Alkaloids from Annona : Review from 2005 to 2016
- 1. Department of Botany, University of São Paulo, Brazil
Abstract
Species of Annonaceae have different popular uses as edible fruits or as traditional medicines. The genus Annona is a rich source of secondary metabolites, which have been isolated and identified from different parts of plant. Among these compounds, alkaloids are known as an important class of chemical constituents. Several chemical and pharmacological investigations on species of Annona indicated the presence of important bioactive compounds, exhibiting various biological activities including anti-acetylcholinesterase, antioxidant, antidepressant, antiepileptic, antimicrobial, antiplasmodial, antiproliferative, antibacterial, antifungal, anti-ulcer, anxiolyticlike effects, cytotoxic, immune-stimulant and larvicidal. In this review, we analyzed published papers from 2005 to 2016 looking preferably for alkaloid composition of species of Annona related to biological activities. Among these papers, 70 alkaloids were reported during the researched period, distributed in 20 species. Aporphine alkaloids were the most common, corresponding to more than half of all alkaloids described. Most of the available data are for leaves; seeds and roots need further studies.
Keywords
Anthracene alkaloids; Aporphine alkaloids; Benzylisoquinoline alkaloids; β-carboline alkaloids; Biological activity.
CITATION
Egydio-Brandão, APM,Novaes, P, Santos, DYAC (2017) Alkaloids from Annona: Review from 2005 To 2016.. JSM Biochem Mol Biol 4(3): 1031
INTRODUCTION
Annonaceae is a pantropical family, which comprises 2400 species distributed in 108 genera arranged in four subfamilies (Annonoideae, Anaxagoreoideae, Ambavioideae and Malmeoideae). Guatteria (210 species) is the largest genera. Annona (around 200 species) also presents a wide number of species, including all previous species placed in Rollinia [1]. The family is recognized as one of the most diverse, even related to richness and abundance of individuals [2]. This family appears into Magnoliales, together with Myristicaceae, Magnoliaceae, Degeneriaceae, Himantandraaceae and Eupomatiaceae [3].
Species of Annonaceae present different popular uses as edible fruits (e.g. A. muricata, A. squamosa, A. cherimola and A. reticulata) or as traditional medicines (e.g. A. crassiflora, A. montana, A. muricata and A. squamosa). A wide diversity of chemical compounds has been isolated from the members of this family. Acetogenins, diterpenes and flavonoids are some of the secondary metabolites isolated from the bark, leaves, fruits and seeds of Annonaceae [4,5] which are related to the biological activities described for the family, e.g. antiprotozoal [6] or cytotoxic [7]. However, one of the most important characteristic of Annonaceae species is the presence of alkaloids [8], mainly benzylisoquinoline.
Despite Annona is only the third genus in number of species in Annonaceae, the most economically important species of this family are placed in this genus (e.g. Annona cherimola Mill. – cherimoya; A. squamosa L. - sugar apple; Annona x atemoya Mabb.- atemoya, a hybrid between A. cherimola and A. squamosa; A. muricata L. - guanabana or soursop; A. reticulata L. - custard apple; A. macroprophyllata Donn. Sm. - ilama; A. glabra L. - pond apple; A. purpurea Moc. & Sessé ex Dunal – soncoya) [9].
Annona species are used in folk medicine for very different treatments. Antdiarrheal use is described for many species includ ing A. crassiflora Mart. [10], A. muricata [11], A. purpurea Moc. & Sessé ex Dunal [12], A. reticulata L. [13] and A. salzmannii A. DC. [14]. Emetic use has been pointed out for A. cherimola [15], A. mu ricata [16,11] and A. squamosa L [11]. Anti-parasitic treatment has been observed for A. cherimola [17], A. muricata [16], A. reticulata [13], A. salzmannii [14], A. squamosa L [11]. A. foetida Mart. [18], A. purpurea [12] and A. reticulata [13] have been traditionally used to treat fever. Rheumatism treatment is described for A. foetida [18], A. glabra [19], A. muricata [16; 11]. Ulcers have been threated us ing A. foetida [18], A. reticulata [13], A. salzmannii [14], A. squamosa L [11], while anti-cancer action is described for A. muricata [11] and A. senegalensis [20]. A. muricata [21,22], A. squamosa L [11], A. vepretorum [23,24] have been used for pain and A. reticulata [13], A. rugulosa (Schltdl.) H. Rainer [25] for infections. Other uses in clude tranquilizer for A. muricata [26], treatment of skin diseases for A. cherimola [27] and A. salzmannii [14], anti-inflammatory for A. salzmannii. [14] and A. vepretorum [28], molluscicide for A. crassiflora [10], insecticide for A. cherimola [17], and antimalarial for A. senegalensis Pers. [29].
