An Overview of the Links between Behavioral Disorders and Alzheimer
- 1. Laboratory of Cellular and Molecular Neurosciences, Faculty of Sciences, University of Chile, Chile
- 2. International Center for Biomedicine (ICC), Chile
- 3. Department of Neurological Sciences, Faculty of Medicine, East Campus, University of Chile, Chile
Abstract
Cumulative evidence shows that innate immunity participates in the pathogenesis of Alzheimer´s disease. This implies that activation of microglia by the so called “damaged signals” triggers a cascade of pathological molecular events thus leading to hyperphosphorylation and oligomerization of the tau protein in the brain, which is associated with cognitive impairment and loss of memory. However, from the pathophysiological point of view, Alzheimer´s disease is significantly more complex in inducing the loss of memory. As initial events in the pathogenesis of this neurodegenerative disease, alterations in the dopaminergic pathway together with serotonin depletion in the elderly lead to late onset depressive phenomena according with recent evidences. These events seem to occur prior to neuroimmunomodulatory alterations that lead to a final oligomerization of tau protein in the course of neurofibrillary tangles formation. It is critical to analyze both affective disorders and mood changes with the cognitive impairment in the context of Alzheimer´s disease.
Keywords
• Alzheimer´s disease
• Dopaminergic system
• Neuroimmuno modulation
• Behavioral disorders
• Mood disorders
CITATION
: Andrade V, Cortes N, Guzman-Martinez L, Maccioni R (2017) An Overview of the Links between Behavioral Disorders and Alzheimer’s Disease. JSM Alzheimer’s Dis Related Dementia 4(1): 1031.
INTRODUCTION
Neurodegenerative disorders including Alzheimer´s disease (AD), constitute a major puzzle to medicine and society when considering their progressive incidence and impact in public health, and the slow progress in searching for diagnosis and therapeutic approaches [1]. Fortunately, significant advances have been achieved about their pathogenesis and the alterations in the signaling pathways involved in communication between glial and neuronal cells in the brain [2,3]. Important information exists on the storage of cognitive information, processes underlying human cognition and acquisition of memory. In AD, for example, the neuroimmunomodulation theory largely explain the sequence of events derived from innate immunities that lead to tau oligomerization and formation of paired helical filaments and tangles [2].
However, Alzheimer’s pathology involves changes not only in memory but also emotional events such as empathy, mood, humor, which appear to integrate well with cognitive processes [4]. In this context, experimental evidence supports the existence of molecular/cellular alterations in sophisticated pathways of molecular connectivity between the dopaminergic cortex and dopamine release with the functional organization of the hippocampus [5]. On the basis of these reports and the multifactorial origin of AD, we hypothesize that behavioral disorders is an important step of the early pathological alterations associated with the symptoms of AD. Here, we briefly reviewed the structural and cellular basis for the functional connections between emotional and cognitive phenomena and their pathological alterations in AD.
ALZHEIMER´S DISEASE AND MOOD DISORDERS
AD has been associated with loss of memory, which is regarded as a main feature and trait of the disorder. However, such non-cognitive symptoms as anxiety, apathy, psychosis, depression appear to be involved in the AD. These disorders negatively affect the life quality of patients and caregivers [4-6]. Moreover, neuropsychiatric symptoms can be present in 80% of AD patients, and the depression is the most frequent alteration among them, with a prevalence of 50% of cases [7]. Controversial evidence, due to the diversity of the design of the studies and the difficulty for distinguish between AD and depression, does not allow a consensus to ascertain if the depression is just a risk factor, and an early event in AD progression [8,9]. In fact, studies show a higher presence of neurofibrillary tangles (NFTs) and senile plaques (SP) in the hippocampus of AD patients with a history of major depression, compared with AD patients without a depressive background. Besides, there is an increase in NFTs and SP of post mortem AD brains that present comorbidity with depression, as compared with those without the neuropsychiatric component [10,11].
