Persistence of Induction Influence for Ebbinghaus, M
- 1. Department of Psychology, University of Southern California, USA
Abstract
Geometric illusions produce distortions of visual space perception, and there are numerous theories about why those distortions occur. Temporal separation of the stimulus elements to be judged from elements that bias the perception may provide additional insight into the nature of illusion mechanisms. Three experiments were conducted wherein respondents were asked to judge size (Ebbinghaus), length (Müller-Lyer), or alignment of segments (Poggendorff). Illusion effects grew weaker with progressively greater delay in the display of illusion-producing elements, but each illusion manifested a different profile of decline. These results contribute to the discussion of processing stages for the respective judgments, and evaluation of which brain areas mediate illusion effects.
Keywords
Ebbinghaus, Müller-Lyer, Poggendorff, Illusion persistence
Citation
Greene E, Simonich S (2018) Persistence of Induction Influence for Ebbinghaus, Müller-Lyer, and Poggendorff Illusions. JSM Anat Physiol 3(1): 1018.
INTRODUCTION
Since purely summative (piece-wise) changes in the stimulus object can bring about qualitative changes in one’s experience, one cannot predict merely from knowledge of the stimulus object what the experience will be. Kurt Koffka [1].
Geometric illusions serve to highlight various aspects of visual processing. The Ebbinghaus and Müller-Lyer illusions pertain to judgments of size and the Poggendorff distorts perception of alignment. It is useful to get information on which neuronal populations mediate the various judgments, and one method for doing so is to examine the duration of persistent interaction between the stimulus elements being judged and those that are biasing the perception. At each stage of processing, the neuronal population requires a certain amount of time to accomplish the judgment, and their activity is subject to bias from the illusion-inducing components. The stimulus information is being transmitted through successive stages of processing, e.g., retina, primary visual cortex (V1), ventral-stream structures, so the extent to which the interaction decays as a function of interstimulus interval can assist in inferring where the major illusion influences are being produced.
The three illusions that were tested require different kinds of judgments: length, size, or alignment. The Müller-Lyer illusion, for example, relies on length judgment. Multiple theories have been put forth, however, to explain the underlying processes in the inaccurate judgment. These include depth perception and size constancy judgments, interactions of the central and peripheral features of the stimulus, gaze fixation, outline orientation detection, and spatial filtration processes [2]. Thus far, no consensus has been reached as to which of these theories best explains the cognitive or neural processes involved in generating the illusion.
The Ebbinghaus illusion, in contrast, relies on judgments of size. One theory for its effect is simply a contrast effect, describing a perceptual exaggeration of the degree to which the test stimulus differs from the inducing stimulus [3]. This contrast effect, or socalled judgmental theory, in which the context circles serve as a basis for relative judgment of the inner circle has been supported experimentally [4]. To our knowledge, there are no other major competing theories.
The judgments involved in perception of the Müller-Lyer and Ebbinghaus illusions are somewhat similar, reflecting what are essentially judgments of size, as length can be considered to be a component of size. The Poggendorff illusion, however, calls for a completely different type of judgment, this being the alignment of the oblique line segments. As with Müller-Lyer, several theories have been put forth to explain its perception. As described by Greist & Grier [5], the majority of theories posit a central basis for the illusion effect. For example, Pressey’s assimilation theory [6], describes a central tendency effect as underlying the misperception in Poggendorff judgment. Gillam [7], applied the misapplied constancy scaling theory/depth processing theory, which was proposed by Gregory [8], extending its application from the Müller-Lyer illusion to the Poggendorff illusion. Weintraub and Krantz [9], proposed a perceived orientationtheory, in which a subjective geometry predicts the intersection of the segments. Once again, consensus has not been reached.
The alternative theories seem to call for a unique basis for producing each of the three illusions, and if so, the time course needed to bias the perception should be different for each. Assessing the decay of induction influence could shed additional light on whether the Ebbinghaus, Müller-Lyer, and Poggendorff illusions are based on common induction mechanisms.