There are a large number of alkaloids that has been identified in different parts of the plant, including fruits, leaves, branches, stems, barks, roots, seeds and twigs [30-34]. Several biological activities described for A. cherimola, A. foetida, A. glabra, A.mucosa and A. muricata are related to alkaloids profiles.
Alkaloids are a very diverse group of low-weighted secondary metabolites with a Nitrogen atom. In plants, most of them present an amino acid as precursor, and they are classified based upon the basic skeletal configuration of their carbon–nitrogen ring systems, which include isoquinoline, indole, pyrrole, pyridine and piperidine structures. Benzylisoquinoline alkaloids are synthesized from tyrosine and make up approximately 2500 compounds, which are common in some families of Ranunculales (Papaveraceae, Berberidaceae, Menispermaceae, Ranunculaceae) and in Magnoliaceae [35,36].
There are two classical reviews about alkaloid distribution in Annonaceae [4,37]. Recently, three reviews on alkaloids from Annonaceae were published. Barbalho et al. [38], presented the compounds of A. crassiflora, A. muricata, A. reticulata, A. cuneata, A. coriacea and A. cherimola and their pharmacological effects. The other two newest reviews were published as book chapters by Rabêlo et al. [39], and Lucio et al., [40]. The first one presented chemical structures and pharmacological activities of alkaloids for species of Annona while the other for Annonaceae in general. However, the access to book chapters is much more difficult than journal articles.
The main goal of our revision is to present alkaloid distribution in Annona associated with their biological activities. For that, we searched for published papers in the period of 2005 – 2016 using a combination of the keywords “Annona”, “alkaloids” and “biological activity” in two scientific databases, ISI ®Web of Science, and ®SciFinder. The search was refined to include only papers with some compound identification. Papers with detection tests only, were not consider.
Alkaloids from Annona
Seventy alkaloids were reported in 42 papers during the searched period (2005 to 2016) from 20 species of Annona with some biological activity associated. Most of them (66) are benzylisoquinoline derivatives. Only few alkaloids reported in Annona, two β-carboline and three antracene derivatives, are not synthesized from tyrosine (Table 1).
