Observational Study by Accelerated Schedules of Cluster Allergen Immunotherapy with House Dust Mites in Patients with Allergic Rhinitis & Bronchial Asthma
- 1. Department of Pulmonology, National Allergy Centre, India
Citation
Kathuria PC, Rai M (2021) Observational Study by Accelerated Schedules of Cluster Allergen Immunotherapy with House Dust Mites in Pa tients with Allergic Rhinitis & Bronchial Asthma. JSM Allergy Asthma 5(1): 1028.
Abstract
Background: Unlike conventional immunotherapy, Cluster immunotherapy achieves maintenance dose in weeks along with omalizumab. However, safety of this method needs to be confirmed before wide spread application. Methods: We have designed open label observational study, to achieve maximum tolerance dose (MTD) in duration of six weeks with cluster Immunotherapy in immunologically significant sensitive patients to house dust mites in perennial rhinitis & Bronchial asthma. Results: With Combined omalizumab (anti-IgE) and cluster Immunotherapy, maintenance maximum tolerance dose (MTD) of 1000/ml achieved in 3 visits in 36 days (> 1month) in 9 patients without an IgE mediated adverse systemic reaction. Cluster Immunotherapy in 40 patients is efficacious, well tolerated than conventional immunotherapy of 4 months of single Allergen Injection as maintenance maximum tolerance dose (MTD) of 1000 - AU achieved in more than 75% in 4 visits of 46 days duration but 20% of patients in cluster Immunotherapy develop grade II/III adverse systemic reaction. Conclusion: Combined omalizumab (Anti - IgE) and cluster Immunotherapy is safe, effective and gives more rapid relief of symptoms.
Keywords
• Cluster immunotherapy
• Immunotherapy
• Omalizumab
ABBREVIATIONS
MTD: Maximum Tolerance Dose; SCIT: Subcutaneous Immunotherapy; D. farinae: Dermatophagoides farina; D. pteronyssinus: Dermatophagoides pteronyssinus; Anti-IgE: Anti Immunoglobulin E
INTRODUCTION
The burden of allergic disease is increasing day by day. Approximately 20-30 % people of India are affected by one or other allergic disease. In Indian population prevalence of allergic rhinitis is around 3.5% [1].
Immunotherapy targeted against specific allergen is the proven disease modifying treatment strategy used to treat condition like allergic rhinitis, allergic asthma etc. In immunotherapy, allergen extracts are given in increasing doses subcutaneously at weekly interval until the Maximum Tolerance Dose (MDT) is achieved. This process helps in desensitizing the allergic individual and increase tolerance against specific allergen. Cluster immunotherapy is another form of immunotherapy in which injection of allergen extract is injected at shorter interval as compared to conventional therapy. The conventional Subcutaneous Immunotherapy (SCIT) is a slow treatment that often leads to poor compliance or discontinuation of treatment. Accelerated Immunotherapy build up schedules may provide a safe alternative to conventional build up schedules to achieve Immuno-tolerance without a significant increase in risks [2]. However, safety of cluster immunotherapy needed to be confirmed before widespread utilization of this method. Therefore, we have designed an observational single center study to find out the safety of cluster immunotherapy in patient with allergic rhinitis and asthma.
MATERIAL AND METHODS
We designed open label observational study, to achieve Maximum Tolerance Dose (MTD) in duration of six weeks with cluster Immunotherapy in immunologically significant sensitive patients to house dust mites in perennial rhinitis & Bronchial asthma. Following were the inclusion criterias. Typical history of perennial rhinitis or Mild to Moderate Bronchial asthma for > 5 yrs; Positive S.P.T > 5-7mm with 10,000 AU of standardized House Dust Mites (D. farinae & D pternoyssinus); Positive level of serum specific IgE to D farinae & D pternoyssinus > 3.5 KU ml, CAP system, Pharmacia and Total IgE > 300 to 700 iu/ml; FEV1/ FVC > 70% & PEFM < 10% Variability with Regular medication (LABA + ICS, ALRI & Ketotifen); Other Allergens (Pollens, fungi etc) positive but not Immunologically significant (HEP). Patients were divided in to three groups as following Group (A) combined Omalizumab (Anti-IgE) + Cluster Immunotherapy Group (B) Cluster Immunotherapy Group (C) Conventional Immunotherapy.
