Central Nervous System Vasculitis in Rheumatoid Arthritis: A Case Report
- 1. Department of Internal Medicine, National University of Rosario, Argentina
Abstract
Central nervous system vasculitis is a rare and extremely severe manifestation of rheumatoid arthritis. We report a case of cerebral vasculitis in a 42-year-old woman with a longstanding deforming and erosive rheumatoid arthritis and extra articular manifestations. She had a previous history of granulomatous pleuritis treated with tuberculostatics without response, and multiple pulmonary complications interpreted as pneumonias and asthma exacerbations. This leaded to under treatment, avoiding methotrexate or other highly effective anti-rheumatic drugs, and using only hydroxychloroquine, prednisone and non-steroidal anti-inflammatory drugs most of the time. She was admitted for respiratory symptoms. Chest computed tomography revealed reticulonodular pulmonary involvement. She was treated with hydrocortisone and broad spectrum antibiotics, and showed a favorable response, but afterwards she suffered a seizure. Magnetic resonance imaging of the brain with T2 and FLAIR sequences showed multiples bilateral hyperintense signal images, suggestive of hemispheric vasculitis. Magnetic resonance angiography was normal. She received phenytoin and immunosuppressive treatment with methylprednisolone pulses, tapering dose of oral prednisone and a 6-month course of intravenous cyclophosphamide, followed by oral azathioprine for 2 years along with low dose aspirin. Treatment was then switched to methotrexate and afterwards leflunomide was added. Brain and thoracic images improved remarkably, and she remained neurologically asymptomatic and without respiratory symptom at a 4-year follow-up. This case illustrates how aggressive rheumatoid arthritis can be if undertreated and the importance of differential diagnosis between infectious and inflammatory manifestations, as treatment may be completely different. It also shows that cerebral rheumatic vasculitis may have a good response to immunosuppressive therapy.
Keywords
• Rheumatoid arthritis
• Extraaticular manifestations
• Pulmonary involvement
• Central nervous system
• Rheumatoid vasculitis
Citation
Lagrutta M, Alle G, Parodi RL, Greca AA (2016) Central Nervous System Vasculitis in Rheumatoid Arthritis: A Case Report. JSM Arthritis 1(1): 1005.
ABBREVIATIONS
RA: Rheumatoid Arthritis; ExRA: Extraarticular Manifestations Of Rheumatoid Arthritis; RV: Rheumatoid Vasculitis; RF: Rheumatoid Factor; CNS: Central Nervous System; AFB: Acid-Fast Bacilli; TB: Tuberculosis; LDH: Lactic Dehydrogenase; TC: Computed Tomography; IV: Intravenous; MRI: Magnetic Resonance Imaging.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune, inflammatory and systemic disease. The spectrum of its clinical manifestations is broad, and encompasses clinical presentations that range from mild, self-limited arthritis to severe erosive polyarthritis, which may lead to progressive joint damage, functional disability [1] and extra-articular manifestations of RA (ExRA) [2]. Among them, rheumatoid vasculitis (RV), an inflammatory condition of small and medium-sized vessels, affects a small percentage of patients (< 1-5%) [3]. RV mainly affects late middle-aged patients with longstanding severe RA, joint erosions, deformities and nodules or other ExRA, and high titers of rheumatoid factor (RF) [3]. It mostly involves skin, peripheral nervous system [3], and lungs [4], while cerebral vasculitis has been rarely described [5-12]. Seizures may be the presenting manifestation of this uncommon and serious complication of RA [3,5,9,10,12], which needs to be promptly recognized and treated. We describe a patient with a longstanding history of RA and chronic pleuritis with pleural effusion, presenting with a respiratory manifestation, and developing afterwards a central nervous system (CNS) vasculitis.
CASE PRESENTATION
A 42-year-old woman with a history of asthma, chronic pleuritis and a longstanding RA was admitted to hospital with a 5-day history of respiratory symptoms (productive cough, increasing dyspnea) and 24-hour fever in April 2012.
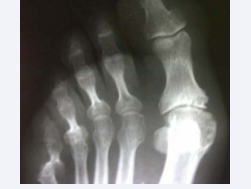
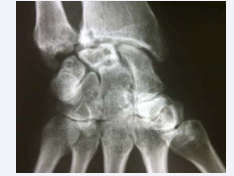
The patient had been diagnosed with RA in 1992, due to erosive seropositive symmetric polyarthritis, affecting wrists, hands and feet, and subcutaneous rheumatoid nodules. She had received an irregular treatment with methotrexate, which had been discontinued for many years due to respiratory complications, so that only hydroxychloroquine, prednisone and non-steroidal anti-inflammatory drugs were used most of the time. Persistent active synovitis lead to joint deformities and erosions on X-rays (Figure 1,2). She had had an articular flair in previous weeks, which improved by increasing prednisone dose from 5 to 20 mg/day.
