Nanomaterials Immobilized Cellulolytic Enzymes and their Industrial Applications: A Literature Review
- 1. Department of Biochemistry, Aligarh Muslim University, India
Abstract
The immobilization of cellulolytic enzymes via nanosupport minimizes the problem of steric hindrances between enzyme and carrier, as it has been frequently observed in case of enzymes immobilized on the surface of bulk supports. Cellulolytic enzymes immobilized on the surface of nanomaterials or entrapped inside polymeric nanospheres showed high catalytic efficiency and yield of immobilization. Nanomaterials bound cellulolytic enzymes were found significantly more stable against heat, pH, storage, operational and several other kinds of denaturants. These immobilized enzyme preparations were found less inhibitory to their inhibitors and products. Immobilized enzymes retained remarkably high activity on repeated uses and the nanocarriers bound cellulolytic enzymes have demonstrated their potential in various fields such as in clarification of juices and wines, extraction of plant oils and coffee, bioconversion of agricultural waste, improving the digestibility of animal feed ingredients. A major application at present is the biodegradation or bioconversion of cellulose to monomeric sugars. Agricultural waste rich in lignocellulosic material has been utilized in the production of large number of industrial products like ethanol, organic acids and other industrially important chemical compounds. Cellobiases immobilized on nanocarriers have also proved their potential as therapeutic agents.
Keywords
Enzymes; Cellulase; Cellobiase; β-1,4-glucosidase; Immobilization; Nanoparticles; Pullulanase; Reusability; Stabilization; Thermostability.
CITATION
Husain Q (2017) Nanomaterials Immobilized Cellulolytic Enzymes and their Industrial Applications: A Literature Review. JSM Biochem Mol Biol 4(3): 1029.
ABBREVIATIONS
3-APTES: 3-aminopropyl- triethoxysilane; CBD: Cellulose Binding Domain; CDI: Carbodiimide; CS: Chitosan; CMC: Car boxymethyl Cellulose; CLEA: Cross-Linked Enzyme Aggregates; EDC: (1-ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Hy drochloride; GA: Glutaraldehyde; NC: Nanocomposite; MNPs: Magnetic Nanoparticles; NMs: Nanomaterials; NPs: Nanoparti cles; MS: Mesoporous Silica; MWCNTs: Multiwalled Carbon Na notubes; PVA: Polyvinyl Alcohol; PMMA: Poly(Methyl Methacr ylate); RSM: Response Surface Methodology
INTRODUCTION
Cellulose is one of the three main components of lignocelluloses. Lignocellulose forms the cell wall and structural tissue of almost all plant systems. It is most abundant regenerative agricultural raw material all over the globe and it is considered as one of most important substrate for the conversion of biomass to biofuels [1]. Cellulose is comprised of hundreds or thousands of glucose molecules and these glucose units are joined together via glucosidic linkages. The first step in the utilization of cellulose into various industrial sectors is its conversion into free glucose. This conversion is brought about by the sequential action of enzymes; these enzymes are known as cellulolytic enzymes [2,3].
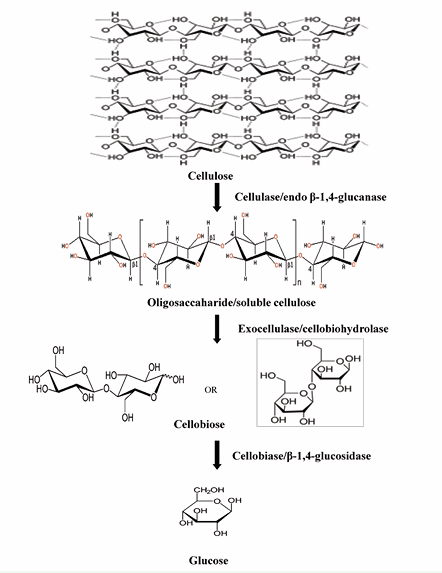
Cellulolytic enzymes are widely present in plants, insects, bacteria and fungi. Both aerobic and anaerobic bacteria are capable of producing cellulolytic enzymes as single enzyme or in the form of cellulosomes, multi-enzyme complexes which are comprised of several cellulolytic enzymes [4,5]. Since past few decades the application of cellulolytic enzymes has attracted a lot of attention due to increasing demand for hydrolyzed cellulose products in various industrial sectors. Numerous kinds of cellulose hydrolyzing enzymes are required for efficient hydrolysis of cellulose and these are mainly three types of synergistically acting enzymes [6,7]. Table 1 and Figure 1 demonstrate enzymatic hydrolysis of cellulose into glucose using various types of cellulolytic enzymes.