On the other hand, patients suffering from depression have showed hippocampal atrophy [12]. In this context, late stage of depression and AD share mutual genetic factors, including the involvement of BDNF, ApoE, IL-1, and methylenetetrahydrofolate reductase (MTHFR), while inflammatory pathways are activated in both disorders [13,14]. Depressive episodes are influenced by dopamine and reduction of serotonin in brain, while AD has been associated with loss of serotonergic neurons and a reduction in the levels of 5-hydrotryptamine (5-HT) of post mortem brains with this disease [15,16]. As it was suggested by Butzlaff and Ponimaskin [17], serotonin receptors 5-HT4R, 5-HT6R and 5-HT7R, could modulate the activity of two essential proteins in tau phosphorylation: GSK-3β and CDK5 respectively, which could lead to NFT formation, triggering microgial activation according with the neuroimmunomodulation theory [2,18,19]. Furthermore, Yun et al. [20], showed that an antagonist of 5-HT6R is capable of rescue memory deficit and attenuate the expression levels of astrocytes and microglia in an AD mouse model, sustaining the role of serotonin in degeneration and microglial activation. Concomitantly, dopamine production is deeply reduced in brains of AD, as well as the levels of its receptors [21]. In addition, it has been recently determined that the loss of dopamine affects memory dysfunction in a transgenic mouse of AD [22]. Because AD has a multifactorial pathogenesis, we hypothesize that depression is an important step of the early pathological alterations which are associated with the symptoms in AD. In a healthy brain, dopamine is continuously released to the hippocampus, which connects mood feelings with cognitive processes [23,24]. In AD, a decrease in the dopaminergic levels plus a serotonin diminution would trigger depression which is regarded as a prodromal symptom of AD. In this context, the alterations generated by late onset of depression appears to have an impact on the hippocampus, thus inducing the inflammatory events, activating microglial cells that trigger overproduction of pro-inflammatory factors, as described in earlier time about the conceptual scheme of our neuroimmunomodulation theory [2,3,18].
CROSS-TALKS BETWEEN THE DOPAMINERGIC CORTEX AND THE HIPPOCAMPAL NEURONS
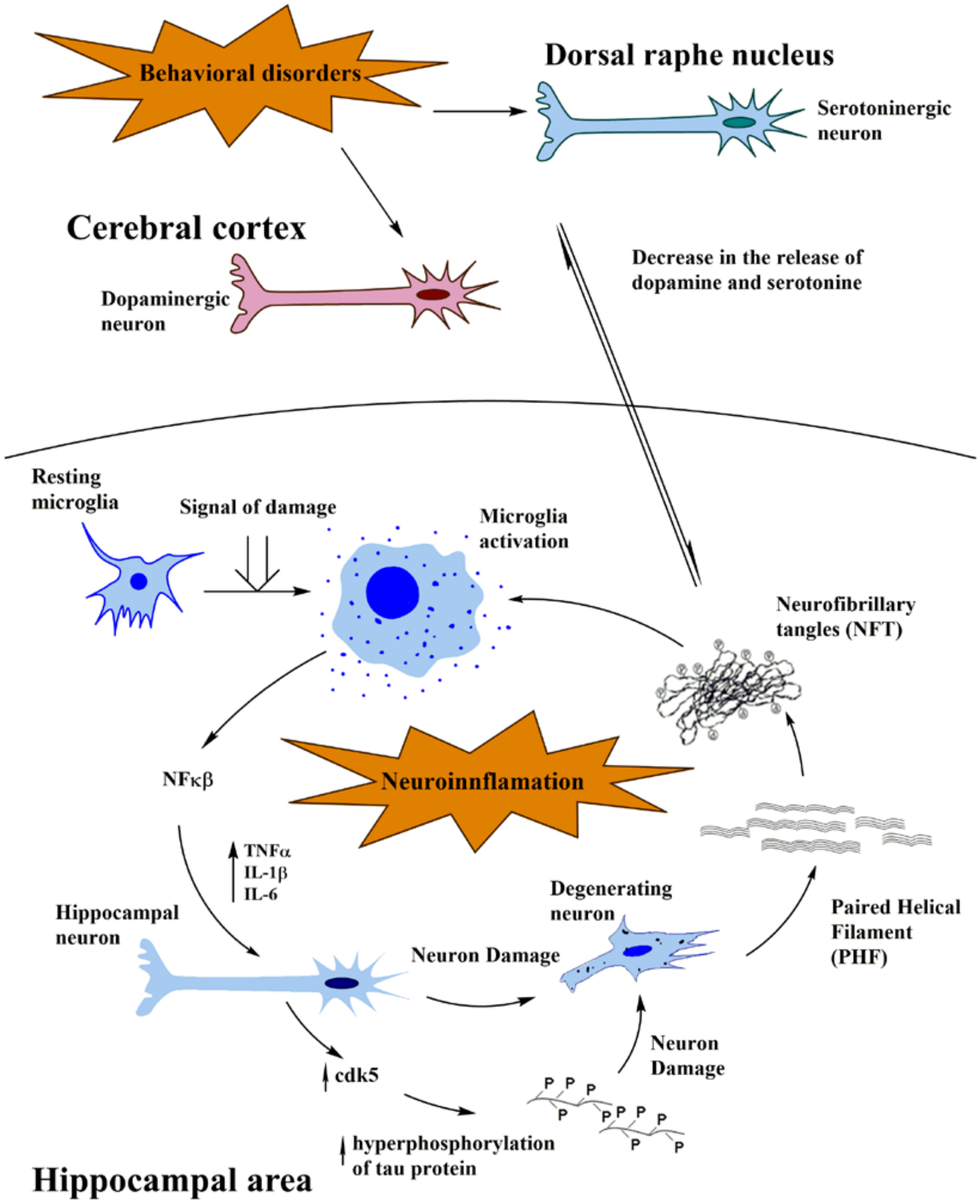
As mentioned about the links between the release of dopamine in the dopamine areas and the neurons from the hippocampus, both brain areas appear to be functionally interconnected. Within a mind-brain perspective, this means a bridge between the brain substrate for emotions and the substrate for rational processes. Recent studies pointed toward deep brain stimulation (DBS) in the medial forebrain bundle, which is associated with the reward system, in order to promote an improvement in a depressive-like rat model. They were capable to obtain not only an anti-depression response but also an increase of dopamine D2 receptors and dopamine transporters, in the areas of the hippocampus and the pre-frontal cortex [25]. These findings suggest a functional mechanism of the dopaminergic system in behavioral disorders of the hippocampal area, which is the primary structure affected by the neuroinflammatory mechanisms triggered by “damage signals” in AD, in agreement with the neuroimmune modulation theory [2,3]. The frontal cortex is also reported as a zone affected in cognition disorders. In this region, the blockade of D3 dopamine receptors has been associated with pro-cognitive activity in rodents and primate models and proposed as a possible therapy for AD [5,26,27]. In the meantime, it seems that improvement in cognition processes is related to cAMP/PKA/CREB signaling in the hippocampus, which also presents D3 receptors [28-32]. In fact, knockout mice for D3 receptor present an improved