An open label, balanced, randomized, two treatments, two sequences, two periods, crossover, single dose, bioequivalence study of lafigin dt (lamotrigine) 200 mg orally dispersible tablets of laboratorios recalcine s.a. Chile and lamictal odt
- 1. AZIDUS Laboratories Ltd.
- 2. Carolina Briceño, Julio Jimenez, Lorena Galeotti. ABBOTT, Chile
ABSTRACT
Lamotrigine is a phenyltriazine used in the treatment of epilepsy and bipolar disorder type I. The purpose of this study was to evaluate the bioequivalence between Lafigin DT (Lamotrigine) 200 mg orally dispersible Tablets of Laboratorios Recalcine S.A. Chile and LAMICTAL ODT® (Lamotrigine) 200 mg orally dispersible Tablets of Glaxosmithkline LLC in healthy, adult, human subjects. An open label, balanced, randomized, two treatments, two sequences, two periods, crossover, single dose study with washout period of 10 days under fasting condition was carried out in 28 subjects in the age group of 26 to 43 years and 27 subjects completed the study. All the subjects included in the study were males and Asians. The pharmacokinetic samples collected from subjects who completed the study were analysed to determine the plasma concentration of Lamotrigine using a validated bio-analytical method. The 90% confidence interval of Cmax and AUC0-72 were 87.03% - 106.32% and 84.23% - 100.64%, respectively which were within the acceptable limits for Cmax of 80.00 % to 125.00 %.
KEYWORDS
• Lamotrigine
• Dispersible tablets
• Bioavailability
• Lamictal
• Bioequivalence
• Pharmacokinetics
CITATION
Srinivas G, Arjun Arumugam O (2022) An open label, balanced, randomized, two treatments, two sequences, two periods, crossover, single dose, bioequivalence study of lafigin dt (lamotrigine) 200 mg orally dispersible tablets of laboratorios recalcine s.a. Chile and lamictal odt® (lamotrigine) 200 mg orally dispersible tablets of glaxosmithkline llc, in healthy, adult, human subjects under fasting condition. JSM Bioequiv Bioavailab 2(1): 1006.
ABBREVIATIONS
AUC: Area under the concentration versus time curve; AED: Anti-Epileptic Drugs; AUC0-72: Area under the plasma concentration versus time curve from zero to truncated 72 hours; BMI: Body Mass Index; Cmax: Concentration Maximum; CV: Coefficient of Variation; ISCV: Intra-subject Co-efficient of Variation; LCMS/MS: Liquid Chromatography Tandem Mass Spectrometry; mg: Milligram; mL: Millilitre; ng/mL: Nanogram per millilitre; PK: Pharmacokinetic; Tmax: Time taken to reach maximum concentration; IRB: Institutional Review Board.
INTRODUCTION
Many drug patents have recently expired or are scheduled to expire in the near future. In response, many drug manufacturers have expanded their generic drug profile, which requires them to conduct clinical trials that demonstrate that their generic equivalents perform similarly to the innovator drug product Regulations introduced by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) over the last thirty-five years have strengthened measures to ensure the bioequivalence of drug products, which may be simultaneously manufactured by multiple drug makers.
Bioequivalence and bioavailability testing standards have also emerged following recognition that bioequivalence and variations in the bioavailability of drug products can result in therapeutic failure and/or toxicity. Lamotrigine, an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Lamotrigine’s chemical name is 3, 5-diamino-6-(2, 3-dichlorophenyl)-astriazine, its molecular formula is C9 H7 N5 Cl2 , and its molecular weight is 256.09. Lamotrigine is a white to pale cream-colored powder and has a pKa of 5.7. Lamotrigine is very slightly soluble in water (0.17 mg/mL at 25°C) and slightly soluble in 0.1 M HCl (4.1 mg/mL at 25°C).
This study was designed to evaluate the relative bioavailability of the test product Lafigin DT (Lamotrigine) 200 mg orally dispersible Tablets of Laboratorios Recalcine S.A. Chile and reference product LAMICTAL ODT® (Lamotrigine) 200 mg orally dispersible Tablets of GlaxoSmithKline LLC in healthy, adult, human subjects under fasting condition.
MATERIALS
Test product, dose and mode of administration
Lafigin DT (Lamotrigine) 200 mg orally dispersible Tablets, 01 x 200 mg, Oral with 200 mL of water in sitting posture under fasting condition, Batch: 19AT289.
Reference product, dose and mode of administration
LAMICTAL ODT® (Lamotrigine) 200 mg orally dispersible Tablets, 01 x 200 mg, Oral with 200 mL of water in sitting posture under fasting condition, Batch: 1914200033.
