Functional Performance and Muscle Strength after Enzyme Replacement Therapy in a Patient with Pompe Disease
- 1. Department of Adult Medical Genetics, University of Colorado Denver, USA
- 2. Department of Physical Therapy, University of Colorado Denver, USA
Abstract
Introduction: Late-onset Pompe disease is a rare glycogen storage disorder characterized by skeletal myopathy and respiratory failure. Enzyme replacement therapy using recombinant alglucosidase alfa improves functional and respiratory status across cohorts, but quantitative measurements of an individual’s response to enzyme replacement therapy are poorly characterized. Here we report quantitative improvements of muscle strength and functional performance in a patient with late onset Pompe disease.
Methods: A 23-year-old patient with late-onset Pompe disease underwent functional performance evaluations and muscle strength testing using handheld and electromechanical dynamometry, at baseline and 24 and 64 months after beginning enzyme replacement therapy.
Results: Quantitative improved functional performance and muscle strength were noted by this approach. Proximal muscle groups gained and retained more strength than did distal muscle groups.
Conclusions: This quantitative approach for tracking individual patient improvements shows promise for monitoring responsiveness to enzyme replacement therapy in late-onset Pompe disease.
Keywords
Pompe disease; Myopathy; Functional performance; Enzyme replacement therapy; Dynamometry.
CITATION
Bovet C, Stevens-Lapsley J, Taylor MRG (2017) Functional Performance and Muscle Strength after Enzyme Replacement Therapy in a Patient with Pompe Dis ease. JSM Biochem Mol Biol 4(1): 1019.
ABBREVIATIONS
LOPD: Late-Onset Pompe Disease; ERT: Enzyme Replacement Therapy; GAA: Acid Alpha Glucosidase; CK: Creatine Kinase; TUG: Timed-up-and-Go Test; SCT: Stair Climbing Test
INTRODUCTION
Pompe disease (OMIM # 232300) is a rare lysosomal glycogen storage disorder that causes a broad spectrum of symptoms in infants, children, and adults, with differing clinical phenotypes for infantile and for late-onset (childhood, adolescence, or adulthood) Pompe disease. The late-onset form runs a protracted course, with minimal cardiac involvement and a steady decline in muscle function leading to eventual respiratory failure [1]. Elapsed time since diagnosis may be more predictive of outcomes than age at onset; the disease progresses at a measurable and significant rate per year [2], but which varies from patient to patient. This gradual reduction in muscle function leads to decreased mobility for many patients, with increasing degrees of disability [3] and reduced quality of life [4]. Patients may have increasing ventilator dependence because of diaphragmatic and respiratory muscle involvement, making late onset Pompe disease (LOPD) life-threatening [5,6]. Prognosis has improved markedly since the approval of enzyme replacement therapy (ERT) for adults in 2010, using recombinant human acid α-glucosidase (alglucosidase alfa). ERT use has become widespread in patients with Pompe disease, and has demonstrated effectiveness through gross functional measures such as the 6-minute walk test, pulmonary function testing, and others [7,8]. These measures provide global assessments of function but do not track specific muscle groups.
Measuring muscle function in patients with adult-type Pompe disease has been proposed as a way to determine disease progression and assess the effectiveness of ERT [9]. Several studies have used manual muscle testing, handheld dynamometry, and activity-based tests to determine the effect of Pompe disease on proximal and distal skeletal muscle strength and functional performance [2,10]. Adults with Pompe disease exhibit a limb girdle pattern of weakness, with a 1.6- to 2.3-percentage point decline in muscle strength per year [2]. One group has also developed a scored test to be performed with a trained physical therapist or physician to rate the severity of muscle involvement in adults [11]. However, there is no consensus on the best means to track an individual patient’s response to ERT over time, particularly given that many patients will receive ERT for many years. In the current report, we used muscle strength and functional performance measures to track an individual patient’s response to ERT and evaluate clinical condition over a 5-year period.
CASE PRESENTATION
Study patient and diagnosis
This case study involved a Caucasian female patient diagnosed with Pompe disease at age 20 and treated with ERT beginning in 2010. She presented at age 20 years with reduced mobility and multiple falls, which were not attributable to her comorbid seizure disorder and mild craniosynostosis. Her diagnosis was made through a muscle biopsy and confirmed using a serum acid alpha glucosidase (GAA) activity assay conducted at Duke University (Durham, NC USA). Her GAA activity level was 2.4 pM/ punch/hr, well below the normal reference range of 10.0 – 49.0 pM/punch/hr.
ERT and Treatment Response: ERT with alglucosidase alfa (Lumizyme) at 20mg/kg biweekly dosing was begun at age 23 years. Her creatine kinase (CK) levels were measured prior to beginning treatment, on the first day of treatment, and subsequently at 6-month intervals for 2 years.