METHODS
The experimental protocols were approved by the USC Institutional Review Board. Twenty-four respondents provided data of illusion strength for each of the three experiments. Participation was completely voluntary and the data from each who was recruited was included in the analysis of treatment effects.
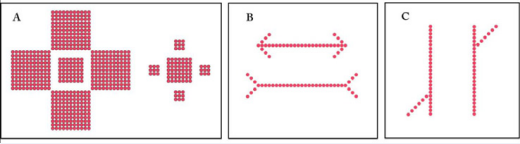
Each illusion pattern was presented as a pattern of discrete dots that were displayed as brief flashes (Figure 1).
Figure 1: Magnitudes of illusion effect were tested with three different geometric illusions, the Ebbinghaus, Müller-Lyer, and Poggendorff, shown in panels A, B, and C respectively. For the Ebbinghaus, the outer squares alter perceived size of the central squares, shrinking the size when the outer squares are larger and enlarging the square when the outer components are smaller. For the Müller-Lyer, the end fins alter the perceived length of the shaft, reducing its length when the outer fins form an arrowhead, and expanding its length when they form tail-feathers. The Poggendorff is an illusion of mis-alignment. The two diagonal arrays are in fact aligned, but appear misaligned under the influence of the parallel arrays.
The elements the Ebbinghaus illusion that were to be judged for relative size consisted of two squares, each being a 7x7 array of 49 dots. The two squares were 30 dots apart, measured from center-to-center. The large induction elements of the illusion were provided by four 11x11 squares. The small induction elements were four 3x3 squares. The edge of each induction square was separated from the edge of its central square by two dots that did not emit any light.
The elements of the Müller-Lyer illusion that were to be judged for length consisted of two 25-dot arrays, hereafter designated as “shafts.” These shafts were parallel and were separated by 10 non-emitting dots. Induction “fins” were provided in pairs at each end, a given fin being a diagonal array of three dots. The fin diagonals were positioned to be above the shaft, forming the classic arrowhead configuration, or tilted away from the shaft, forming the classic tail feather configuration.
The Poggendorff illusion provides two diagonal elements that are to be judged for collinearity, i.e., alignment. An array of six dots provided each diagonal, and were presented on a common diagonal of the 64x64 array, thus assuring alignment. The parallels of the Poggendorff were 25 dots in length, separated by 11 non-emitting dots.
The dimensions of stimuli have been described as dot counts. However, viewing distance was 3.5 m, so the visual angle subtended by each dots was 4.92 arc′, and dot-to-dot spacing was 9.23 arc′. From these dimensions one can calculate the visual angle provided by each stimulus element.
Each of the three experiments used the same basic protocol. The dots of a given illusion pattern were delivered as flashes from the LEDs of a custom designed display board. The LEDs of this board emit at a peak wavelength of 630 nm (red). Flash intensity was 1000 µw/sr, and flash duration for a given pattern element was 10 µs. Room illumination was dim (10 lx), so the pattern being displayed was readily perceived. Display timing was under the control of a Mac G4 Cube that was programmed with Tk/ tcl instructions. The instructions were further implemented as machine language through a PropoxMMnet101 microcontroller, running at 16 Mhz. This system provides for control of flash duration and timing with a temporal resolution of 1µs.
The treatment variable was the time between display of the elements to be judged and the induction elements that followed. Six temporal intervals were used, these being: 0, 50, 100, 200, 400, and 800 ms of separation. A seventh treatment condition displayed only the elements to be judged, i.e., the induction elements were not provided. Each treatment was displayed 50 times. The treatment to be administered on a given trial was selected at random.
Locations at which the patterns were presented on the display board were varied from trial to trial at random. The Müller-Lyer shafts were displayed with horizontal or vertical alignment, this being chosen at random for a given trial, and the same was done for the parallels of the Poggendorff configuration.
The display board was mounted on the wall and approximately centered on the line of sight of the respondent. Respondents were asked to keep their vision centered on the board, noting that the patterns could appear briefly at various locations and trying to fixate the stimuli would not contribute to accurate judgments. For the Ebbinghaus experiment, they were asked to judge the relative size of the two central squares, responding by saying either “same” or “different” after the stimuli were displayed. For the Müller-Lyer experiment they judged whether the two shafts appeared to have the same length, and for the Poggendorff they judged whether the two diagonals appeared to be aligned.