METHODOLOGY
The study protocol with annexes was prepared and IRB approval was obtained before initiation of the study. The subjects were screened and enrolled in the study as per the IRB approved protocol. Written informed consent was obtained from each volunteer in screening visit to initiation of screening procedure and for the study prior to enrolment. Individual counselling was then given to the willing volunteers by the Investigator in private and any questions and concerns were addressed prior to obtaining consent. The Principal investigator/sub-investigator/ physician reviewed all the screening results to assess eligibility of each volunteer. Subjects were enrolled in the study based on the inclusion and exclusion criteria.
This study was designed based on the known pharmacokinetic profile of the investigational product and general accepted standards for the conduct of bio-equivalence study.
Twenty-eight subjects who met the eligibility criteria were enrolled and dosed with a single dose of either test or reference product in sitting posture at a fixed time in each period. Washout period of 10 days was maintained between each period, in order to minimize any possibility of carryover effect from preceding treatment. The blood samples were collected at pre-defined time intervals for the measurement of concentration and pharmacokinetic parameters of Lamotrigine in both the periods.(Figure 1-2).
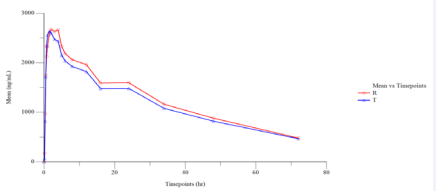
Figure 1 Linear Plot of Mean Plasmatic Lamotrigine Concentration vs. Time Points (N=27).
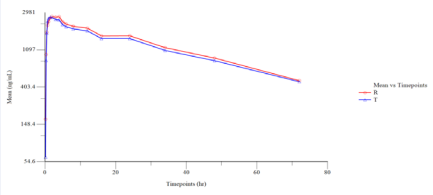
Figure 2 Semi log Plot of Mean Plasmatic Lamotrigine Concentration vs. Time Points (N=27).
Data obtained from 27 subjects who completed the study were used for the pharmacokinetic statistical analysis of Lamotrigine.
Bioequivalence was determined by statistical comparison of Ln-transformed data of Cmax and AUC0-72 of the test and reference formulations using SAS version 9.4.
Study Criteria for inclusion/exclusion of subjects
Healthy volunteers, aged 18 to 45 years, and with BMI of 18.50 - 29.99 Kg/m2 and weight > 50 Kg were eligible to be enrolled in the study.
Inclusion criteria encompassed no evidence of cardiac, pulmonary, gastrointestinal, hepatic, renal, hematologic, or neurologic disorders, or any acute or chronic disease, no history of drug or alcohol addiction, normal laboratory tests (complete blood counts, urinalysis, liver and kidney function, and blood sugar); and serological negativity HIV, hepatitis B.
Subjects were informed by an investigator about the purposes and risks of the study. They were asked to abstain from using concomitant medications, including over-the-counter products, dietary supplements and natural products which potentially modify kinetics / dynamics of Lamotrigine, 14 days prior to dosing and throughout the end of the study. Consumption of grapefruit and/or its products were not allowed within 10 days prior to the start of the study. Caffeine and/or xanthinecontaining products or alcohol were not allowed 48 hours prior the first administration of the study medications and throughout the blood sampling periods.
Sample size and power
An Intrasubject CV of 16% was considered for estimation of sample size. This was obtained from the earlier studies conducted. A desired power of 90% was opted to have adequate data for evaluation of bioequivalence. Difference between formulation was considered to be 5%. The sample size deemed required is about 18 subjects. However, to account for possible dropouts during study conduct due to adverse events or any other reasons, a sample size of 28 subjects was considered.
Subjects drug administration and blood sampling
After an overnight fasting of 10 hours, subjects were administered with a single oral dose of either test product or reference product with 200 mL of water according to the sequence assigned to each subject in sitting posture in each period.
A single oral dose of either test or reference tablet according to randomization schedule was placed on the subjects’ tongue and was asked to move around the mouth. Subjects were instructed to not chew or swallow the tablet. The tablet rapidly disintegrates inside the mouth. Once the tablet completely disintegrated, subjects were instructed to swallow the same with allotted 200 mL of water.
Compliance to drug administration was assessed by examination of the oral cavity and hands of the subject immediately after dosing.