Figure 1 Erosions on foot X-rays
Figure 2 Erosions on wrist X-rays.
She smoked 15 cigarettes per day for 20 years, and had a 15-year history of asthma, treated with inhaled salmeterol and fluticasone. On November 2000, 12 years before admission, she developed an exudative sterile pleural effusion. Tuberculin test was negative. Pleural fluid showed low pH and glucose level, and high lactic dehydrogenase (LDH) and leucocytes count, with neutrophil predominance. Biopsy revealed a fibrous pleura with a chronic inflammatory process consisting of neoforming vessels, infiltration of lymphocytes, plasma cells, and epithelioid histiocytes, with tendency to aggregate, and occasional Langhans giant cells, concluding intense chronic pleuritis with areas of granulomatous reaction. Ziehl-Neelsen and PAS stainings, as well as cultures for acid-fast bacilli (AFB) and mycosis, were negative. A possible rheumatoid pleuritis was considered, but since tuberculosis (TB) could not be ruled out, a 9-month TB treatment was indicated. In spite of this treatment, chronic recurrent pleural effusion persisted. Pleural fluid characteristics persisted as described, with cellular debris finally replacing the neutrophil predominance. A new biopsy was performed, showing chronic inflammatory sclerosis. AFB was not found on biopsy specimens or cultures, and corticosteroid treatment was started. Finally in 2008, after multiple thoracocentesis requirement, a pleurodesis was performed. In 2011 a chest computed tomography (CT) scan revealed residual right loculated pleural effusion with pleural thickening and subpleural nodules. Recurrent pulmonary complications interpreted as pneumonias and asthma exacerbations were also frequent in the past years. She received multiple antibiotic treatments for respiratory infections and corticosteroids due to bronchial spasm.
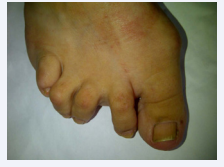
On admission her blood pressure was normal, and she was subfebrile, but she presented respiratory insufficiency with hypoxemia that required intensive care. Clinical examination revealed diffuse wheezing, dullness percussion and decreased breath sound of the right lower lung zone, with bilateral crackling sounds. Typical joint deformities of RA were evident mostly on feet (Figure 3),
Figure 3 Foot rheumatoid deformities
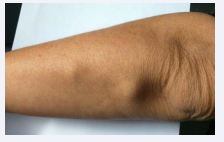
range of motion of elbows and wrists were limited, and she had subcutaneous nodules on fingers and arms (Figure 4).
Figure 4 Rheumatoid subcutaneous nodules
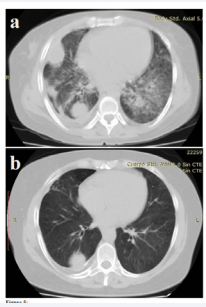
Mild synovitis was noted only on both hands. White cell count was 16,300/mm3 , erythrocyte sedimentation rate was 80 mm/h, and procalcitonin level was 0.12 ng/ml. Chest CT reports showed bilateral reticulonodular opacities with subpleural nodules, and bilateral laminar pleural effusion (Figure 5a)
Figure 5 a. Reticulonodular pattern and subpleural nodules, and laminar pleural effusion on chest computed tomography. b. Post treatment improvement of reticulonodular pattern and subpleural nodules on chest computed tomography
. Sputum samples were negative for AFB, as well as blood cultures. She received intravenous (IV) hydrocortisone (400 mg/ day) for bronchospasm and broad spectrum antibiotic treatment, with good response.
On the 10th day of hospitalization, having improved respiratory symptoms, and showing normal oxygen level and no fever, she suffered a seizure. It began as a partial seizure characterized by involuntary clonic movements of the right side of the face and right arm, spreading to a generalized tonic-clonic seizure with loss of consciousness and urinary incontinence, which required IV lorazepam administration. After recovering from postictal phase, the neurological examination was unremarkable.
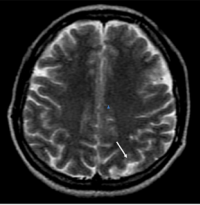
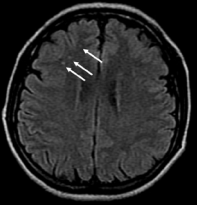
Laboratory tests revealed: erythrocyte sedimentation rate 20 mm/h, C-reactive protein 18 mg/l (normal: < 5), hemoglobin 10.4 mg/dl and white blood cell count 21.740/mm3 . High level of RF were founded (408,5 UI/ml), since tests were negative for antinuclear antibodies, antineutrophil cytoplasm antibodies, antiphospholipid antibodies and VDRL. Serum complement was normal. CT scan of the brain was normal. Magnetic resonance imaging (MRI) scan of the brain with T2 and FLAIR sequences, disclosed bilateral hyperintense signal images at cortico-subcortical regions and periventricular white matter, suggesting hemispheric vasculitis (Figure 6,7)
Figure 6 Cerebral magnetic resonance imaging, High signal lesion in the left occipital lobe on T2-weighted sequence.