spatial memory and an increased CREB phosphorylation in the hippocampus, suggesting an enhancement in memory consolidation [33]. Other brain regions which constitutively express D3 receptors, seem to regulate memory processes, attention, emotions, motivation and reward. Neurons projecting their neurites from the nucleus accumbens (NAc) are enriched in D3 receptors and are innervated by dopaminergic neurons from the ventral tegmental area (VTA), which in turn, also receive NAc projections. Moreover, NAc processes reach the entorhinal and PFC and, receive projections from the cortex, hippocampus and the amygdala [34]. In other AD models, dopamine has been a target for the enhancement of memory tasks and the control of the associated cognitive impairment. In 2012, Guzman-Ramos and their collaborators [35] performed the microdialysis of dopamine reuptake blocker in cortical and hippocampal regions of a triple transgenic mouse model of AD (3xTg-AD). Moreover, cortical release of this neurotransmitter specifically in the insular cortex was able to attenuate the memory and cognitive impairment [35]. Furthermore, a recent study indicated that the gradual loss of dopaminergic neurons in an AD mouse model (Tg2576) characterized by memory and reward dysfunction [26]. It is known that dopamine D1 and D2 receptors are expressed in specific hippocampal areas, suggesting their role together with acetylcholine in memory processes [36,37]. More interesting D2 receptor antagonists have been proved as neuroprotective agents against tau toxicity and its aggregation [38] (Figure 1)
(Figure 1 Schematic representation of concerted action of dopaminergic/serotonergic decrease-neuroinflammation hypothesis on Alzheimer´s pathogenesis. Molecular and cellular events occurring at the dopaminergic area and dorsal raphe nucleus, linked with behavioral and mood alterations results in dopamine/serotonergic reduction. This event appears to activate the neuroinflammatory cascade at the hippocampal level, by stimulating microglial cells and as a consequence, promoting the release of pro-inflammatory factors that in turn activate protein kinases such as CDK5, tau phosphorylation, oligomerization into paired helical filaments and neuronal death. This may explain that late depression involving decrease in dopamine and serotonin decrease occur as early events, prior to deregulation of microglia-neuronal cells cross talks and activation of the neuroinflammatory cascade)
These observations seem to be connected with a series of evidence linking electric and magnetic induction in some regions of the brain, not only with the emergence of a minimal stages of consciousness or vegetative state but also with differential levels of serotonin and dopamine agents [39-42]. Previous reports have linked the dopaminergic system with brain damage and cognitive disorders [43-45]
DOPAMINERGIC SYSTEM IN BEHAVIORAL DISORDERS AND AD
According with the ideas outlined in the precedent paragraphs, an important cross- talk exists between the dopaminergic pathway involved in mood activities and neurons from the hippocampal domain and entorhinal cortex. Research shows that behavioral and mood disorders have been associated with AD phenotypes. In 1993, Rohling and Scogin [46] reported the correlative effect between depression and memory deficiencies. Today, we know that there are many reports with data related to the same phenotypes, giving us insights on the possible effects of the early AD event in behavioral or mood disorder conditions [47]. Since we have linked the hippocampal deterioration with a compromised behavioral state and mood disorders, it is interesting to pay attention to the evidence of the involvement of the glutamate system. Recent research has suggested ketamine, as a glutamatergic promoter, aimed to improve depressive or bipolar conditions [48].