Figure 1: Demonstrates enzymatic hydrolysis of cellulose into glucose.
|
Table 1: Summarizes various types of cellulolytic enzymes, their systematic names, EC number, substrates and products. |
||||
|
Name of enzyme |
Systematic name |
EC number |
Substrates |
Product
|
|
Cellulases or Endocellulases |
1,4-β-D-glucan-4-glucano-hydrolase |
EC 3.2.1.4 |
Amorphous cellulose |
Microcyrstalline cellulose, oligo- & soluble polysaccharides |
|
Exocellulases or cellobiohydrolases |
1,4-β-D-glucan cellobio-hydrolase |
EC 3.2.1.91 |
β-glucans. Microcrystalline |
Tetrasaccharide/disaccharides or cellobiose |
|
Cellobiases or β-glucosidases |
1,4-β-D-glucoside-gluco-hydrolasease |
EC 3.2.1.21 |
Cellobiose |
Glucose
|
Endoglucanases hydrolyze glycosidic bonds in the amorphous part of the substrate and produce water soluble oligo and polysaccharides. Cellobiohydrolases cleave crystalline ends of cellulose producing cellobiose and tetrasaccharides. Cellobiase is also known as β-glucosidase which is responsible for the hydrolysis of cellobiose into glucose monomers [8,9].
These enzymes are employed in a large number of industrial processes, such as in cotton and paper manufacturing, food and fuel industry, extraction and clarification of fruits juices, brewery and wine, animal feed additives, detergents, agriculture and research [10,11]. Moreover, cellulolytic enzymes are gaining more and more interest for agriculture, biotechnology and bioenergy uses, especially in the utilization of cellulosic biomass for the production of renewable liquid biofuels like ethanol, butanol or other fermentative products of sugar [4,12]. The use of cellulolytic enzymes during bio-ethanol production from biomass has led to the development of an environmental friendly and sustainable technology. The development of bio-refinery is getting attention because already existing petro-refinery will be exhausted in near future. With such uses, these have the potential to become the largest group of industrially-used enzymes worldwide [13,14].
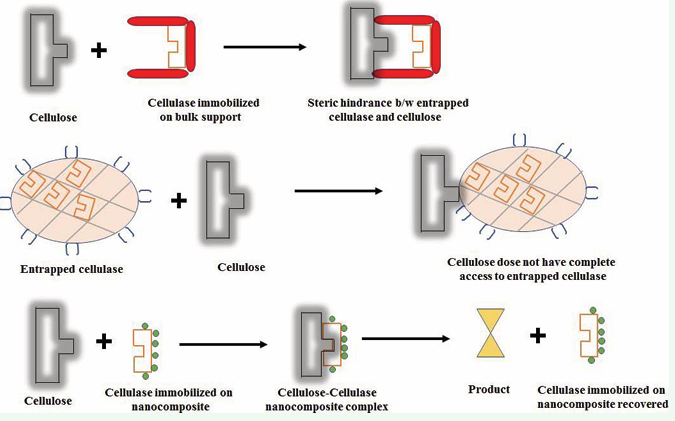
Indeed, the demand for these enzymes is more rapidly growing than ever before, that has focused to the research on stable and reusable cellulolytic enzymes. With the opening of new avenues in biotechnology, the application of cellulolytic enzymes has increased into several other fields such as environmental and analytical chemistry [15,16]. In order to produce soluble sugars from cellulose, cellulolytic enzymes from varying sources such as microbial, plant, insects and recombinant organism have extensively been obtained. The use of cellulolytic enzymes in cellulose based industries has been prevalent since last several decades but the use of soluble enzymes, limits their applications due to certain inherent problems such as high cost, low stability, poor reusability and difficulty of their use in continuous reactors. Therefore, alternative technology is required for economizing system and using enzymes at large scale; enzyme immobilization is a preferred technology to apply enzymes at industrial level in batch processes as well as in continuous reactors [17,18]. Since the advent of enzyme immobilization technology, a large number of methods have been developed to immobilize cellulolytic and other enzymes and many of them have successfully been applied at commercial level [19]. Moreover, the immobilization of cellulolytic enzymes on the surface of bulk support via adsorption or covalent attachment or by entrapment is not suitable particularly for cellulases, a major enzyme required for cellulose hydrolysis. The substrate for cellulase is insoluble and very large macromolecule, and thus it creates steric hindrances in the binding with the active site of enzyme. There are also some more demerits of traditional immobilization of cellulases such as poor yield of immobilization in case of surface binding and problem of accessibility of substrate for entrapped and microencapsulated enzymes [20,21]. Figure 2 represents steric hindrances between active site of cellulase bound to bulk and nanosupport and cellulose substrate. The separation of immobilized enzyme from reaction medium is also problematic due to insoluble nature of substrate, cellulose.
Figure 2: Schematic representation of steric hindrance between active site of cellulase bound to bulk and nanosupport and cellulose substrate.
Nanoparticles (NPs) have unique properties such as large surface area and their surfaces can be easily modified for the purpose of enzyme immobilization. NP supports have demonstrated their potential in immobilizing enzymes in high yield and the presence of NP-support with enzymes poses least chances of steric hindrances [22,23]. In one of the study, the author’s group has investigated the immobilization of β-galactosidase on native and nano ZnO, the immobilization yield and stability of this enzyme on nano ZnO was remarkably higher compared to immobilization obtained on native ZnO [24]. Enzymes immobilized on nanosupprts have demonstrated their applications in analytical, industrial, environmental and medical fields [25,26]. In addition to properties of nanomaterial, the magnetic nanosupports has an additional advantage, the magnetic supports can simply be removed from the reaction mixture by applying magnetic field after the reaction is over. [27,28]. MNPs based supports have proved their potential in the immobilization of diverse types of cellulolytic enzymes [29,30]. In addition, with the advent of nanobiotechnology, nanocarriers having ideal physicochemical properties, such as mass transfer resistance, specific surface area, modifiable surfaces and high enzyme binding, can be employed as effective and useful nanoscaffolds for enzyme immobilization.