Pulmonary Function Testing: The patient underwent clinical pulmonary evaluation with pulmonary function tests (PFTs) at approximately 1, 2 and 4 years after beginning ERT. The same center performed PFT testing and the same standard reference values were used. No baseline PFT values prior to initiation of ERT were available.
Manual Muscle Testing and Functional Evaluation: The patient underwent a full evaluation including manual muscle testing and functional performance just before beginning ERT (September 2010), in October 2012, and again in February 2015. The same trained physical therapist (JS-L) performed all measurements and collected all data.
Functional performance was assessed using the timed-up and-go (TUG) test, stair climbing test (SCT) and the number of completed sit-to-stands (STS) in 30 seconds, as previously described [12,13]. The TUG measures the time it takes a patient to rise from an arm chair (seat height of 46 cm), walk 3 m, turn and return to sitting in the same chair without physical assistance. The SCT measures the time it takes a patient to ascend and descend 12 stairs (17 cm high, 30 cm deep) without the use of the handrail; time to ascend and time to descend were recorded separately. The STS requires patients to perform as many sit-to stands as possible from a standard chair (seat height of 46 cm) within a 30 second interval without upper extremity assistance.
Muscle strength was measured using a hand-held dynamometer (HHD)(Lafayette Manual Muscle Test System, Quebec Canada)in combination with isometric “make” and “break” tests in all individuals, as previously described [13,14]. A single physical therapist, JS-L, performed manual muscle strength tests for each muscle until 2 maximal attempts were within 5% of each other, and the highest value was used in analysis. Plantarflexion strength was assessed through up to 20 heel lifts while standing on one foot and balancing with light touch hand-held assist as necessary. Isometric quadriceps muscle strength was measured using an electromechanical dynamometer (Humac Norm, CSMI, Stoughton MA USA) as described [13]. Up to three maximal voluntary isometric contractions were performed against the dynamometer’s force transducer, and the trial with the largest output was used for data analysis.
Muscle Activation Testing: Quadriceps muscle activation testing was performed using a doublet interpolation test, as described previously [15]. This measure indicates ability to recruit the tested muscle on cue and can elicit the cause of muscle weakness in a particular area. An activation value of 100% represents full voluntary muscle activation, and values less than 100% indicate incomplete motor unit recruitment or decreased discharge rates.
Data Analysis: For functional measures, the same researcher recorded one trial of each measure at each evaluation, and these data were used to calculate percent change from baseline. For manual muscle and isometric strength testing, left and right sides were averaged where applicable, and then used to calculate percent change from baseline. For CK data, r2 was calculated using the standard formula and adjusted for sample size and number of independent variables.
RESULTS
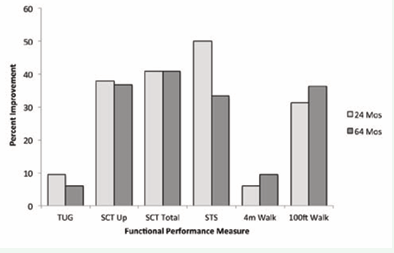
All measures of functional performance improved after treatment with ERT, and showed a sustained response at 64 months after beginning treatment (Figure 1).
Figure 1: Percent improvement in functional performance measures after 24 and 64 months of ERT. Percent change calculated from pre treatment values. TUG, timed up-and-go; SCT, stair-climbing test; STS, sit-to-stand test; reps, repetitions in 30 seconds.
In no areas did the patient’s functional ability decline after 5 years of ERT, in contrast to the natural history of untreated LOPD. While improvement was seen in all measures at 24 months, it is notable that several measures, including total stair-climbing time, 4-meter walk, and 100-foot walk, continued to improve at 64 months as well.
In other areas, the patient had improved at 24 months and then experienced a slight worsening in performance at 64 months, but in all cases functional performance remained above the pre treatment baseline.
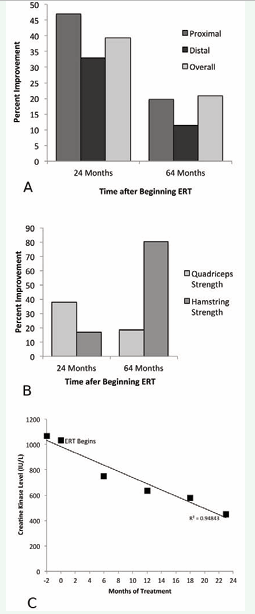
Since LOPD has typically been considered a proximal and axial muscle disorder [16], we categorized manual muscle testing results into proximal and distal muscle groups to examine the effect of ERT by muscle group (Figure 2A). The proximal muscle category included shoulder flexion, shoulder abduction, hip flexion, and hip abduction; the distal muscle category included ankle plantar- and dorsiflexion. At 24 months, both categories showed improvement, and both remained above baseline at 64 months. Notably, proximal muscles showed more improvement over baseline than did distal muscles at both 24 months (46.85% for proximal group vs. 32.97% for distal group) and 64 months (19.72% for proximal group vs. 11.37% for distal group).