Safety assessment
Patients are observed after injection of allergen. Adverse events were graded according to the European Society of Allergy and Clinical Immunology recommended systemic reactions grading system as following: Grade 0 asymptomatic or nonspecific symptoms? Grade I mild? Grade II moderate? Grade III severe? Grade IV anaphylactic shock.
RESULTS AND DISCUSSION
Immunotherapy is newer avenue in the management of allergic condition and now it’s increasing in the developing countries like India. Conventional immunotherapy usually taken longer time to reach to maintenance maximum tolerance dose (MTD) and this leads to treatment interruptions and poor patient compliance. Cluster immunotherapy is a newer means to achieve the maximum tolerance dose in shorter period of time as compared to conventional immunotherapy. With cluster regimen there is saving of the cumulative dose used and also treatment time reduces which helps in improving the adherence to the treatment.
This observation study shoes that both conventional and cluster immunotherapy leads to similar improvement in symptoms scoring. Few patients received omalizumab showed greater improvement in symptoms scoring.
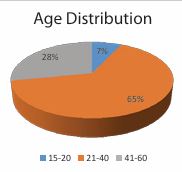
Figure 1 Age Distribution. 65% of the patients were between the 21-40 years of age, followed by 28% belonging to 41-60 years of age and 7% belonging to 15-20 years of age.
In this study with Cluster immunotherapy MTD was reached at 46 days far earlier than conventional therapy which took 140 day to reach MTD. When omalizumab prescribed along with cluster immunotherapy, it further reduces the time to reach MTD, reaching MTD at 36 days. Study by Tabart et al showed that the cluster schedule reduced the time to maintenance dose by 46%. Cluster immunotherapy led to decreases in asthma and rhinitis symptoms, reduced the cutaneous reactivity, and produced the increase in specific IgE and IgG 4 levels on reaching the maintenance dose in the sixth week, 6 weeks earlier than with the conventional schedule [3]. 20 percent (8/40) of the patients receiving cluster immunotherapy developed grade ii (3) or iii (5) systemic reaction while 18.7% patients developed grade ii (2) or iii (1) systemic reaction who received conventional immunotherapy.
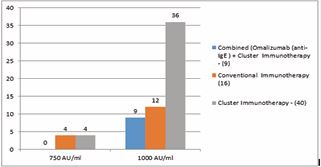
Figure 2 MTD (Maximum Tolerance Doses)
ALLERGEN EXTRACTS (1000 AU/ml) (Standardized HD Mites - 50% of D.farinae, & D pternoyssinus)
Source - Greer Allergy Immunotherapy lenoir USA.
In a similar retrospective study by Nieto García A et al., titled “Safety of cluster specific immunotherapy with a modified high dose house dust mite extract.” Show that Cluster schedule with high dose hypoallergenic mite-SCIT was safe and well-tolerated in routine clinical practice. Therefore, its use could reduce the costs and time needed to achieve the desired maintenance dose and increase compliance [4].