In this manuscript an effort has been carried out to review the latest work on the use of varying types of NMs for the immobilization of cellulolytic enzymes. Each immobilized enzyme preparation has been critically evaluated for binding efficiency and yield of immobilization. The stabilities of these immobilized cellulolytic enzymes have been investigated against a number of denaturants, those influencing the activities of such enzymes. The reusability and operational stability of such immobilized enzymes have been discussed. A comparative analysis of various immobilized cellulases with their soluble counterparts has been done. In this manuscript NMs-immobilized cellulolytic enzymes; cellulases, cellobiohydrolases and cellobiases or β-1,4 glucosidases have extensively been discussed.
Cellulase
Cellulase (EC 3.2.1.4) catalyzes hydrolysis of cellulose and other related polysaccharides. It hydrolyzes β-1,4-D-glycosidic bonds in cellulose, hemicellulose, lichenin and cereal β-D glucans. This enzyme is produced by various bacteria, fungi and protozoans. Cellulases hydrolyze cellulose into monosaccharides; simple sugars such as β-glucose, shorter polysaccharides and oligosaccharides [10,11]. There are various synonyms, derivatives and specific enzymes associated to the name “cellulase” include endo-1,4-β-D-glucanase, β-1,4-glucanase, β-1,4-endoglucan hydrolase and cellulase A etc. Table 2 depicts cellulases from various sources immobilized on different nanosupports and their improved properties/applications.
|
Table 2: MNPs immobilized cellulases, their mode of immobilization and improved properties. |
||||
|
Name of enzyme |
Name of support |
Mode of immobile zation |
Propert/properties Enhanced |
Reference/s |
|
Cellulase |
CS-MNPs |
Covalent Binding |
High heat & storage stability |
38 |
|
Cellulase |
CDI- Fe3O4 MNPs |
Covalent binding |
Better catalytic efficiency, stability and reusability |
32-34 |
|
Cellulase |
Supermagnetic MNPs & GA |
Covalent binding |
High affinity and activity in a broad range of pH & temperature; effectively hydrolyzed steam-exploded corn stalks |
40 |
|
Cellulase |
PVA)/Fe2O3 |
Adsorption |
Good reusability |
36,37 |
|
Cellulase |
CS-Fe3O4 & GA |
Covalent binding
|
Very high loading, stabilityover a borad range of pH & temperature, & reusability |
50 |
|
Cellulose |
Functionalized MWCNTs |
Adsorption |
High binding efficiency & loading, reusability |
53 |
|
Cellulase from T. reesei |
Activated magnetic support |
Covalent binding |
Km decreased, High temperature-optima, good reusability |
52 |
|
Cellulase |
Molecular imprinted supermagnetic Fe3O4@SiO2 NPs |
Adsorption |
Higher catalytic efficiency & temperature optima, better thermal stability |
54 |
|
Cellulase |
Functionalized magnetic nanosphere, APTES & GA |
Entrapment |
Very high loading, stability and reusability, effective use in biofuel production |
56,57 |
|
Cellulase from A. niger
|
β-cylcodextrin-MNPs via silani-zation & reductive amidation |
Covalent binding |
Increased continuous hydrolysis of raw straw, high biding efficiency, stability & reusability |
58 |
|
Cellulase A. fumigates |
MnO2 NPs |
Adsorption |
High stability in a broad range of pH & temperatures, high heat stability, reusability & cellulose hydrolysis |
59 |
|
Cellulase |
AgNPs |
Adsorption |
Quite efficient in cellulose hydrolysis |
60 |
|
Cellulase |
AgNPs & AuNPs |
Adsorption |
High heat stability & reusability |
61 |
|
Cellulase |
CLEA-amine functionalized Fe3O4-@silica coreshell MNPs |
Covalent binding |
Improved heat & operational stability, & reusability |
55 |
|
Cellulase A. niger |
TiO2 NPs & 3-APTES |
Adsorption & Covalent binding |
Covalently bound enzyme found superior in stability & reusability than adsorbed enzyme |
51 |
|
Cellulase |
Fe3O4@SiO2 NPs |
Adsorption |
High immobilization yield, half life & reusability |
68 |
|
Cellulase |
Vinyl functionalized cubic MS |
Adsorption |
NPs support far superior in activity, stability & reusability |
67 |
|
T. reesi cellulase |
CS-MNPs & GA |
Covalent binding |
Better thermal & storage stability & very high CMC hydrolyzing reusability |
65 |
|
Cellulase |
MNPs |
Adsorption |
Effective bioethanol production from Sesbania aculeate biomass |
66 |
|
Cellulase |
Nano-PEGylated GO |
Covalent binding |
Efficiently hydrolyzed raw straw slurry |
69 |
|
Cellulase |
AF-CoFe2O4-MNPs, EDS & NHS |
Covalent binding |
Superior thermal stability & good reusability |
63 |
|
Cellulase |
Attapulgite@CS (ATP@CS) NC & GA |
Covalent binding |
High pH & heat stability & reusability, effective hydrolysis of wheat straw |
64 |
|
Cellulase |
Silica-coated MNPs |
Adsorption |
High colloidal stability, loading & reusability |
62 |
|
Cellulase |
CS-Fe3O4 |
Covalent binding |
Improved stability and reusability |
72 |