|
Table 1: Alkaloids described for Annona species between 2005-2017. |
|||
|
Species |
Part |
Alkaloids |
References |
|
A. amazonicaR.E. Fr. |
Stems |
cassythicine liriodenine |
[68] |
|
A.cherimolaMill. |
Leaves |
anonaine liriodenine nornuciferine 1,2-dimethoxy-5,6,6a,7-tetrahydro-4h-dibenzoquinoline-3,8,9,10-tetraol |
[69] |
|
|
Roots |
corytenchine isocoreximine |
[49] |
|
Annona × atemoyaMabb. (is an unresolved name) |
Leaves |
anonaine asimilobine lanuginosine liriodenine lysicamine pronuciferine stepharine |
[70] |
|
|
Seeds |
Atemoine cleistopholine |
[34] |
|
A. crassifloraMart |
Leaves |
anonaine annoretine romucosine xylopine |
[71] |
|
A. diversifoliaSaff. |
Embryos, radicles, and roots at early developmental stages |
liriodenine |
[43, 72]
|
|
|
Seeds |
liriodenine |
[42] |
|
A. foetidaMart. |
Barks |
annomontine liriodenine N-hydroxyannomontine O-methylmoschatoline |
[53] |
|
|
Branches |
annomontine atherospermidine liriodenine O-methylmoschatoline |
[33] |
|
A. glabraL. |
Leaves |
actinodaphnine anolobine 7-hydroxyactinodaphnine roemeroline |
[44] |
|
|
Stems |
asimilobine actinodaphnine dehydrocorydalmine dehydrocorytenchine hydroxynornantenine, hydroxynornuciferine liriodenine lysicamine 5-O-methylmarcanine N-methylcorydine norushinsunine oxonantenine palmatine pseudocolumbamine pseudopalmatine pycnarrhine reticuline
|
[69] |
|
A.hypoglauca Mart. |
Stems |
actinodaphine anonaine isoboldine nornuciferine |
[73] |
|
A. leptopetala(R.E.Fr.) H.Rainer |
Leaves and branches |
corypalmine laurotetanine anonaine nornuciferine norannuradhapurine |
[74] |
|
A. lutescensSaff. (Synomym of A. reticulata L.) |
Leaves, roots andstems |
liriodenine |
[30] |
|
A. mucosaJacq. (synonym of R. mucosa (Jacq.) Baill. |
Leaves |
atherospermidine
liriodenine |
[32] |
|
A. muricataL. |
Leaves |
anonaine annonamine asimilobine coclaurine isoboldine isolaureline liriodenine N-methylcoclaurine norcorydine O-dimethylcoclaurine O-methylcoclaurine remerine xylopine |
[56, 75, 76, 77]
|
|
A. pickelii(Diels) H. Rainer |
Bark |
anolobine anonaine asimilobine atherospermidine coclaurine discretamine juziphine liriodenine lysicamine orientaline stepharine stepholidine |
[59] |
|
|
Leaves |
asimilobine liriodenine lysicamine nornuciferine |
[78] |
|
A. purpureaMoc. &SesséexDunal |
Roots |
annomontine |
[51] |
|
A. reticulataL.
|
Leaves |
lanuginosine liriodenine lysicamine
|
[47] |
|
A. rugulosa(Schltdl.) H.Rainer |
Leaves |
anonaine asimilobine isoboldine liriodenine lanuginosine litseferine magnococline norisocorydine nornantenine N-methylcoclaurine N-nornuciferine reticuline xylopine |
[79] |
|
A. salzmanniiA. D.C.
|
Barks |
anonaine asimilobine cleistopholine liriodenine oxolaureline reticuline xylopine |
[31, 41, 60]
|
|
|
Leaves |
anonaine asimilobine liriodenine norcorydine |
[80] |
|
A. senegalensisPers. |
Leaves |
anonaine isoboldine coclaurine liriodenine nornuciferine roemerine |
[50, 81]
|
|
|
Aerialparts |
anonaine asimilobine nornantenine |
[82] |
|
A. sericeaDunal |
Leaves |
hydroxynornuciferine isoboldine lysicamine N-methylcoclaurine nornantenine nornuciferine oxonantenine |
[42] |
|
A. squamosaL. |
Leaves |
annonaine corydine lanugiosine liriodenine lysicamine N-methylcoclaurine oxophoebine O-methylarmepavine reticuline roemerine |
[50, 54, 61, 63, 82, 83]
|
|
|
Twigs |
anomuricine isocorydine lanuginosine N-methylcorydaldine O-methylarmepavine |
[48, 64]
|
|
|
Barks |
N-nitrosoxylopine norlaureline roemerolidine |
[69] |
|
A. vepretorumMart. |
Leaves |
lanuginosine liriodenine lysicamine oxonantenine 1,3,6,6-tetramethyl-5,6,7,8-tetrahydro-isoquinolin-8-one |
[80] |
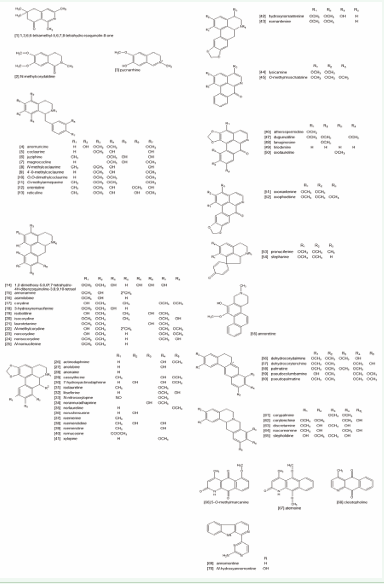
The structures of all alkaloids are in Figure 1. There are three isoquinolines (1-3) 10 benzylisoquinolines (4-13), 39 apophines (including oxoaporphines) (14-52), two proaporphines (53-54), one phenanthrene (55), 10 protoberberines (56-65). Among the non-tyrosine derivative alkaloids there are three anthracenes (66-68) and two β-carbolines (69-70).