For the Poggendorff experiment, one respondent apparently failed to understand the task instructions, providing judgments that were exactly opposite of the normal illusion effect. The general practice in this laboratory is to include the data from all respondents who are tested once the experimental protocols have been finalized. However, it was clear that this was not a matter of weak or inconsistent treatment effects, so this respondent was replaced.
Responses were recorded by the experimenter as a keystroke. Respondents were not informed as to how the treatments might alter the perception of size, length, or alignment, and neither the experimenter nor the respondent was told what treatment was provided on a given trial. All were able to complete the task, providing a judgment for each trial, with a given session lasting approximately 45 minutes.
RESULTS
In the Ebbinghaus illusion, the two central squares are the same size, but the surrounding squares produce the perception that their sizes are different. If a given treatment display provides suitable conditions for producing the illusion, it will decrease the number of “same” judgments. To better reflect the size of the illusion effect, one can form a ratio with the judgments made to the treatment that did not provide any surrounding squares. That provides an index that can be described as percent illusion effect. The same data summary was applied to the Müller-Lyer and Poggendorff judgments, i.e., with fins being absent for the former, and parallels being absent for the latter.
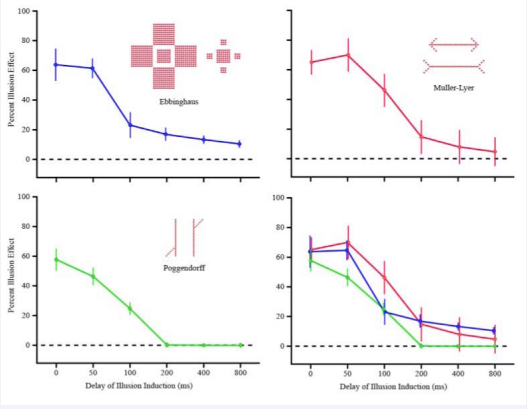
Figure 2 shows plots of mean illusion effect for each of the three experiments along with the 95% confidence interval for each mean.
Figure 2: Respondents judged the size of central squares for the Ebbinghaus, the length of shafts for the Müller-Lyer, and the alignment of oblique segments for the Poggendorff. Illusion effects declined in each of the three experiments as a function of delay for presentation of the induction elements, i.e., surrounding squares, fins, and parallels, respectively. Vertical bars show the size of confidence intervals of the plotted means.
For each configuration, a decline of illusion effect was manifested as a function of the temporal separation of the induction elements from the elements being judged. These treatment effects were evaluated using one-way repeatedmeasures Anovas. The decline of induction influence was significant at p < 0.001 for the Ebbinhaus illusion and at p < 0.0001 for the Müller-Lyer and Poggendorff illusions.
Although monotonic declines were manifested for each of the three illusions, there were significant differentials in the rate of decline that can be inferred from the confidence intervals. At 50 ms of delay the strength of Ebbinghaus and Müller-Lyer illusions were as strong as for zero ms, where there was simultaneous display of induction and judged elements. But with this relatively brief delay the alignment judgments of the Poggendorff were significantly below the others. By 100 ms the Ebbinghaus manifested a very steep decline, and percent illusion strength was significantly below the Müller-Lyer for both the Ebbinghaus and Poggendorff. From that interval onward, it dropped very slowly and remained well above chance level with very little variability in the judgments at longer intervals. By 200 ms the Poggendorff had dropped to chance and remained there, with the confidence intervals being only slightly greater than the token size from that point onward. The means the Müller-Lyer were above chance at 400 and 800 ms, but the confidence intervals reached that threshold, so one cannot be sure that any illusion effect was still present. The Ebbinghaus illusion remained well above chance out to the longest interval tested (800 ms), but did not differ significantly from the Müller-Lyer illusion.