Figure 7 Cerebral magnetic resonance imaging, multiples dot-like pattern of high signal intensity images on FLAIR sequence.
. Angio-MRI was normal.
Administration of phenytoin, and combined immunosuppressive treatment with IV methylprednisolone pulses (1 g/d for 3 consecutive days) and cyclophosphamide (1 g), was followed by oral prednisone (1 mg/kg/day). Prednisone dose was tapered, and a 6-month course of IV cyclophosphamide (1 g/ month) was completed, followed by oral azathioprine for 2 years. Treatment was then switched to methotrexate 22.5 mg/week, and, after a new articular flare, leflunomide was added. Low dose prednisone was maintained to control articular symptoms. Low dose aspirin was also added, and the patient stopped smoking. RMI images had clearly improved after three month. Chest TC also markedly improved (Figure 5b). She remained neurologically asymptomatic and showed no respiratory symptoms at a 4-year follow-up.
DISCUSSION
Extra-articular manifestations of rheumatoid arthritis (ExRA) occur in many patients with RA (~20 to 40%) [13,14]. They are associated with particularly severe and disabling disease, frequent life-threatening comorbidities and poor outcomes [14]. The most frequent of such manifestations are subcutaneous rheumatoid nodules. On the other hand, severe ExRA, such as vasculitis, rheumatoid lung disease, pericarditis and pleuritis, are seen more frequently in patients with rheumatoid nodules, and in longstanding active and severe RA [14,15]. Smoking and RF also predict the development of severe ExRA [16]. Our patient had all predictive factors for ExRA, and particularly for its severe forms.
Rheumatoid pleural effusion is an uncommon complication of RA, found in 2-5% of patients [17]. Pleural involvement in this patient raised the question of differential diagnosis between TB and RA-associated pleural effusion. As mentioned above, she was at risk for this ExRA. Pleural fluid characteristics were typical of rheumatoid effusion: exudative and sterile, with low glucose and pH, and high LDH values and cell count with neutrophil predominance in acute state [18,19]. However, the cytological finding of elongated macrophages and multinucleated giant cells alongside with granulomatous debris, considered pathognomonic of rheumatoid effusions [18,19] was not present (only debris were described). Regarding pleural biopsy, the findings were also consistent with rheumatoid pleuritis, in spite of the absence of typical surface layer of fibrinoid necrosis with necrotic nuclear debris [20]. Nevertheless, the observation of granuloma on pleural biopsy is often considered diagnostic for TB [21], and previous immunosuppression constituted a risk factor for this chronic infection. Tuberculin test was negative, but this test can be negative in one third of TB cases [21]. However, the whole clinical picture, including the pleural fluid characteristics, the biopsy description, the negativity of staining and cultures for TB and tuberculine test, as well as the absence of prolonged fever and the lack of response to TB therapy, made this diagnosis unlikely and argued in favor of rheumatoid pleuritis with pleural effusion. Although rheumatoid pleural effusions often resolve spontaneously over time [18], oral, parenteral, and intrapleural corticosteroids, pleurodesis and decortication, have been used for its treatment [22].
On the other hand, the later lung reticulonodular pattern and subpleural nodules, which improved with immunosuppression, further support RA-related lung involvement. The question of whether the pulmonary involvement on the latest hospitalization was related to infection or to lung parenchymal rheumatoid involvement remains unknown. Low procalcitonin levels abrogate against a bacterial infection but doesn´t exclude it [23]. Probably a combination of both processes lead to respiratory insufficiency that required intensive care. Treatment with IV broad spectrum antibiotics as well as corticosteroid therapy was prescribed, and respiratory symptoms improved. However, corticosteroid treatment probably was not sufficient to stop the systemic inflammatory burden, and finally CNS rheumatoid vasculitis, expressed by seizures, occurred.
The lack of intensive treatment for RA in this patient, in whom methotrexate was avoided due to persistent respiratory complications interpreted as possible infections and asthma exacerbations, probably lead to an aggressive disease. No other highly effective anti-rheumatic drugs were used. This may explain the erosive deforming arthritis, the torpid evolution of her rheumatoid lung involvement, and the late occurrence of a systemic rheumatoid vasculitis [4].