Figure 1: Structures of all Annona alkaloids reported in the period of 2005-2016.
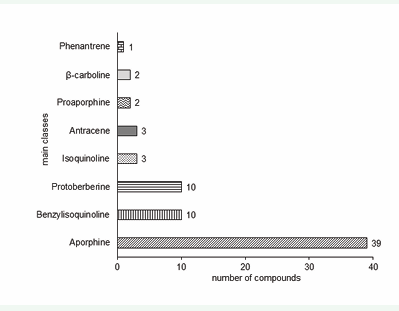
As shown in Figure 2, aporphine alkaloids are in majority, corresponding to 55% of all alkaloids reported in the period. Among this class, 24% of the alkaloids are oxoapophine-type derivatives (44-52). In contrast, only one phenanthrene skeleton (55) was reported. Some aporphine (including oxoaporphine) and benzylisoquinoline alkaloids have been suggested as chemotaxonomic markers in Annona [41,42]. No-tyrosine derivatives correspond to 7.5% of the alkaloids.
Figure 2: Number of Annona alkaloids reported in the period of 2005-2016 arranged in main classes based on basic skeleton. Drawing bars: tyrosine derivative alkaloids. Solid bars: no-tyrosine derivative alkaloids.
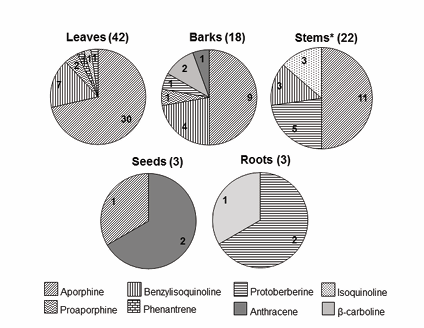
According to literature data (2005-2016), a wide variation on alkaloid composition of Annona species is observed through plant parts (Figure 3).
Figure 3: Distribution of the main classes of alkaloids described for species of Annona (2005-2016) in different plant organs. Following the organ name is the number of total alkaloids described. Numbers inside the graphic correspond to compounds of each main class. * Stems data include twig and branches from Table 1. Drawing slices: tyrosine derivative alkaloids. Solid slices: no-tyrosine derivative alkaloids.
Aporphine alkaloids are absent only in roots. All other plant organs present this alkaloid type, being the major class of constituents in leaves, barks and stems. Benzylisoquinoline alkaloids, the second more abundant class reported, presented the same distribution of the aporphine alkaloids except for seeds. Protoberberine-type is the main alkaloid in roots, also occurring in stems (22.7%), but with less prominence in barks (5%). Some alkaloid-types presented a very restrict distribution, and were detected only in one or two plant organs. Annoretine (55), the only one phenanthrene skeleton, was reported exclusively in leaves. The no-tyrosine derivative alkaloids were also restricted. Antracene skeleton was reported in barks and seeds, and β-carboline in barks and roots.
Besides the alkaloid variation, an enormous difference in plant organs content can be noted. While almost 46% of the alkaloids were reported in leaves, only 3.5% of them were obtained from seeds or roots (Figure 3). It is worthy to notice that both, seed and roots, are important sources of uncommon alkaloids in Annona. In addition, liriodenine (49), an oxoaporphine alkaloid, was described for young tissues as embryos, radicles, and roots at early developmental stages [43] (Table 1).