All the subjects remained in sitting posture for 02 hours after dosing. During this restriction period, the subjects were permitted to walk for reasons such as but not limited to the following: natural exigencies. Subjects were restricted from consumption of water for 01 hour before and 01 hour after dosing in each period and allowed to drink water ad libitum thereafter.(Table 1,2)
| Table 1: Statistical Results of Test Product-T versus Reference Product - R for Lamotrigine. | ||||||
| Parameters | Antilog Least Square Mean | Point Estimate (%) | 90% Confidence Interval | ISCV (%) | Power (%) | |
| Test Product (T) | Reference Product (R) | |||||
| Ln (Cmax ) | 2862.3778 | 2975.5473 | 96.20 | 87.03% - 106.32% | 21.77 | 97.70 |
| Ln (AUC0-72) | 82402.833 | 89499.719 | 92.07 | 84.23% - 100.64% | 19.31 | 99.18 |
| Table 2: Summarized Demographic Profile of Subjects who Completed the Study (N=27). | ||||
| Parameter | Mean | SD | Minimum | Maximum |
| Age (years) | 35 | 5 | 26 | 43 |
| Height (m) | 1.668 | 0.041 | 1.580 | 1.730 |
| Weight (Kg) | 67.7 | 7.1 | 55.6 | 79.3 |
| BMI (Kg/m2) | 24.31 | 2.18 | 19.75 | 28.84 |
The pharmacokinetic profile of both test and reference products (in terms of rate and extent of absorption) were evaluated based on measured concentration of drug in the human plasma samples collected during the clinical phase. Blood samples for pharmacokinetic analysis were designed appropriately for characterizing the pharmacokinetic profile for the given treatments at the dose administered.
Blood samples, of 04 mL each one, were collected at 00.00 (Pre-dose), 00.17, 00.33, 00.50, 00.75, 01.00, 01.50, 02.00, 03.00, 04.00, 05.00, 06.00, 08.00, 12.00, 16.00, 24.00, 34.00, 48.00 and 72.00 hours post dose for measurement of pharmacokinetic parameters.
The samples collected were subjected to centrifugation and separated to 02 aliquots which were stored at -70º C. During bio analysis, the samples were thawed and a validated method was used to analyse the samples.(Table 3,4)
| Table 3: Summary of Pharmacokinetic Parameters for Lamotrigine of Reference Product -R. | ||
| Parameter | N |
Reference (R) (Mean ± SD) |
| Cmax (ng/mL) | 27 | 3016.764 ± 541.323 |
| AUC0-72 (ng.hr/mL) | 27 | 91024.762 ± 17359.061 |
| *Tmax (hr) | 27 | 2.00 (0.50 - 4.00) |
| *Expressed in terms of median (range) | ||
| Table 4: Summary of Pharmacokinetic Parameters for Lamotrigine of Test Product -T. | ||
| Parameter | N | Test (T) (Mean ± SD) |
| Cmax (ng/mL) | 27 | 2923.998 ± 627.449 |
| AUC0-72 (ng.hr/mL) | 27 | 84476.191 ± 17896.168 |
| *Tmax (hr) | 27 | 1.00 (0.33 - 4.00) |
| *Expressed in terms of median (range) | ||
Lamotrigine was selectively extracted from human plasma by Liquid-Liquid extraction as per the in house procedure. Separation was achieved by reverse phase chromatography and quantification was done using LC-MS/MS method.
Agilent 1290 Binary Pump with Agilent 6460 Triple Quad LC/ MS detector was used for bio analysis and the validated analysis was performed using Lamotrigine and Lamotrigine 13CD3 as reference standards.
Tolerability
Subjects were monitored for adverse events during both periods of the study and ambulatory sampling visits. Subjects were instructed to inform clinical personnel of any untoward medical symptoms and/or events that arose during the study. Prior to check in of each period, subjects were questioned concerning unusual symptoms that may have occurred after the previous administration of the study drug. The Principal Investigator/sub-investigator/ physician also evaluated the subjects for subsequent dosing.
Subjects’ Safety was assessed via continuous monitoring and scheduled recording of safety measurements throughout the study through clinical examinations, vital signs assessment, 12- lead Electrocardiogram (ECG), Chest X - ray, clinical laboratory parameters (e.g., Haematology, Biochemistry, Urine analysis and Serology test) and monitoring subjects’ well-being, symptoms and signs for adverse events.
Pharmacokinetic and Statistical Analysis
The pharmacokinetic and statistical analysis of Lamotrigine was performed using the concentration data obtained from 27 subjects who completed both the periods of the study. In order to test the two one-sided tests for bioequivalence, ratio analysis,90% confidence intervals for the difference between treatments’ least-square mean was calculated for Ln-transformed Cmax and AUC0-72 of Lamotrigine.
Pharmacokinetic parameters were calculated using noncompartmental model of Phoneix® WinNolin® version 8.1 and statistical analysis was carried out using the SAS® statistical software, version 9.4 of SAS Institute Inc, USA.