DISCUSSION
The present experiments confirm that illusion induction effects can persist for hundreds of milliseconds after the displays to be judged are no longer present, and establishes the rates at which influence declines for the Ebbinghaus, Müller-Lyer, and Poggendorff illusions. Although the declines in persistence were monotonic for size, length, and alignment judgments, there were differences in the rate of decline and the interval across which the inducing elements were able to generate illusion effects.
A long persistence window argues against a retinal basis for the induction effects, or at least against all of the induction being provided within the retina. Upon stimulation, photoreceptors produce a “receptor potential” that can last a hundred milliseconds or more, depending on ambient lighting and other conditions [10,11]. This laboratory assessed elemental flash fusion and found the duration of persistence to be 122 milliseconds in the dark and 88 milliseconds in a lighted room [12]. For the dim conditions of the present work, one would expect an intermediate duration in the vicinity of 100 milliseconds. Numerous studies of perceived brightness using a flash summation protocol have found that the summed impact on brightness declines to the oneflash level within 100 milliseconds of separation [13-15]. Greene and Visani [16], found that two threshold-level intensities could sum to provide recognition of letters, but the maximal interval across which the flashes could sum was 100 milliseconds. Based on these prior findings, we view a rapid decline in perceived illusion effect to be from a decay of visible persistence that can be attributed to the retina.
For the Poggendorff configuration, 50 milliseconds of separation of the parallels from the diagonal components produced a large and significant drop in illusion magnitude. This suggests that a substantial portion of the illusion influence is being generated by retinal mechanisms. There is, however, strong evidence of a cortical contribution to Poggendorff illusion effect, manifested here as a moderate level of persistence at 100 milliseconds, declining to zero by 200 milliseconds. The fMRI results of Shen and associates [17], suggest a role for the middle occipital cortex and the left premotor cortex. These investigators used classic Poggendorff configurations as well as Kanizsa-like versions that induced the illusion with cognitive contours, comparing judgments of these configurations with decisions about bare aligned diagonals. Left precentral gyrus and right middle occipital cortex were specifically involved in the Poggendorff illusion induced by the real contours. The Kanizsalike configurations activated bilateral intraparietal sulcus (IPS) and right lateral occipital complex (LOC). Cognitive contours provide similar activation of the lateral occipital complex [18,19]. Lateral occipital complex is thought to be associated with representation of objects, object fragments, and figure-ground segmentation [20-22]. Harris and associates [23], suggested that it integrates local elements involved in optical illusions.
The declines in illusion strength for the two size illusions were substantially different from what was seen for the Poggendorff. Neither the Ebbinghaus or the Müller-Lyer illusions manifested a decline in illusion effect when inducing components were separated from judged components by 50 or 100 milliseconds.
One might infer, therefore, that the neuronal activities that provide for these illusion effects are not being generated within the retina.
However, the findings of Song and associates [24], suggest otherwise. They tested for strength of induction for the Ebbinghaus and also the Ponzo illusion under binocular, monocular, or dichoptic viewing conditions. The dichoptic conditions provided the configuration to be judged to one eye and the illusion-inducing configuration to the other eye. The dichoptic condition produced significant reduction of induction influence for the Ebbinghaus components, relative to the monocular viewing condition, but did not reduce illusion strength for the Ponzo components.
The Ponzo is also a size illusion, but it is more clearly related to depth perception and likely depends on cortical systems that evaluate depth cues. Depth might not be relevant in producing the Ebbinghaus illusion, and the findings of Song and associates [24], argue for a substantial role of retinal mechanism in the production of illusion influence. The lack of decline in illusion magnitude with separations of up to 100 milliseconds of separation may simply mean that there are cortical systems that can sustain the influences that begin in the retina. The substantial similarity of declines for both the Müller-Lyer and Ebbinghaus suggests common mechanisms, and one would thus assume similar processing at retinal and cortical sites.
Schwarzkopf and associates [25,26] used standard retinotopic mapping methods to determine the sizes of visual cortices of their volunteers. They then measured degree of illusion influence produced by Ebbinghaus and Ponzo illusions on each of the observers. Illusion magnitude for both were significantly correlated with the size of V1, not the sizes of V2 or V3. Correlation for Ponzo was somewhat larger.