Biological activities
Few papers described some biological activity investigation for isolated alkaloids or, at least, for enriched alkaloid extract (Table 2).
|
Table 2: Bioactivities of isolated alkaloids from Annona species reported between 2005-2017. |
||
|
Alkaloids |
Bioactivity |
References |
|
annomontine |
Antileishmanial |
[53] |
|
|
Tripanocidal |
[33] |
|
anonaine |
Moderate neurotoxicity against human neuroblastoma cell line Antimicrobial
|
[56, 60, 82]
|
|
|
Antiepileptic agent |
[13] |
|
anolobine |
Inhibitors of acetylcholinesterase |
[60] |
|
|
Antimicrobial |
|
|
asimilobine |
Antioxidantwith ORAC Antimicrobial |
[60, 82]
|
|
coryntechine |
Cytotoxic |
[49] |
|
|
Antimicrobial Antioxidantwith ORAC |
[60] |
|
discretamine |
Antimicrobial Antioxidante with ORAC |
[59] |
|
isocoreximine |
Cytotoxic |
[49] |
|
isocorydine |
Anti-ulcer |
[48] |
|
lanuginosine |
Antiproliferativeeffect |
[63] |
|
liriodenine |
Antileishmanial |
[53] |
|
|
Tripanocidal |
[33] |
|
|
Antileishmanial Antioxidant with ORAC Citotoxicity to mice peritoneal macrophage |
[32, 60]
|
|
|
Antimicrobial |
[60] |
|
|
Antiproliferative effect against HTLV-I-infected T-cell lines |
[63] |
|
|
Antifungal |
[43] |
|
lysicamine |
Antiproliferativeeffect |
[63] |
|
7-hydroxyactinodaphnine |
Inhibitorsofacetylcholinesterase |
[44] |
|
N-hydroxyannomontine
|
Antileishmanial |
[53] |
|
O-methylmoschatoline |
Antileishmanial |
[53] |
|
O-methylarmepavine |
Anti-ulcer |
[48] |
|
|
Tripanocidal |
[33] |
|
|
Leishmanicidal |
[54] |
|
N-methylcorydaldine |
Anti-ulcer |
[48] |
|
nornantenine |
Antimicrobial |
[82] |
|
palmatine |
Inhibitorsofacetylcholinesterase |
[58] |
|
pseudocolumbamine |
Inhibitorsofacetylcholinesterase |
[58] |
|
pseudopalmatine |
Inhibitorsofacetylcholinesterase |
[58] |
|
roemeroline |
Inhibitorsofacetylcholinesterase |
[58] |
Several pharmacological activities have been described for these compounds including anti-acetylcholinesterase [44], antioxidant, antidepressant [31], antiepileptic [45], antimicrobial, antileishmanial [32], anti-Trypanosoma [33], antiplasmodial [46], antiproliferative [47], antibacterial, antifungal [43], anti ulcer [48], cytotoxic [49], immune-stimulant, larvicidal [50], and anxiolytic-like [51].
Antileishmanial activity
Leishmaniasis is a disease caused by Protozoan species of Leishmania, which are transmitted by the bite of an infected female of Phlebotomines and flies. There are three main forms of the leishmaniasis: visceral (also known as Kala-azar, the most serious form of the disease), cutaneous (the most common) and mucocutaneous. The disease affects some of the poorest people on Earth, and is associated with malnutrition, population displacement, poor housing, a weak immune system and lack of financial resources [52].
The antileishmanial activity was related to benzylisoquinoline (O–methylarmepavine), oxoaporphine (O-methylmoschatoline and liriodenine) and β-carboline (annomontine and N-hydroxyannomontine) alkaloids against promastigote or amastigote forms of L. amazonensis, L. braziliensis, L. chagasi and L. guyanensis [53,54,32].