Results of quadriceps isometric muscle strength testing showed a similar pattern (Figure 2B), with initial improvement of 37.72% at 24 months, with maintenance of a smaller degree of increased strength relative to baseline (18.53%) at 64 months. Interestingly, hamstring isometric strength showed modest improvement at 24 months but then continued to improve, reaching an 80.16% improvement over baseline by 64 months of ERT. We also examined quadriceps activation, and found only minor changes in muscle activation in response to ERT (Table 1).
|
Table 1: Quadriceps muscle activation 2 and 5 years after ERT. |
||||
|
Pre-Treatment |
24 Months |
Percent Change |
64 Months |
Percent Change |
|
81.2% |
84.6% |
4.2% |
75.9% |
-6.5% |
|
Activation data represent an average of left and right sides. An activation measure of 100% represents the activation reached by healthy controls. |
||||
Our patient’s activation was 81.2% before treatment, and remained near this value through the treatment course.
There was a downward trend in serum CK measured before beginning ERT and every 6 months for 2 years (Figure 2C).
Figure 2: Strength and biochemical changes after beginning ERT. 2A: Strength improvement by proximal and distal muscle groupings as measured by hand held dynamometry testing 2 and 5 years after ERT. Proximal muscle measurements include: shoulder flexion, shoulder abduction, hip flexion and hip abduction. Distal muscle measurements include: ankle dorsiflexion and ankle plantarflexion. Overall figure is an average of all muscle testing data, including elbow flexion and extension and neck flexion as well as the groups listed above. Calculated as the combined average of percent change for each muscle grouping above.2B: Changes in isometric muscle strength using an electromechanical dynamometer at 2 and 5 years after ERT. Values are an average of left and right sides. 2C: Decrease in serum creatine kinase over time. All creatine kinase values measured in IU/L. r2 = 0.95, adjusted r2 = 0.94. Note that ERT was started at the second data point, Month 0.
By 23 months of treatment, serum CK level was at 450 IU/L, less than half of the starting value. Additionally, this decline is highly linear, with r2 = 0.95. These data are consistent with a biochemical response to ERT in addition to the functional and muscle-strength responses detailed above. Finally, the patient’s pulmonary function remained relatively unimpaired at 1, 2 and 4 years after beginning ERT (Table 2).
|
Table 2: Pulmonary function tests at 1, 2 and 4 years after ERT initiation. |
|||
|
Pertinent Values |
Value, 1 Year (% expected) |
Value, 2 Years (% expected) |
Value, 4 Years (% expected) |
|
FVC, liters |
3.40 (96) |
3.58 (102) |
3.44 (98) |
|
FEV1, liters |
2.54 (83) |
3.01 (99) |
2.71 (90) |
|
FEV1/FVC, % |
75% |
84% |
79% |
|
TLC, liters |
4.88 (103) |
4.98 (105) |
5.41 (114) |
|
VC, liters |
3.40 (96) |
3.58 (102) |
3.44 (98) |
|
DLCO* |
18.4 (65) |
21.3 (76) |
22.3 (80) |
|
*mL/mmHg/min, adjusted for Denver, CO USA barometric pressure of 640 mmHg FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; VC, vital capacity; DLCO, diffusing capacity of lungs for carbon monoxide. |
|||
DISCUSSION
Our study examined the effects of long-term ERT on quantitative functional performance and muscle strength, two factors that predict patient outcomes and independence [17,18]. Our data showed measurable improvements at both 2 and 5 years after beginning therapy. In addition, we correlate biochemical response to ERT with improvements in strength and function, and demonstrate the use of handheld dynamometry and functional measures to track patient response to ERT. The patient did not receive regular physical therapy in between evaluations; the goal was to assess strength but not provide an additional intervention besides ERT.
Finally, we demonstrate sustained normal respiratory function in this patient on ERT. There are few published reports of functional performance data in patients with Pompe disease, but functional performance testing has been shown in other populations to identify patients at risk for reduced independence [18,19]. The timed tests used in this investigation correlate with strength [20], functional mobility [18], risk of falls [18], and oxygen consumption [21]. In addition, they test movement sequences the patient is likely to need in activities of daily living, including climbing stairs, walking on level ground, and rising from a chair. The patient’s dramatic improvement in many of these functional performance tests confirms the benefits of ERT on an individual level, and also suggests that combining ERT with rehabilitation focused on functional abilities may be successful in the long term for these patients.