|
Table 1: Gender Distribution. |
|||
|
|
Combined Omalizumab + Cluster Immunotherapy (9) |
Cluster immunotherapy (40) |
Conventional Immunotherapy (16) |
|
Female |
6 |
30 |
12 |
|
Male |
3 |
10 |
4 |
|
Total 48 females and 17 males included in the study. Male female ration was 1:3 in both cluster and conventional immunotherapy groups. Male female ration was 1:2 in group where patients received omalizumab in addition to cluster immunotherapy. |
|||
|
Table 2: Group wise study visits and dose administration. |
||||||||||||||
|
Omalizumab + Cluster Immunotherapy (9) |
Cluster Immunotherapy (40) |
Conventional Immunotherapy (16) |
||||||||||||
|
Visit |
Day |
Concs |
Doses |
Volumes |
Visit |
Day |
Concs |
Doses |
Volumes |
Visit |
Day |
Concs |
Doses |
Volumes |
|
1 |
0 |
50% |
500 AU |
0.1 0.2 0.2 |
1 |
0 |
30% |
300 AU |
0.5 0.10 0.15 |
1 |
0 |
5% |
50 AU |
0.05 |
|
2 |
3 |
10% |
100 AU |
0.10 |
||||||||||
|
3 |
6 |
20% |
200 AU |
0.20 |
||||||||||
|
2 |
15 |
75% |
750 AU |
0.2 0.2 0.35 |
2 |
10 |
50% |
500 AU |
0.15 0.15 0.20 |
4 |
12 |
30% |
300 AU |
0.30 |
|
5 |
22 |
40% |
400 AU |
0.40 |
||||||||||
|
6 |
35 |
50% |
500 AU |
0.50 |
||||||||||
|
3 |
36 |
100% |
1000 AU |
0.30 0.35 0.35 |
3 |
25 |
75% |
750 AU |
0.20 0.25 0.30 |
7 |
50 |
60% |
600 AU |
0.60 |
|
8 |
68 |
70% |
700 AU |
0.70 |
||||||||||
|
8 |
89 |
80% |
800 AU |
0.80 |
||||||||||
|
4 |
46 |
100% |
1000 AU |
0.30 0.35 0.35 |
10 |
113 |
90% |
900 AU |
0.90 |
|||||
|
11 |
140 |
100% |
1000 AU |
1CC |
||||||||||
|
Cluster immunotherapy was started with the dose of 300AU of Allergen extracts (Standardized HD Mites- 50% of D. farinae, & D pternoyssinus (1000 AU/ml)) on first visit. Dose is Total volume injected was 0.30 ml in divided doses at three different sites. On the subsequent visit dose is increased to 500, 750 and 100 on Visit 2, 3 and 4 respectively. MTD was achieved after 4 visits that is day 46. MTD of 1000 AU with [Allergen extracts (1000 AU/ml) (Standardized HD Mites - 50% of D. farinae, & D pternoyssinus)] achieved with in three visits (36 days) when cluster immunotherapy prescribed along with omalizumab. In this group of patients starting dose of 500 AU (50% concentration) was used at three different injection sites. While with standard immunotherapy MTD of 1000 was reached after 140 days and took 11 visits of patient. |
||||||||||||||
|
Table 3: Summary of study results. |
|||
|
|
Omalizumab + Cluster Immunotherapy (9) |
Cluster Immunotherapy (40) |
Conventional Immunotherapy (16) |
|
Total Visits |
3 |
4 |
11 |
|
Duration |
36 Days (1month) |
46 Days (1½month) |
140 Days (>4½month) |
|
↓ Repeat Skin Prick Tests (7 mm) after maintenance dose |
3mm / 7mm |
3mm / 7mm |
4mm / 7mm |
|
↓ Symptoms Scoring (VAS) |
>70% |
>50% |
>50% |
|
Systemic Reactions |
Non - specific Reaction |
(20%) 8/40 (IgE specific Reaction) |
(18.7%) 3/16 (IgE specific Reaction) |
|
Total 11 visits [140 Days (>4½month)] are taken by conventional immunotherapy as compared to 3 [36 Days (1month)] with cluster immunotherapy plus omalizumab and 4 [46 Days (1½month)] with cluster immunotherapy. Overall symptomatic relief was maximum, with more than 70% reduction in VAS symptoms scoring in patients receiving cluster immunotherapy along with omalizumab. Both conventional and cluster immunotherapy responded similarly with more 50 percent reduction in VAS symptoms scoring. |
|||
|
Table 4: Subcutaneous House Dust Mites Immunotherapy Systemic reaction Grading System. |
||
|
Omalizumab + Cluster Immunotherapy (9) |
Cluster Immunotherapy (40) |
Conventional Immunotherapy (16) |
|
3/9 (33.3%) (Headache, Pharyngitis acute appendicitis Non - specific Reaction |
IgE mediated reaction 8/40 (20%) grade II, (3 ) grade III, (5) |
IgE mediated reaction 3/16 (18.7%) grade II, (2) grade III, (1) |
|
(Cough, sneezing, Running nose, wheezing urticaria, Anaphylaxis, abdominal cramps, vomiting or diarrhea & less than 40% PEF or FEV1 drop) 20 percent (8/40) of the patients receiving cluster immunothrepay developed grade ii (3) or iii (5) systemic reaction while 18.7% patients developed grade ii (2) or iii (1) systemic reaction who received conventional immunotherapy. Out of nine patients who were treated with both cluster immnotherpy and omalizumab three patients (33.3%) developed non-specific reaction such as headache, pharyngitits, acute appendicitits. |
||
Local side effects like edema, pain at the site of injection and pruritis are comparable in the all the three groups. In another study published in 2015 showed that the incidence of local and systemic adverse reactions during the incremental-dose phase and maintenance-dose phase compared with conventional immunotherapy were not significantly different (P > 0.05) [5].