Lima et al. [32], reported the antileishmanial activity of liriodenine isolated from leaves of Annona mucosa against promastigote forms of L. amazonensis, L. guyanensis and L. braziliensis, with IC50 values of 1.43 μg.mL-1, 0.84 μg.mL-1 and 55.92 μg.mL-1, respectively. Despite this activity, this alkaloid was also considered toxic when tested for cytotoxicity after 96 h incubation of peritoneal macrophages (LC50 = 19.11 μg.mL-1). Pentamidine, a standard antileishmanial drug, presented IC50 values of 0.07 μg.mL-1 and 5.58 μg.mL-1 against promastigote forms of L. amazonensis and L. braziliensis, respectively. Liriodenine was also active against intracellular amastigote forms of L. amazonensis at a concentration of 25 μg.mL-1. These authors also showed that liriodenine was highly selective against L. guyanensis, but not for L. braziliensis. Costa et al. [53], had already demonstrated the higher sensitivity of L. guyanensis to liriodenine (IC50 of 21.5 μM).
O-methylarmepavine, an alkaloid isolated from leaves of A. squamosa, showed EC50 = 23.3 μg.mL-1 and 25.3 μg.mL-1 against promastigote and amastigote forms of L. chagasi. Pentamidine and glucantime, reference standard drugs, showed EC50 values of 1.63 μg.mL-1 and 17.4 μg.mL-1, respectively. The EC50 value for the cytotoxicity assay of this alkaloid was 79.7 μg/mL [54].
Annomontine was the most active alkaloid against promastigote forms of L. braziliensis, with an IC50 = 34.8 μM, while O-methylmoschatoline and N-hydroxyannomontine presented lower activity [53].
Trypanocidal activity
Chagas disease, also known as American trypanosomiasis, is a potentially life-threatening illness caused by the protozoan parasite of the genus Trypanosoma. About 6 to 7 million people worldwide, mostly in Latin America, are estimated to be infected with this parasite [52].
Trypanocidal activity assays revealed that the compounds liriodenine, O-methylmoschatoline, and annomontine, isolated from A. foetida, were active against epimastigote and trypomastigote forms of T. cruzi. The three alkaloids showed high level of activity against trypomastigote forms of T. cruzi with EC50 values of 4.0 μg.mL-1, 3.8 ± 1.8 μg.mL-1and 4.2 ± 1.9 μg.mL-1, respectively. These compounds were less active against epimastigote forms (EC50 =177.0 ± 10 μg.mL-1, 92.0 ± 18.4 μg.mL-1, 198.0 ± 4.2 μg.mL-1, respectively). The three alkaloids were more effective against T. cruzi trypomastigotes than the positive control crystal violet (EC50 = 12.8 ± 0.9 μg/mL) [33].
Antiplasmodial activity
Malaria is a disease caused by parasites transmitted to people by the bite of infected female mosquitoes. Plasmodium falciparum is the most deadly malaria parasite. Since the year 2000, malaria mortality rates have declined by 60% globally. In Africa, malaria mortality rates reduced about 66% among all age groups and 71% among children under 5 years old. However, we are far from reaching the complete elimination. Nearly half of the world’s population, 3.2 billion people, remains at risk of malaria. Over 200 million new cases of malaria were reported in 95 countries and more than 400,000 people died due to this disease in 2015 [52].
Antiplasmodial activity of some aporphine alkaloids from the bark of A. squamosa has been demonstrated. N-nitrosoxylopine, roemerolidine and duguevalline presented IC50 values ranging between 7.8 and 34.2 μg.mL-1. However, N-nitrosoxylopine, but not roemerolidine and duguevalline, also showed cytotoxic effect in Chinese hamster ovarian cell line [46].
Central Nervous System (CNS) activity
Common mental disorders are increasing throughout the world. From 1990 to 2013, the number of people suffering from depression and/or anxiety diseases had an increase of 50% (from and 416 million to 615 million of people). These two mental disorders affect around 10% of the world’s population, while other mental problems are responsible for about 30% of the global non-fatal disease burden [52].