Phadtare and coworkers [31], carried out synthesis of polyurethane microsphere-AuNP “core shell” structures and this nanocomposite (NC) was used for the immobilization of endoglucanase. The attachment of AuNPs on the surface of polymer microspheres occurs via interaction of nitrogens in the polymer with NPs. Thus endoglucanase binds to the AuNPs decorating polyurethane microspheres, leading to a highly stable biocatalyst with excellent reusability. The immobilized cellulase maintained its activity and showed improved thermal stability in comparision to free enzyme. The high surface area of the host AuNPs made immobilized enzyme “quasi free”, while at the same time maintaining benefit of immobilization such as ease of reuse and enhanced heat stability etc. Some investigators have used carbodiimide (CDI) activated Fe3 O4 MNPs for covalent immobilization of cellulase. The maximum binding was 90% at loadings of 1-2 mg enzyme and enzyme-to-support saturation point took place at a weight ratio of 0.02. Immobilzed enzyme showed optimum-temperature at 50°C and demonstrated very high heat and storage stability. The ionic forces between enzyme and support resulted in the shifting of pH-optimum from 4.0 to 5.0 [32,33]. In a further study, Jordan and Theegala [34] investigated the immobilization of cellulase on CDI activated MNPs (diameter of 13 nm). The immobilized enzyme retained 30.2% of the initial activity and was successfully reused 6-times by loosing some of its activity after each reuse. There was a loss of 47.5% activity after initial hydrolysis reaction. A protein assay showed varying degrees of detachment of enzyme after each repeated use. However, immobilized enzymes had higher stability than the free enzyme and bound enzyme retained 57.9% of its activity, which was slightly better than the 51.2% retained by free enzyme. NPs bound enzyme produced 76.8% of total reducing sugars after 96 h that produced by a single reaction with free enzyme.
A new soluble and easily separable nanocarrier, polyvinyl alcohol (PVA)/Fe2 O3 NPs has been used to immobilize cellulase. The activity retention of immobilized enzyme was 42% in a buffer of pH 6.0. The immobilized cellulase preserved 50% activity after 5-repeated uses [35]. In a further study same group have used PVA/Fe2 O3 NPs bound cellulase along with wet ball milling to degrade microcrystalline cellulose and it has successfully produced 1.89 mg mL−1 glucose, at least 3-times than the sum of individual yield. The immobilized cellulase retained 40% of its original activity after 4-recycles use. Here, the results showed that the use of immobilized of cellulase alongwith wet ball milling has high potential in improving the efiiciency of cellulose hydrolysis [36]. Ho et al. [37], developed a one-step procedure for constructing cellulase-immobilized NPs that made up of well-defined poly(methyl methacrylate) (PMMA) cores and cellulose shells. The immobilized cellulase demonstrated a wide working pH range and better thermal stability. Thus, this method has opened new avenues for the immobilization of heat stable enzyme to form nanoenzyme particles. Zhou [38], demonstrated the immobilization of cellulase on chitosan (CS)-coated MNPs modified by α-ketoglutaric acid. The immobilized cellulase showed a broader pH range of high activity and lower loss of activity than the soluble enzyme. The optimal-temperature for immobilized cellulase was 50°C while the free enzyme had at 40°C. The immobilized cellulase exhibited high thermal and storage stability.
Superparamagnetic NPs was used for the immobilization of cellulase via ionic bonds. Bound enzyme showed 95% immobilization efficiency and adsorption capacity of 31 mg g-1 NPs. The maximal enzyme activity was determined using carboxymethyl cellulose (CMC) as a substrate and was 0.1 U, μmol min-1 mL-1. The stability and activity of the cellulase were enhanced after immobilization. It is evident from the findings of the work that immobilized enzyme has successfully been employed in various application in the broad ranges of pH and temperatures [39]. Xu et al. [40], reported covalent immobilization of cellulase on MNPs via glutaraldehyde (GA). The immobilized enzyme showed remarkably high activity in the broad ranges of pH and temperature and storage stability. The immobilized enzymes exhibited higher affinity towards its substrate, cellulosic compounds, as it is confirmed by its lower Km compared to soluble enzyme. The immobilized cellulase hydrolyzed more efficiently steam-exploded corn stalks compared to bleached sulfite bagasse pulp. Hung et al. [41], developed a method to immobilize cellulase using an electrospun polyacrylonitrile nanofibrous membrane as support. The immobilized cellulase showed a protein loading of 30 mg g-1support and a specific activity of 3.2 U mg-1 protein. The bound cellulase exhibited improved stability against pH and heat exposure and was used to hydrolyze cell wall of microalgae for production of reducing sugars. Analyses using response surface methodology (RSM) showed that the hydrolysis yield was affected by the temperature, pH and substrate/cellulase mass ratio, and a hydrolysis yield of 60.86% was at 47.85°C, pH 5.82, and a substrate/cellulase mass ratio of 40 g substrate g-1 cellulase. This result demonstrated that the proposed scheme for the cellulase immobilization has a great future in the production of reducing sugar. Lupoi and Smith [42], demonstrated the adsorption of cellulase on the 40 nm silica NPs and compared hydrolysis of microcrystalline cellulose with free enzyme. The immobilized cellulase produced 1.6 times (p=0.01) more glucose than the free enzyme in 96 h at pH 4.8 and 35°C. There was no significant accumulation (<250 µg) of soluble cellooligomers in either the solution or immobilized enzyme reactions. It revealed that immobilized enzyme demonstrated production of more glucose compared to free enzyme.