In summary, cluster immunotherapy achieves faster MTD and not significantly different form conventional immunotherapy with respect to safety. When omalizumab is used along with cluster immunotherapy, leads to faster clinical onset. However, the sample size of the study was very less and patient receiving omalizumab along with cluster immunotherapy were very less. Further evaluation is required with regards to omalizumab and cluster immunotherapy.
|
Table 5: GroupWise local reaction and treatment. |
|||||
|
Type of Side Effects |
% of Allergen Vaccine Reaction Which Induced Local Side Effects |
Time of Incidence |
Management |
||
|
Omalizumab + Cluster Immunotherapy (9) |
Cluster Immunotherapy (40) |
Conventional Immunotherapy (16) |
|||
|
local oedema (5-10cm) |
3/9 (33%) |
15/40 (37.5%) |
4/16 (25%) |
Late 6-24hrs. |
Spontaneously resolves |
|
local oedema (>10cm) |
0/9 (0%) |
10/40 (25%) |
3/16 (18.75%) |
Late 6-48hrs. |
Antihistamine (Fexofenadine) + Methylpredisolone |
|
PRURITUS at the site of allergen vaccine Injection |
7/9 (77%) |
30/40 (75%) |
10/16 (62.5%) |
Late 6-48hrs. |
Cold Compresses |
|
PAIN at the site of allergen vaccine Injection |
4/9 (44%) |
10/40 (25%) |
2/16 (12.5%) |
Late 6-48hrs. |
Cold Compresses Antihistamine (Fexofenadine) |
|
No Early reaction Late Reaction after 6hrs. < 10cm = 22/65 (33.8%), > 10cm = 12/65 (18.4%) Large Local reaction > 10cm predicts the systemic reaction and was given Fexofenadine 180mg & Methyl - prednisolone 8mg |
|||||
CONCLUSION
Combined omalizumab (Anti-IgE) and cluster Immunotherapy is safe, effective and gives more rapid relief of symptoms. Cluster Immunotherapy has reduced the time to 6 weeks to achieve MTD (Maximum tolerance Dose) rather than 4½ months in conventional Immunotherapy and provide a balance between convenience & safety.
ACKNOWLEDGEMENT
The authors were assisted in the proof reading of the manuscript by Bharat Bhushan, an MSL, working with Novartis India.
REFERENCES
- Prasad R, Kumar R. Allergy situation in India: what is being done?Indian J Chest Dis Allied Sci. 2013; 55: 7-8.
- Pfaar O, Leitzbach S, Hörmann K, Klimek L. Cluster protocols in SCIT: enough evidence for practical use? Curr Opin Allergy Clin Immunol. 2010; 10: 188-193.
- Tabar AI, Echechipía S, García BE, Olaguibel JM, Lizaso MT, Gómez B, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005; 116: 109-118.
- Nieto García A, Nevot Falcó S, Carrillo Díaz T, Cumplido Bonny JA, Izquierdo Calderón JP, Hernández-Peña J. Safety of cluster specific immunotherapy with a modified high-dose house dust mite extract. Eur Ann Allergy Clin Immunol. 2013; 45: 78-83.
- Luo Z, Li B, Wan L, Peng C. [Comparative study on cluster and conventional immunotherapy in patients with allergic rhinitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015; 50: 105-109.