Patient underwent video assisted thoracoscopic surgery and was found to have marked discoloration of the lingula [Figure 3] and wedge resection of the lingula was performed.
Figure 3: Gross specimen of the lingula
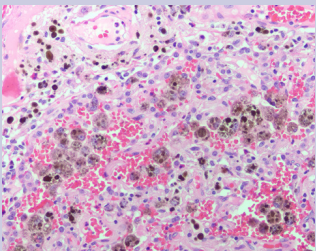
Pathology sections from the left lingula resection revealed prominent areas of hemosiderin deposition both within alveolar macrophages and within the interstitium [Figure 5], especially hemorrhage with no evidence of vasculitis or capillaritis.
Figure 5: Significant hemosiderin deposition within alveolar macrophages and the interstitium (200x). Hemosiderin deposition would not be expected if these collections of blood were an artifact of the surgical resection. There is no evidence of vasculitis or capillaritis
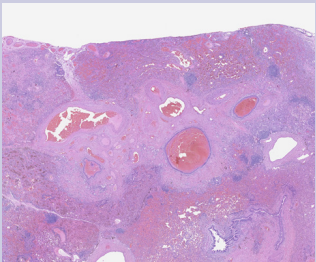
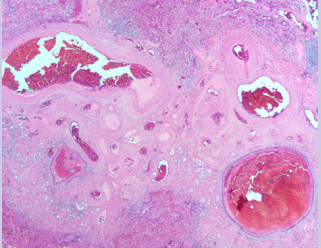
The most prominent hemosiderin deposition was seen in a portion of lung that contained an abnormal collection of small arteries and veins with associated fibrosis that forms a vascular tangle. This vascular tangle was located at the periphery of the lung near the pleural surface and surrounds a broncho-vascular structure that displays traction bronchiectasis [Figure 4 & 6].
Figure 4: An abnormal collection of blood vessels with various sizes in the subpleural lung parenchyma (20x). Prominent collections of hemorrhage are seen in surrounding alveolar airspaces and distended bronchioles.
Figure 6: This abnormal vascular tangle contained mostly small veins and venules with a few small arteries and arterioles (20x). The lack of associated airways with some of the small arteries and arterioles also suggested an abnormal vascular malformation. Classic features of arteriovenous malformation were not identified
Some of the vessels showed evidence of prior thrombosis with multiple recanalized lumens. A few of the vessels had changes, that represent several episodes of recurrent thrombosis and recanalization [Figure 4 & 6]. Some of these vessels showed abnormal aneurysmal dilatation. These findings were most suggestive of a vascular malformation and not normal lung as they lack the associated airways. Focally, the pleural surface adjacent to this malformation showed an increased numbers of variably sized arteries and veins, which may be part of the same process [ Figure 4 & 6].
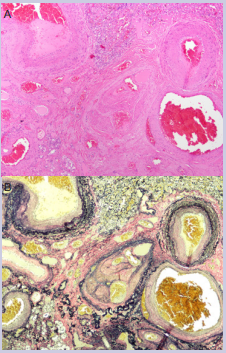
EVG elastic stains in three blocks that contained the vascular malformation confirmed the presence of mostly veins with a few arteries in the malformation [Figure 7].
Figure 7: A) Higher power view of the abnormal vascular malformation containing various blood vessels (40x). Rare small veins (center) contained multiple vascular lumens and other features suggestive of recurrent thrombosis and recanalization. B) Elastic Van Gieson (EVG) stain (40x).
Classic features of an arterio-venous malformation, a capillary free communication between the pulmonary arterial and pulmonary venous circulations, were absent.
DISCUSSION
Pulmonary vascular anomalies are abnormal vascular communications between pulmonary circulations or between pulmonary and systemic circulation. They are classified as pulmonary arterio-venous malformations, systemic to pulmonary vascular communications, pulmonary sequestration, pulmonary varices, and pulmonary artery aneurysms.
Pulmonary arterio-venous malformations are direct capillary free communications between pulmonary arterial and venous systems creating right to left shunts, thereby resulting in hypoxemia and paradoxical embolism. They can be hereditary or acquired [1,2,5]. On histopathology, simple arterio-venous malformations’s have an aneurysmal venous sac supplied by a single artery and drained by a single vein. Complex pulmonary arterio-venous malformations consist of a group of venous sacs supplied by multiple vessels arising from adjacent segmental or sub segmental pulmonary artery branches and drain into multiple veins [1,5,6]. Walls of venous sacs are of varying degrees of thickness, with disorganized adventitia, and areas of focal thickening with abundant elastin tissue with a variable contribution of smooth muscle cells being prominent. They may show up as a circumscribed, round, soft tissue nodule of any size associated with enlarged feeding and draining vessels on chest radiographs, Chest CT or catheter pulmonary angiography. [1,5,6]. Percutaneous transcatheter embolization is the preferred treatment modality [7,8] especially if vessels are greater than 2 to 3 mm in diameter [1,6,9,10]. Small pulmonary arterio-venous malformations with feeding artery less than 2 mm are usually not treated, unless there is a concern for neurologic complications and if not amenable for embolization, thoracoscopic resection is an option.
Thoracoscopic resection can also be considered when there are ongoing ischemic strokes or transient ischemic attacks following maximal embolization. Lobectomy or pneumonectomy is considered in cases of massive hemoptysis [11,12,13].
Our case is unique in that it has features of vascular anomaly with abnormal collection of arteries and veins along with abnormal aneurysmal dilatation, however lacks the classic features of direct capillary free communication between the pulmonary arterial and pulmonary venous circulations of pulmonary arterio-venous malformation.
CONCLUSION
Pulmonary vascular anomaly is an unusual cause of hemoptysis. There should be high index of suspicion for these kinds of lesions especially in cases of chronic persistent hemoptysis and appropriate diagnostic work up and treatment should be pursued.
DISCLOSURE
The authors, Sravanthi Nandavaram, Bisma Alam and Ioana Amzuta, certify that we have no affiliations with or involvement in any organization or entity with any financial interest in subject matter discussed in this manuscript.
No commercial support or funding was received for this manuscript.