Several species of Annona are used in traditional medicine by their anti-anxiety, anticonvulsant and tranquilizer properties, as presented by Martinéz-Vásquez et al., [55]. These authors demonstrated that the administration of the total alkaloid extract (TAE) from aerial parts of A. cherimola in mice, at doses from 5 to 10 mg/kg, induces antidepressant-like effects. The TAE presented antidepressant effects on serotonergic 5-HT1A receptors and modulated dopaminergic transmission, which are involved in depressive disorders. The alkaloids 1,2-dimethoxy 5,6,6a,7-tetrahydro-4H-dibenzoquinoline-3,8,9,10-tetraol, anonaine, liriodenine, and nornuciferine were identified as the main constituents of this extract. The effect produced by TAE was similar to those of imipramine and clomipramine, two classical antidepressant drugs widely used in the clinical therapy.
The effects of annomontine, an alkaloid isolated from roots of A. purpurea, have been studied on anxiety disorder. The administration of this alkaloid to mice with doses of 1, 10, and 30 mg/kg induces, at least with the higher doses, anxiolytic-like effects comparable to those of diazepam, a clinical commonly used drug, at 1mg/kg. The authors suggested that the anxiolytic effect of annomontine might be due to its interaction with the benzodiazepine-binding site GABA receptors [51].
Anonaine has already has been considered an antiepileptic agent by acting on GABA receptor in rats. GABA is an important neurotransmitter inhibitor of the central nervous system. Epileptic rats treated with anonaine for 15 days (250 mg/ kg) presented improved therapeutic effect by reversing the alterations in the GABA receptor. This alkaloid showed similar effect to that of carbamazepine, a standard drug used for the treatment of epilepsy [45]. However, anonaine showed moderate neurotoxicity (IC50 = 34.6 μM) against the human neuroblastoma SH-SY5Y cell line which is a well-characterized catecholaminergic cell line often used as a neuronal model in Parkinson’s disease researches [56].
Anti-Acetylcholinesterase activity
Alzheimer’s disease is a neurodegenerative disease characterized by progressive memory loss and cognitive impairment. This disease is the most common type of dementia in ageing populations causing a severe loss of cholinergic neuron in a specific brain area. Acetylcholinesterase inhibitors (AChEI) have been used to treat early stages of Alzheimer’s disease [57].
Anolobine and roemeroline, two aporphine alkaloids, showed moderate inhibitory activity on acetylcholinesterase with IC50 values of 22.4 and 26.3 μM, respectively [44]. All dehydroprotoberberine alkaloids reported for the stem of Annona glabra (dehydrocorydalmine, dehydrocorytenchine, palmatine, pseudocolumbamine, and pseudopalmatine) inhibited acetylcholinesterase activity with IC50 ranging from 0.4 – 8.4 μM [58].
Antibacterial and antifungal activities
Discretamine, a protoberberine alkaloid isolated from stem bark of A. pickelii, had moderate in vitro antifungal activity against Candida parapsilosis and C. dubliniensis with minimal inhibitory concentration (MIC) of 125 µg.mL-1. Ketoconazole was used as positive control, and presented MIC values of 12.5 µg.mL-1 [59]. Asimilobine, anonaine, and liriodenine isolated from bark of A. salzmannii showed antimicrobial activity with MIC values ranging from 25 to 100 µg.mL-1, some of them were even more active than the positive control [60]. Reticuline and oxophoebine have been reported for their bactericidal activity against organisms related to a common Food borne Disease [61].
Annona alkaloids also contribute to the plant defense mechanisms against phytopathogens. Liriodenine from A. diversifolia, exhibited antifungal activity against the phytopathogenic fungi Rhizopus stolonifer and Aspergillus glaucus [62].
Antiproliferative effects/anticancer
According to WHO [52], 14.1 million of new cancer cases, 8.2 million cancer deaths and 32.6 million people living with cancer for at least 5 years were registered worldwide in 2012. In all these situations, around 50% of the cases occurred in less developed regions of the world. Treatment options include surgery, chemotherapy and radiotherapy depending on tumor stage and available resources. The search for new drugs looking forward cancer treatment is always an important topic, including natural products investigations.