Chang et al. [43], studied the synthesis of two mesoporous silica (MS) NPs having different particle size, pore size and surface area. The cellulase was immobilized on the nanosupports by two different methods; physical adsorption and chemical binding. The findings of this work revealed that the cellulase attached covalently to MSNPs containing carboxyl groups and a large pore size was quite effective in the hydrolysis of cellulose into glucose. The immobilized cellulase showed significantly high stability and yield of cellulose conversion, 80%. Cellulase immobilized on magnetic beads was successfully used in a broad range of temperature and pH. Immobilized enzyme retained high storage stability and was easily reused and recovered from the reaction mixture. The native celluclast BG cellulase complex comprised of varying enzymes which degraded native celluloses. This enzyme complex has been covered by a few nanometers thick, polymer layer, in order to improve its stability. It has been demonstrated that the polymer layer around the enzyme molecules did not alter hydrolysis of crystalline cellulose. The stability of the enzyme NPs conjugate was markedly increased compared to free enzyme. The bound enzyme retained about 10-times higher activity in a temperature range of 20 and 37°C compared to soluble enzyme. The pretreated enzyme complex maintained about 50% activity at 80°C for 12 h, whereas the soluble cellulase lost its full activity within 6 h. The activity of layered enzyme was significantly more stable over a broad range of pH 1.5-12 [44].
Amine and aldehyde functionalized Fe2 O3 MNPs supports were considered for the immobilization of cellulases. These immobilized cellulases were easily recycled and reused in fermentative biofuel process [45]. Cellulase from Trichoderma viride was coupled to polystyrene nanospheres and the immobilized enzyme was employed for the hydrolysis of celluloses from purified and natural sources. Immobilized cellulase showed higher affinity for its substrates; microcrystalline and natural celluloses obtained from thickened walls of cultured wood cells compared to free enzyme. These finding demonstrated that NP bound cellulases demonstrated improved catalytic efficiency on physically intractable substrates in biofuels production [46]. Cho et al. [47], used AuNP and Au-MSNP for the immobilization of 3-cysteine-tagged cellulases and employed the immobilized enzyme preparations for the hydrolysis of cellulose. The stabilities, activities and reusabilities of these co-immobilized enzymes preparations were remarkably higher than the mixtures of free cellulases. In a further study cellulase was mmobilized on silica via l-cysteine functionalized by AuNPs and the immobilized enzyme was used for continuous production of glucose from waste bamboo chopsticks powder in a medium of pH 8.0 and at 50°C. A 4-day reaction with an initial 0.3 g L-¹ waste bamboo chopsticks powder, a feed containing 0.2 g L?¹ waste bamboo chopsticks powder at a continuous feed and draw rate of 0.5 mL min?¹ and an enzyme loading of 40 mg cellulose g-1silica, has 72.0-76.6% conversion rates on repeated hydrolysis that corresponded to a total production of 630.5-671.2 mg glucose and was much better than batch hydrolysis. At higher enzyme loading 117 mg cellulose g-1 silica, higher initial concentration (0.5 g L-1), and higher feed concentration (0.42 g L-1) the conversion rate increases to 82.9% and a total amount of 1418 mg glucose. The immobilized cellulase was recovered simply by filtration and used repeatedly at least 6-times during a time period of more than 3 months with the obtained activity about the same as or superior than earlier reported [48].
Gokhale et al. [49], developed pH tunable, temperature sensitive magnetoresponsive graphene-based nano-biocarriers and used them for the immobilization of cellulase. The supramolecular assembly of oppositely charged quenched polyelectrolytes and maghemite-magnetite NPs on 2-D graphene supports followed by covalent immobilization of cellulase exhibited a marked improvement in bio-receptivity of graphene supports. The incorporation of MNPs has provided an oppotunity of recovery of the enzyme over repeated applications. Cellulase was covalently attached to CS coated Fe3 O4 MNPs support by GA. The amount of enzyme bound to MNPs was 112.3 mg g-1. The immobilized cellulase had higher operational stability than the soluble enzyme over the broad spectrum of temperature and pH and retained good activity on its repeated uses [50]. T. reesei cellulase covalently bound to the activated MNPs maintained nearly 94% of its activity. Immobilized enzyme showed same pH-optima, 4.0, higher temperature-optima at 60°C and lower Km value compared to native enzyme. The immobilized enzyme retained 50% of its original activity after 5-reuses and hydrolyzed higher quantity of pretreated hemp hurd biomass than the soluble enzyme. The immobilized enzyme found much superior in terms of reusability and storage stability than the free enzyme [51].