The benefit of ERT in late-onset patients was formally established in a 90 patient 1.5 year multicenter trial [8]. Improvements in mean 6-minute walking and on forced vital capacity on PFTs separated treatment from placebo groups. Patients in this trial were slightly more impaired than our patient. A smaller, prospective report of 8 Italian juvenile cases reported slight improvements in 6-minute walk and forced vital capacity, with substantial variability among patients [22]. Neither trial performed quantitative muscle testing as an outcome parameter.
Our patient demonstrated improved strength after 24 and 64 months of ERT on both manual muscle tests and isometric strength tests. Although these findings were not as consistent as the functional performance data, they nonetheless support a relationship between strength and functional performance. The marked improvement of the hamstring isometric strength over time also indicates these patients may continue to show long term benefits from ERT, an important finding given the lack of studies lasting longer than 5 years. These improvements suggest a more optimistic prognosis for this patient than that found in previous studies [23], although individual prognosis varies and the relatively young age of this patient may have influenced her ability to respond to ERT.
We also found a greater increase in the patient’s proximal muscle strength compared to distal strength at both points of measurement. This trend suggests a greater involvement of proximal muscles in Pompe disease, as these muscles showed greater strength gains following ERT. Proximal weakness such as limb-girdle weakness is often the first presenting symptom of late-onset Pompe disease [24], and is linked to worse outcomes and poor quality of life [17]. This theory is supported by our patient’s lack of change in quadriceps muscle activation, suggesting that the improvements in muscle function were not mediated by improved muscle activation. Other patients with Pompe disease have also undergone single time point, activation testing at our institution (unpublished data), and have shown almost no activation deficits, suggesting that targets for intervention should not be focused on strategies to improve muscle recruitment. Consequently, effective physical therapy could be targeted toward strength and functional performance, with less emphasis on muscle recruitment techniques.
Serum CK levels measured at 6-month intervals also show a definite response to ERT, with 2 high levels measured before beginning ERT and a linear decline thereafter. This drop in enzyme levels is consistent with observations in other studies [25,26] and supports a biochemical response to treatment, which correlates well with the strength and function improvements described here.
Finally, this patient demonstrated sustained relatively normal pulmonary status as evidenced by pulmonary function tests at 1, 2 and 4 years after beginning treatment. Untreated patients are expected to show reduced TLC and VC [27], and may experience a gradual decline in FVC without treatment. Treatment with ERT can stabilize or improve PFT parameters in some patients [28]. This patient’s pulmonary stability on ERT is consistent with results of other studies [29], and suggests that respiratory weakness was not a significant confounding factor in her muscle strength testing. Her data are consistent with relatively stability of PFT values over a nearly 4-year period.
This study’s principal limitation was its design as a case study of a single patient, a constraint imposed by the rarity of LOPD. Yet the combination of muscle strength and functional improvements, along with biochemical changes, measured in this single patient suggest that other individual patients could be tracked for ERT response in a similar fashion. We recognize the potential difficulties of reproducing manual muscle testing results, and have limited the inter-observer variability by ensuring a single trained physical therapist performed all manual muscle testing in this study. Isometric strength measures are easily reproducible [14,30], and the isometric strength data here supported the results of manual muscle testing. The patient was also quite young and the results here may be more favorable due to an intervention at an earlier stage of disease. A recent systematic review of the literature, collating data on over 400 patients, noted improved survival in patients undergoing ERT along with favorable changes in lung function [28]. A prior study demonstrates similar stabilization or improvement in respiratory function and strength after starting ERT, thus providing confidence of an overall group benefit of ERT [31], but does not specifically address strategies to measure improvement in individual patients, such as was demonstrated in this case.
CONCLUSIONS
Our study measured long-term functional and strength gains in a single patient with LOPD. These data indicate feasibility for an individual improvement measure approach in response to ERT and show quantifiable gains in functionality and muscle strength over a 5-year period after beginning ERT. Furthermore, our study indicates that these measures are reliable ways to determine a patient’s clinical status on ERT in the physical therapy or medical office. Most studies have not identified ways to track an individual patient’s response to ERT; these data suggest that HHD matches well with muscle activation results and with functional performance. HHD can be applied quickly in a standard physical therapy office and would be a valuable addition to standard care for adults with LOPD on ERT.
Proximal muscle groups show a disproportionate strength increase after beginning ERT that persists through 64 months of treatment, supporting the concept of Pompe disease as a proximal and axial myopathy. In addition, muscle weakness appears not to be a function of any activation deficit. Future studies may examine strength and functional abilities over periods beyond 5 years of treatment and study the role of rehabilitation in maintaining strength and reducing disability. Our study suggests that successful physical therapy may target proximal and axial muscle groups, as well as functional activities that support patient independence, as this patient was able to improve in these areas using ERT alone. Early detection of proximal muscle weakness may enable more rapid diagnosis, earlier treatment, and perhaps prevent progression of this debilitating disorder.
ACKNOWLEDGEMENTS
We extend our thanks to the patient and family who participated in this study.