Alkaloid-rich extracts from aerial parts of A. reticulata and A. squamosa showed significant antiproliferative effects with EC50 values ranging from 0.1 to 1 µg.mL-1. Citotoxic effects toward normal peripheral blood mononuclear cells were observed only at 100 µg.mL-1. Three apophine isolated alkaloids – lysicamine, lanuginosine and liriodine – were assayed on two T-cell lines (MT 1 and MT-2) infected with HTLV-I (human T-cell lymphotropic virus type I). EC50 values for MT-1 cell line were 31.61 µM, 1.34 µM, and 3.09 µM for lisicamine, lanuginosine and liriodenine, respectively, while for MT-2 cell the values were 16.25 µM, 4.49 µM, and 3.62 µM. Doxorubicin, the positive control, presented EC50 values of 0.017 µM for MT-1 and 0.023 µM for MT-2 [63].
Immune-stimulant activity
Lanuginosine, O–methylarmepavine, and N-methylcorydal dine, alkaloids isolated from A. squamosa, were evaluated in vivo for their immune modifier activities after oral administration in BALB/c mice at three doses, 0.3, 1.0 and 3.0 mg/kg, showing dose dependent immune stimulating activity. N-methylcorydaldine presented the higher activity at 3.0 mg/kg oral dose. Picroliv, a standard immunostimulant compound, showed similar efficacy at 1 mg/kg [64].
Anti-ulcer activity
Peptic ulcer illness refers to encircling gastric and duodenal ulcers. These diseases affect a large part of the population throughout the world and appears when an imbalance occurs between gastro protective agents (mucin, prostaglandin, bicarbonate, nitric oxide and growth factors) and aggressive factors (acid, pepsin, Helicobacter pylori) [65]. The alkaloids N-methylcorydaldine, O-methylarmepavine and isocorydine isolated from twigs of Annona squamosa showed anti-ulcer activity, inhibition of the gastric H+/K+-ATPase activity with IC50 values of 111.83 μg.mL-1, 60.98 μg.mL-1 and 88.42 μg.mL-1, respectively, and reduction of the plasma gastrin level [48].
Antioxidant activity
Free radicals are responsible for a large number of human health problems including cancer, cardiovascular diseases, neural disorders, Alzheimer’s disease, mild cognitive impairment, Parkinson’s disease, alcohol induced liver disease, ulcerative colitis, aging and atherosclerosis [66]. Due to the antioxidant activity of naturally occurring substances from higher plants, there was an increased interest in the protective activity of these natural antioxidants against chronic disorders caused by the oxidative process [67].
The antioxidant activity of discretamine and asimilobine has been pointed out with the ORAC (Oxygen Radical Absorbance Capacity) assay with values of 2.10 and 2.09 µmol of trolox equivalents.g-1, respectively [59].
CONCLUSION
Although a wide variety of alkaloids has been identified in species of Annona, most of them were not investigated for biological activities. Within this period (2005-2016), among the 70 alkaloids reported to several species of this genus, less than 30% have some biological activity associated to the isolated compound. Moreover, up to 45% of the references of our research focused on leaves. Seeds and roots are still poorly studied. Comparing the scientific action from the investigation concerning the folk uses, several traditional indications could be related to alkaloids, mainly by in vitro tests. On one hand, antiplasmodial, antileishmanial, anti-anxiety, antidepressant, and anti-ulcer are some biological activities associated to these alkaloids. On the other hand, few investigations were carried out to test possible toxic effects of these compounds. The investigation of alkaloids from Annona species is promising not only for discovering new compounds, but also for finding new other biological activities.
ACKNOWLEDGEMENTS
The authors thank to FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for financial support (07/06511-6) and APMEB postdoctoral fellowship (2013/19398-4). DYACS (308742/2013-3) is fellow researcher of CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and PN has postdoctoral fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).