Aspergillus niger cellulase was immobilized on TiO2 NPs via two different methods; adsorption and covalent attachment. For covalent immobilization the TiO2 NPs were functionalized by 3-APTES. The adsorbed and covalently immobilized enzyme preparations retained 76% and 93% of the initial activity, respectively. The catalytic efficiency Vmax /Km increased from 0.4 to 4.0, 10-fold after covalent attachment, whereas there was only a slight increase in Vmax /Km in case of adsorption method from 0.4 to 1.2, 3-fold. The reusability and operational stability results exhibited that the covalent immobilization maintained higher activity and heat stability compared to physically adsorbed enzyme [52]. Mubarak et al. [53], described the immobilization of cellulase onto functionalized multiwalled carbon nanotubes (MWCNTs) via physical adsorption in order to yield a stable and ease of separate enzyme. The efficiency of enzyme immobilization reaches an optimal value when 4 mg ml-1 enzyme was used in which approximately 97% enzyme loading was noticed. The immobilized enzyme showed its maximum activity at pH 5.0 and 50°C. The findings of the work have shown that MWCNTs-cellulase composite retained 52% of its original activity after 6-repeated CMC analysis. This feature is beneficial to the industrial applications because of its potential to be easily separated from its product at the end of the reaction, reuse as multiple times.
The molecular imprinted supermagnetic Fe3 O4 @SiO2 NPs support has been employed for fast and specific adsorption of cellulase. The adsorption yield was 95% and t1/2 of the immobilized cellulase was 3.3-fold of the soluble-enzyme at 70°C. The immobilized cellulase exhibited identical pH-optima, higher temperature-optima, heat stability and catalytic efficiency than the free enzyme. The obtained results have demonstrated that the immobilized cellulase can be successfully employed in various fields such as in the production of bioethanol, paper and pulp, and pharmaceutical industry [54]. The cellulase cocktail in the form of cross-linked enzyme aggregates (CLEA) was immobilized onto NH2-Fe3 O4 @silica core-shell MNPs. There was no change in temperature-optimum of the immobilized enzyme while the pH optimum was slightly shifted towards acidic side. The immobilized enzyme demonstrated greater carboxymethyl cellulase (CMCase) activity at higher side of pH and temperature-optima compared to free enzyme. Cellulase CLEA-MNP maintained nearly 45% activity at pH higher than 4.8 whereas the free cellulase completely lost its activity. Immobilized cellulase maintained nearly 65% activity at higher temperatures up to 80°C and showed an improvement in heat and operational stability. The bound enzyme exhibited 30% CMCase activity after 6-repeated uses [55]. Three different kinds of functionalized magnetic nanospheres were prepared by co-condensation of tetraethylorthosilicate with different amino-silanes: 3-(2-aminoethylamino propyl)-triethoxysilane (AEAPTES), 3-(2-aminoethylamino propyl)-trimethoxysilane (AEAPTMES) and 3-aminopropyltriethoxysilane (APTES) and used for immobilization of cellulase. Cellulase bound to functionalized magnetic nanospheres with core-shell morphologies exhibited higher capacity for compared to enzyme immobilized on non-functionalized magnetic nanospheres. With the increase in the surface charge of functionalized magnetic nanospheres resulted into higher immobilization of cellulase immobilization. AEAPTMES functionalized magnetic nanospheres demonstrated 87% activity recovery and the optimum amount of immobilized cellulase was 112 mg g-1 support at concentration of initial cellulase of 8 mg mL-1. Cellulase immobilized on AEAPTMES functionalized magnetic nanospheres showed higher temperature and pH stability compared to other immobilized preparations and free cellulases [56]. Cellulase was covalently immobilized on functionalized magnetic silica nanospheres via GA crosslinking. The immobilized cellulase exhibited remarkably high heat and pH stability compared to free enzyme. Moreover, the enzyme immobilized via cross-linking agent showed greater binding of its protein and operational stability. The amount of immobilized cellulase with the cross-linking agent was 92 mg g-1 support. The immobilized cellulase retained 85.5% its activity even after 10 repeated uses [57].