Vasculitis is a group of chronic inflammatory diseases in which blood vessels are the target of an immune reaction. Vascular involvement is an integral part of the pathogenesis of RA, as autopsy and blind biopsy studies have shown widespread inflammatory involvement of blood vessels [3]. These vascular lesions are mostly asymptomatic, but occasionally may cause severe life-threatening manifestations [3]. Capillaries, arterioles, and venules are involved from early stages of RA by an inflammatory process. However, clinically apparent vasculitis occurs only in the context of an inflammatory infiltrate associated with destructive lesions of the wall of medium-sized arteries, arterioles, and venules [3]. Damage to the endothelium may trigger the formation of thrombi, with resulting ischemia in the territory served by the occluded vessels [3].
In practice, RV is considered when clinical manifestations of vasculitis unexplained by conditions such as diabetes, atherosclerosis, infection, drug hypersensitivity, malignancy, or other primary systemic vasculitis, occur in a patient with an stablished diagnosis of RA [3]. This is a relatively rare complication, consideredas a consequence of uncontrolled inflammation, which usually occurs, as in this case, in patients with long-standing erosive nodular RA and high titers of RF [3,4].
Longitudinal studies showed that the incidence of systemic RV has dramatically declined since the mid-1990s [3,4,14]. Possible explanations include the increased use of methotrexate and other immunosuppressive agents together with improved strategies to control systemic inflammation, and decreasing cigarette smoking [4]. As previously mentioned, the avoidance of methotrexate or other highly effective anti-rheumatic drugs in this patient, along with smoking habit, may had a role in the occurrence of this CNS vasculitis.
RA involvement of the CNS is rare. It have been described traditionally in seropositive patients with long-standing, active and erosive RA, with subcutaneous nodules and extra-articular manifestations [11,12], and occasionally with minimal concomitant joint manifestations [8], as in our case.Patients may present with seizures, neuropsychiatric symptoms, confusional state, dementia, blindness, hemiparesis or headaches [3,8,11,12]. The diagnosis often remains challenging, as the histopathological demonstration of vasculitis, considered the gold standard for the diagnosis of RV, is not always possible or practical. There is a wide spectrum of clinical and pathological manifestations, and lack of specific signs and symptoms, as well as reliable tests [3]. Magnetic resonance imaging is useful, but it has to be interpreted in the adequate context. It is superior to CT in detecting vascular and microvascular CNS disease, and it is a sensitive though nonspecific test for CNS vasculitis [5]. Arteriography or angio-RMI may be normal, reflecting the small vessel distribution of the vasculitis [5,11]. Therefore the demonstration of ischemic lesions in different territories, in patients without any other etiologic explanation, usually sustains the diagnosis of RA CNS vasculitis. As in our case, the diagnosis of CNS vasculitis due to RA is supported by the clinical symptoms, MRI findings, high titers of RF and finally the good response to immunosuppressive therapy [5,9].
However, caution should be taken regarding a possible atherosclerotic origin of the ischemic features. It must be reminded that RA patients have a 1.5 to 2-fold increase in risk of acute myocardial infarction, cerebrovascular disease and cardiovascular mortality [24], which is probably related to chronic inflammatory burden, so atherosclerotic cause of ischemia must always be considered. In this case, a young woman with no other atherosclerotic risk factors, and multiple high intense images on T2 and FLAIR MRI, the diagnosis of CNS vasculitis was established. In view of the extremely poor prognosis [5,11], most authors agree that these cases should be treated aggressively like other vasculitic conditions [10]. A treatment regimen including high dose IV methylprednisolone, and cytostatic drugs such as cyclophosphamide must be used [3,10]. Our patient had an excellent response to therapy with methylprednisolone and cyclophosphamide, and is doing well with a more intense RA treatment 4 years after the CNS vasculitic episode.
CONCLUSIONS
Our patient showed a severe form of RA. She suffered from articular damage, and from severe extraarticular manifestations: initially rheumatoid pleuritis with recurrent sterile exudative pleural effusion that intensively affected her quality of life, then reticulonodular lung involvement, and finally CNS vasculitis, a life threatening condition. Differential diagnosis between RA activity and infection is crucial, as treatment options differ substantially, and may even be opposite. Once CNS vasculitis diagnosis was made, and aggressive treatment was established, a remarkable improvement of her disease activity and quality of life was achieved. Close monitoring of disease activity is always required, to avoid severe articular and systemic complications of RA that results from chronic uncontrolled inflammation.
ACKNOWLEDGEMENTS
We wish to acknowledge Dr. Maria de Lujan Navas for his assistance in neuroimaging interpretations.