Altogether, these reports suggest the importance of several neurotransmitters related to the interest regions in AD, indicating a paramount cross-talking between these neurotransmitters and functions such as memory, behavioral and cognition affections. It has already been shown that the progress of AD associated with neuronal death processes are preceded by pathological tau aggregation [2]. Therefore, the greatest interest from the therapeutic point of view is to search for compounds being capable of interfering with abnormal tau aggregation, as well as compounds that have a neuroprotective capacity, in order to ameliorate the degree of injury and prevent continuous cell damage. These studies on the connectivity between the dopaminergic pathway and the hippocampal area will be critical in the search for therapeutic solutions for AD.
ACKNOWLEDGEMENTS
This research has been supported by INNOVA Corfo project 12IDL4-13071 to RBM and by the International Center for Biomedicine (ICC).
REFERENCES
- Maccioni R, Farias G, Rojo L, Jimenez J. Diseases and Disorders of the Human Brain. “Brain Disorders” When Things Go Wrong. New York: Medical Publishers, Ámsterdam; 2012. 125-35.
- Maccioni RB, Rojo LE, Fernandez JA, Kuljis RO. The role of neuroimmunomodulation in Alzheimer's disease. Ann N Y Acad Sci. 2009; 1153: 240-260.
- Morales I, Jimenez JM, Mancilla M, Maccioni RB. Tau oligomers and fibrils induce activation of microglial cells. J Alzheimer's Dis. 2013; 37: 849-856.
- Seignourel PJ, Kunik ME, Snow L, Wilson N, Stanley M. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008; 28: 1071-1082.
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, et al. The potential role of dopamine D(3) receptor neurotransmission in cognition. Eur Neuropsychopharmacol. 2013; 23: 799-813.
- Jacus JP. Awareness, apathy, and depression in Alzheimer's disease and mild cognitive impairment. Brain Behav. 2017; 7: e00661.
- Tsuno N, Homma A. What is the association between depression and Alzheimer's disease? Expert Rev Neurother. 2009; 9: 1667-1676.
- Chi S, Yu JT, Tan MS, Tan L. Depression in Alzheimer's disease: epidemiology, mechanisms, and management. J Alzheimers Dis. 2014; 42: 739-755.
- Gasser AI, Salamin V, Zumbach S. Late life depression or prodromal Alzheimer's disease: Which tools for the differential diagnosis?. Encephale. 2017.
- Rapp MA, Schnaider-Beeri M, Grossman HT, Sano M, Perl DP, Purohit DP, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006; 63: 161-167.
- Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am J Geriatr Psychiatry. 2008; 16: 168-174.
- Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord. 2011; 134: 483-487.
- Ye Q, Bai F, Zhang Z. Shared Genetic Risk Factors for Late-Life Depression and Alzheimer's Disease. J Alzheimers Dis. 2016; 52: 1-15.
- Stefano P, Concetta C, Luigi D, Marco C, Antonis P, Ioannis L, et al. Role of neurodevelopment involved genes in psychiatric comorbidities and modulation of inflammatory processes in Alzheimer's disease. J Neurol Sci. 2016; 370: 162-166.
- Kovacs GG, Kloppel S, Fischer I, Dorner S, Lindeck-Pozza E, Birner P, et al. Nucleus-specific alteration of raphe neurons in human neurodegenerative disorders. Neuroreport. 2003; 14: 73-76.
- Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CP, Francis PT, Lasheras B, et al. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer's disease. Neuropsychologia. 2005; 43: 442-449.
- Butzlaff M, Ponimaskin E. The role of serotonin receptors in Alzheimer’s disease. Opera Med Physiol. 2016; 2: 77-86.
- Morales I, Farias G, Maccioni RB. Neuroimmunomodulation in the pathogenesis of Alzheimer's disease. Neuroimmunomodulation. 2010; 17: 202-204.
- Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farias GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer's disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014; 8:112.
- Yun HM, Park KR, Kim EC, Kim S, Hong JT. Serotonin 6 receptor controls Alzheimer's disease and depression. Oncotarget. 2015; 6: 26716-28716.
- Storga D, Vrecko K, Birkmayer JG, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci lett. 1996; 203: 29-32.
- Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer's disease. Nat Commun. 2017; 8: 14727.
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013; 14: 609-625.
- Rocchetti J, Isingrini E, Dal Bo G, Sagheby S, Menegaux A, Tronche F, et al. Presynaptic D2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol Psychiatry. 2015; 77: 513-525.
- Dandekar MP, Luse D, Hoffmann C, Cotton P, Peery T, Ruiz C, et al. Increased dopamine receptor expression and anti-depressant response following deep brain stimulation of the medial forebrain bundle. J Affect Disord. 2017; 217: 80-88.
- Loiseau F, Millan MJ. Blockade of dopamine D(3) receptors in frontal cortex, but not in sub-cortical structures, enhances social recognition in rats: similar actions of D(1) receptor agonists, but not of D(2) antagonists. Eur Neuropsychopharmacol. 2009; 19: 23-33.
- Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC. Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology. 2012; 37: 770-786.
- Basile J, Houston M, Ferrario CM. Treating the cardiometabolic syndrome: an opportunity to provide comprehensive cardiovascular risk reduction. J Cardiometab Syndr. 2006; 1: 358-361.
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain res. 1991; 564: 203-219.
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A. Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol. 1998; 40: 353-371.
- Richtand NM, Kelsoe JR, Segal DS, Kuczenski R. Regional quantification of D1, D2, and D3 dopamine receptor mRNA in rat brain using a ribonuclease protection assay. Brain Res Mol Brain Res. 1995; 33: 97-103.
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000; 295: 1223-1231.
- Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-dependent neuroprotection and cAMP response element-binding protein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J neurosci. 2005; 25: 1137-1148.
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006; 5: 25-43.
- Guzman-Ramos K, Moreno-Castilla P, Castro-Cruz M, McGaugh JL, Martinez-Coria H, LaFerla FM, et al. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer's disease. Learn Mem. 2012; 19: 453-460.
- Gangarossa G, Longueville S, De Bundel D, Perroy J, Herve D, Girault JA, et al. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus. 2012; 22: 2199-3207.
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005; 46: 703-713.
- McCormick AV, Wheeler JM, Guthrie CR, Liachko NF, Kraemer BC. Dopamine D2 receptor antagonism suppresses tau aggregation and neurotoxicity. Biol Psychiatry. 2013; 73: 464-471.
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007; 448: 600-603.
- Sesia T, Bulthuis V, Tan S, Lim LW, Vlamings R, Blokland A, et al. Deep brain stimulation of the nucleus accumbens shell increases impulsive behavior and tissue levels of dopamine and serotonin. Exp Neurol. 2010; 225: 302-309.
- Tsubokawa T, Yamamoto T, Katayama Y, Hirayama T, Maejima S, Moriya T. Deep-brain stimulation in a persistent vegetative state: follow-up results and criteria for selection of candidates. Brain Inj. 1990; 4: 315-327.
- Van Gompel JJ, Chang SY, Goerss SJ, Kim IY, Kimble C, Bennet KE, et al. Development of intraoperative electrochemical detection: wireless instantaneous neurochemical concentration sensor for deep brain stimulation feedback. Neurosurg Focus. 2010; 29: E6.
- Clauss RP. Neurotransmitters in disorders of consciousness and brain damage. Med Hypotheses. 2011; 77: 209-213.
- Fridman EA, Calvar J, Bonetto M, Gamzu E, Krimchansky BZ, Meli F, et al. Fast awakening from minimally conscious state with apomorphine. Brain Inj. 2009; 23: 172-177.
- Zafonte RD, Watanabe T, Mann NR. Amantadine: a potential treatment for the minimally conscious state. Brain Inj. 199; 12: 617-621.
- Rohling ML, Scogin F. Automatic and effortful memory processes in depressed persons. J Gerontol. 1993; 48: 87-95
- Masters MC, Morris JC, Roe CM. "Noncognitive" symptoms of early Alzheimer disease: a longitudinal analysis. Neurology. 2015; 84: 617-622.
- Lener MS, Kadriu B, Zarate CA Jr. Ketamine and Beyond: Investigations into the Potential of Glutamatergic Agents to Treat Depression. Drugs. 2017; 77: 381-401.