Aspergillus niger cellulase was immobilized on β-cyclodextrin coupled MNPs via silanization and reductive amidation. Immobilized cellulase showed 90% of the original activity. The rate of hydrolysis of rice straw by immobilized enzyme was raised from 1.629 to 2.739 g h−1 L−1 in the presence of an ionic liquid. Magnetized cellulase can be recycled by magnetic field more easily than free enzyme and immobilized enzyme exhibited 85% activity in presence of high concentration of glucose, 15 g L−1. The findings of the work have revealed that the greater amount of rice straw was hydrolyzed by immobilized cellulase compared to free enzyme. The immobilized cellulase showed no change in its activity even after 16 repeated uses. The amount of glucose harvested by bound enzyme was 20-times greater than the glucose produced by native enzyme [58]. Cherian et al. [59], described immobilization of cellulase from Aspergillus fumigates JCF onto MnO2 NPs, which improved the activity of cellulase and offer a superior support. The maximum cellulase binding efficiency was 75%. The immobilized cellulase retained higher stability at operational pH and temperature compared to native enzyme. It was found that cellulase immobilized on MnO2 NPs has been used to hydrolyze cellulosic substrates over a broad range of temperature and pH. MnO2 NPs bound cellulase maintained very high activity on repeated uses. Herein findings showed that cellulase immobilized on MnO2 NPs was highly effective in the hydrolysis of cellulose. The cellulase immobilized onto the surface of AgNPs synthesized via reduction of silver nitrate by extracts of 5 medicinal plants (Mentha arvensis var. piperascens, Buddleja officinalis Maximowicz, Epimedium koreanum Nakai, Artemisia messer-schmidtiana Besser, and Magnolia kobus) was quite useful in the degradation of cellulose; these enzyme preparations have a lot of scope in diverse industrial applications [60]. The cellulase assisted synthesized AgNPs and AuNPs were further employed as immobilization matrix for the same enzyme. Thermal stability analysis exhibited that the NPs immobilized cellulase retained 77-80% activity as compared to free enzyme. The immobilized cellulase was repeatedly used 6 times without a significant loss in its activity [61]. In a further study, Roth et al. [62], obatined a simple and fast electrostatic assembly of cellulase on low-priced silica-coated MNPs, which showed strong enzyme bonding and very high colloidal stability. The high cellulose loading, 0.43 g g−1, without leaching of biocatalyst and high recovery yields, 75 %, after 10 repeated uses were obtained. The important finding of this work was the retention of remarkably high catalytic activity and stability.
Amine functionalized cobalt ferrite (AF-CoFe2 O4 ) MNPs were used for immobilization of cellulase via 1-ethyl-3-[3 dimethylaminopropyl] carbodiimide hydrochloride (EDS) and N-hydroxy-succinimide (NHS) coupling reaction. The immobilized cellulase had superior thermal stability than free cellulase, which might be due to covalent interaction between cellulase and AF-CoFe2 O4 surface. The immobilized cellulase also showed good reusability after recovery. Therefore, AF-CoFe2 O4 MNPs appeared as a useful support for enzyme immobilization [63]. An attapulgite@CS (ATP@CS) NC was prepared by CS coating onto naturally needle-like nanoscale attapulgite clay. Cellulase was covalently immobilized on ATP@CS support via GA and compared with ATP-APTES support. The amount of cellulase on the ATP@ CS and ATP-APTES was 88.3 and 76.5 mg g−1, respectively. The ATP@CS immobilized cellulase exhibited higher pH and heat stability as well as good reusability compared to the ATP-APTES immobilized cellulase and the free cellulase. The immobilized cellulase preparations were also utilized to hydrolyze wheat straw in order to understand its practical applications [64]. Sánchez-Ramírez et al. [65], covalently immobilized T. reesei cellulase on CS-coated MNPs using GA. The average diameter of MNPs before and after enzyme immobilization was about 8 and 10 nm, respectively. The immobilized enzyme retained about 37% of its initial activity and also showed better heat and storage stability than free enzyme. Immobilized cellulase retained about 80% of its activity after 15 repeated uses in CMC hydrolysis and was easily separated by applying an external magnetic field. However, in this reaction, Km was increased 8-times. The immobilized enzyme was able to hydrolyze lignocellulosic material from Agave atrovirens leaves with yield close to the amount detected with free enzyme and it was reused in vegetal material conversion up to 4 cycles by maintaining 50% of its original activity. It provides an opportunity to reduce the enzyme consumption during lignocellulosic material saccharification for bioethanol production. Cellulase bound MNPs was used as nanobiocatalyst for the hydrolysis of Sesbania aculeate biomass. The maximum bioethanol yield of 5.31 g l-1 was obtained using Sesbania aculeate biomass hydrolysate under optimal conditions. The produced bioethanol was confirmed by GC-MS analysis [66]. Magnetic Fe3 O4 @SiO2 NPs were prepared with molecular imprinting method using cellulase as the template and the surface of NPs was chemically modified with arginine. The modified NPs were employed as a carrier for specific and selective immobilization of cellulase. The immobilization yield and efficiency obtained were more than 70% after optimization. Immobilized cellulase exhibited identical pH and temperature optima as shown by soluble enzyme. The half-life of the immobilized cellulase was 2-fold higher than the free enzyme at 50oC. The immobilized enzyme still retained 77% of the original activity after 7 repeated uses. These findings have demonstrated that the obtained imprinted NPs have the potential industrial applications for the purification/immobilization of enzymes [67].
Cellulase was immobilized on silica materials having cubic mesostructured with two different sizes; micron size around 8 µm and nano size approximate 300 nm. NPs bound enzyme exhibited a marked improvement in its activity, stability and reusability compared to the cellulase bound to micron sized silica. These workers reported the superiority of vinyl functionalized NPs support over the common large particle cubic MS materials [68]. In order to immobilize cellulase for utilizing it in lignocellulosic biorefinery a functional nanoscale carrier, PEGylated graphene oxide (GO) composite was successfully fabricated by chemical binding of 4-arm-PEG-NH2 and GO. The PEGylated GO-cellulase retained 61% of the initial activity in 25% (w/v) 1-butyl-3 methylimidazolium chloride ([Bmim][Cl]) while free cellulase retained only 2% activity under identical treatment. The ionic liquid (IL) stability was enhanced more than 30 times. After treating rice straw with [Bmim][Cl] and dilution to a final IL concentration of 15% (w/v), the slurry was directly hydrolyzed using PEGylated GO-cellulase without IL removing and a high hydrolysis rate of 87% was achieved [69].