The mean, standard deviation, standard error, geometric mean, coefficient of variation, minimum, median, maximum and range were calculated for Cmax, AUC0-72, and Tmax.(Table 5)
| Table 5: p -Value for Cmax and AUC of Lamotrigine. | |||
| Parameters | Cmax | AUC0-72 | Significance |
| Sequence effect | 0.2259 | 0.5796 | Insignificant for Cmax and AUC0-72 |
| Period effect | 0.3400 | 0.6313 | |
| Treatment (Formulation) effect | 0.5142 | 0.1255 | |
| Subjects nested within sequence | 0.8090 | 0.0926 | |
| P < .10 for Sequence effect and P < .05 for all other effects considered to be significant | |||
RESULTS
Twenty-eight subjects in the age group of 26 to 43 years, who met the study eligibility criteria, participated in the study and twenty-seven subjects completed the study. All the 28 subjects enrolled in the study were males and Asians.
One subject (S06) did not report for period II of the study, hence was withdrawn. The clinical study was conducted over a period of 15 days. Blood sampling was done at pre-defined intervals up to 72.00 hours in both the periods, separated by a washout period of 10 days. The pharmacokinetic plasma samples collected from 27 subjects were analysed to determine concentration of Lamotrigine using a validated bio-analytical method in LCMS/MS.
The pharmacokinetic and statistical analyses of Lamotrigine were performed using the concentration data obtained from 27 subjects who completed both the periods of the study. The 90% confidence interval of Cmax and AUC0-72 were 87.03% - 106.32% and 84.23% - 100.64%, respectively, for Lamotrigine, which were within the acceptable limits of 80.00 % to 125.00 %.
DISCUSSION
The study was carried out in healthy male subjects. The tolerability of Lamotrigine was already well established. In this study, no adverse events are reported indicating that the drug is safe and tolerable at this dose level in healthy subjects.
As indicated in the package insert of the product, the study was conducted under fasting conditions. Lamotrigine is expected to be less variable and the ISCV reported in the literature indicates the same. Hence, a conventional two way crossover design is opted to evaluate the bioequivalence with optimal number of subjects. The washout period opted did not result in any carryover of the drug to subsequent period. Hence, the washout selected is considered adequate and accurate. The pharmacokinetic parameters estimated in the study are comparable with that of the published data. The sponsor, Abbott had included this study in the dossier submission for the generic drug approval in Chile.
CONCLUSION
Bioequivalence was demonstrated between Lafigin DT (Lamotrigine) 200 mg orally dispersible Tablets of Laboratorios Recalcine S.A. Chile and LAMICTAL ODT® (Lamotrigine) 200 mg orally dispersible Tablets of Glaxosmithkline LLC, in healthy, adult, human subjects under fasting condition. The 90 % CI of Lafigin DT (Lamotrigine) 200 mg orally dispersible tablets was within the acceptable limits of 80.00 % to 125.00 %. There were no adverse events reported during the study. Thus, it can be considered that both the test and reference products were well tolerated in healthy adult subjects at selected dose level.
REFERENCES
1. International Conference on Harmonization (ICH): Harmonized Tripartite Guideline-Guideline for Good Clinical Practice (GCP) - E6 (R2), 2016.
2. CDSCO’s New Drugs and Clinical Trials Rules 2019 G.S.R. 227(E).
3. Structure And Content of Clinical Study Reports E3 Current Step 4 version dated 30 November 1995.
4. 21 Code of Federal Regulations, USFDA.
5. ISP Chile – Technical guide G-BIOF 01 - Guide for performance of Comparative Bioavailability studies in solid pharmaceutical forms of oral administration and systemic action.
6. Formulario F BIOF - 03: presentación De Resultados De Estudio De Biodisponibilidad/Bioequivalencia Para Estableer Equivalencia Terapéutica.
7. Prescribing information of LAMICTAL ODT® (Lamotrigine) 200 mg orally dispersible Tablets of Glaxosmithkline LLC.
8. Sane RT, Francis M, Deo A (1998) Bioequivalence study of lamotrigine tablets in healthy male human volunteers. Indian drugs.1998; 35: 570-573.
10.Brodie MJ. Lamotrigine. Lancet. 1992: 339: 1397-1400.
12.Garnett WR. Lamotrigine: pharmacokinetics. J Child Neurol. 1997; 12: 10-15.








































































































