There are other indications that V1 is involved in illusory size perception. Murray and associates [27], examined perceived size in a corridor illusion, finding that depth cues that enlarged the perceived size of an object modified the size of the area of fMRI activation on V1. Size adaptation produces similar effects [28]. Fang and associates [29] found that the corridor-induced enlargement of a “distant” ring modified the location of fMRI response in V1 by shifting the activation to be more eccentric. The “near” ring was shifted to a more foveal location. Others have reported similar effects [30].
The ability of illusions to modify size and location mapping in V1 is most likely produced by feedback from brain structures that are involved in depth perception. Weidner and associates [31,32], found fMRI activation of the right intraparietal sulcus and LOC in response to the Müller-Lyer illusion, and Tabei and associates [33], found LOC activation from a number of illusions, including the Müller-Lyer and the Ebbinghaus. Others have reported similar results [34-38]. Mancini and associates [39], was able to suppress illusion strength by application of trans-magnetic stimulation to LOC. This suggests that the LOC may contain a sizescaling module that transforms the retinal image into perceived size. It should be noted, however, that Tabei and associates [33], also found that illusory configurations produced activation of numerous other brain structures, so it much additional work is needed to delineate the exact contribution of each.
CONCLUSIONS
The present experiments found differentials of decline in strength of illusion for the Ebbinghaus, Müller-Lyer, and Poggendorff configurations with temporal separation of illusioninducing elements. Each showed a different profile of decline across intervals ranging from 50 to 800 milliseconds. The two size illusions were most similar, with no indication of reduced illusion magnitude when the elements to be judged were separated from the inducing elements by only 50 milliseconds. This likely reflects sustained activation from photoreceptors which generates a perceptual response described as “visible persistence.” Though there were other significant differences as the illusion-inducing elements were further separated, both size illusions continued to show perceptual bias for many hundreds of milliseconds. This argues for sustained activation of neuronal mechanisms in primary visual cortex and/or subsequent processing stages along the ventral route.
The Poggendorff manifested a simple linear decline of illusion strength, reaching zero induction effect by 200 milliseconds and remaining there at longer intervals. The immediate decline in induction at the shortest test interval suggests that most of the illusion effect comes from cortical rather than retinal mechanisms. The lack of extended persistence, i.e., beyond 200 milliseconds, suggests that all induction is provided by the first cortical processing site, most likely primary visual cortex.
ACKNOWLEDGMENTS
Programming of experimental protocols was done by Jack Morrison, Digital Insight, Somis, California. Funding for this research was provided by the Neuropsychology Foundation and the Quest for Truth Foundation.
REFERENCES
1. K Koffka. ZurGrundlegung der Wahrnehmungspsychologie. Ein Auseinandersetzungmit V. Benussi. Z Psychol. 1915; 73: 11-90.
2. Bulatov A, Bertulis A, Strogonov V. Müller-Lyer illusion and visual field anisotropy. Perception. 2001; 30: 86-87.
3. Gillam B. Geometrical illusions. Sci Amer. 1980; 242: 102-111.
27.Murray SO, Boyaci H, Kersten D. The representation of perceived angular size in human primary visual cortex. Nat Neurosci. 2006; 9: 429-434.
28.Pooresmaeili A, Arrighi R, Biagi L, Morrone MC. Blood oxygen leveldependent activation of the primary visual cortex predicts size adaption illusion. J Neurosci. 2013; 33: 15999-16008.
34.Hirsch J, DeLaPaz RL, Relkin NR, Victor J, Kim K, Li T, et al. Illusory contours activate specific regions in human visual cortex: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1995; 92: 6469-6473.
35.Seghier M, Dojat M, Delon-Martin C, Rubin C, Warnking J, Segebarth C, et al. Moving illusory contours activate primary visual cortex: an fMRI study. Cereb Cortex. 2000; 10: 663-670.
39.Mancini F, Bolognini N, Bricolo E, Vallar G. Cross-modal processing in the occipito-temporal cortex: A TMS study of the Müller-Lyer illusion. J Cogn Neurosci. 2011; 23: 1987-1997.