Biofluorescent nanogels were used for the immobilization of cellulase. The biocatalytic activity of cellulase-conjugated nanogels was simply tuned by controlling their degree of cross linking. The secondary structure of the immobilized cellulase was changed with respect to α-helix contents as analysed by circular dichroism. The fluorescence resonance energy transfer based study further revealed that nanogels with lower cross linking degree enable higher substrate transport rate, thus it make easy access to the active site of the enzyme. The biohybrid nanogels bound cellulase demonstrated significantly improved stability compared to soluble enzyme. The obtained method allows precise control of the enzyme activity and improvement in resistance against harsh environmental conditions, chaotropic agents, and organic solvents. The functional biohybrid nanogel immobilized cellulase with improved stability is a potential candidate for biomass conversion [70]. Fruit and vegetable waste (FVW) is a big disposal problem in developing countries. Enzymatic method is a useful method for the treatment of FVW. Magnetic molecular imprinting immobilized cellulase was obtained by using magnetic modified CS and Fe3 O4 . The immobilized enzyme showed improved stability and reusability. The bound cellulase maintained almost all its activity even after 60 days storage whereas the soluble enzymes lost its full activity within 30 days. The findings of the work exhibited that the immobilized enzyme developed excellent capacity and five anthocyanins were collected [71]. Selvam et al. [72], immobilized cellulase onto Fe3 O4 MNPs via GA. Using RSM and Box-Behnken design, the variables such as the concentrations of MNPs, GA and enzyme, and cross linking time were optimized. The Box-Behnken design analysis showed a reasonable adjustment of the quadratic model with the experimental data. The immobilized cellulase exhibited enhanced activity and stability against various types of denaturants compared its free form.
The polycatalytic clusters of cellulases on colloidal polymers NPs exhibited an increased rate of hydrolytic reactions on cellulose, but it was noticed mainly at relatively low cellulase-to cellulose ratios. An enhancement in efficiency was mainly due to increased local concentrations of cellulases on the scaffolds and their polyvalent interactions with cellulose [73]. Furthermore, a same group has investigated that these polycatalytic cellulase complexes showed increased binding affinity for the substrate but due to their larger size, these complexes were unable to access to the internal surfaces of cellulose. The bound enzyme exhibited significantly lower binding capacity compared to unbound enzyme. Analysis of released soluble sugars demonstrated that the formation of complexes may promote the prospect of having consistent, multiple attacks on cellulose substrate. The polycatalytic complexes remained compactly bound on the surface with very limited mobility due to their strong, multivalent binding to cellulose. Thus, the overall performance of polycatalytic complexes is limited by its substrate accessibility as well as mobility on the substrate surface [74].
MgNPs supplemented cellulase from a psychrophilic strain of Bacillus subtilis was immobilized on GO nanosupport via GA. The immobilized enzyme exhibited 2.98 folds increase in its activity at 8°C and more than 3.5 folds activity increment at 90°C. The MgNPs-cellulase on GO showed a decrease in Km by 6.7 folds at 8°C and 34 folds at 90°C. Immobilized cellulase demonstrated 5 fold and 4.7 fold increases in Vmax at 8°C and 90°C, respectively than the free enzyme. GO-MgNPs-cellulase had half life and Ed increased by 72.5 fold and 2.48 fold, respectively at 90°C; and 41.6 fold and 2.19 fold, respectively at 8°C compared to soluble enzyme. GO-MgNPs-cellulase retained almost all activity even after 12 repeated uses and showed storage stability at 4°C for more than 120 days. This NP assisted immobilization of cellulase has a great future in bioprocessing industries which require functioning at extreme conditions of temperature [75]. The cellulase was immobilized on MNPs encapsulated in polymeric nanospheres. The polymer nanospheres containing encapsulated MNPs showed superparamagnetic behavior and intensity average diameter about 150 nm. Immobilized cellulase showed very high thermo- stability and retained 69% of its original activity even after 8-repeated uses. The magnetic support bound enzyme was quite easy to recollect from the reaction mixture [76]. A novel magnetic cross-linked cellulase aggregate was developed and applied for biomass conversion. The crosslinked aggregate was purified and used to immobilize cellulase in a single operation and was combined with MNPs. The immobilized cellulase exhibited higher activity in a broad range of temperature and pH compared to its soluble form. The immobilized cellulase retained 74% of CMC hydrolyzing activity after 6-repeated uses. Moreover, the immobilized cellulase was successful in hydrolyzing bamboo biomass with a yield of 21% and was re-used in biomass conversion 4-times with retention of 38% activity; it suggested that immobilized enzyme has good potential in biomass conversion [77].
In a recent study cellulase was linked to the magnetic MgO Fe3 O4 via xylan aldehyde. In order to improve the hydrolysis process, MgO was blended with Fe3 O4 and the obtained complex showed a marked improvement in immobilization yield, activity recovery and hydrolysis of cellulose. Addition of xylan aldehyde as a linking molecule enhanced the enzyme binding onto metal NPs. Here findings showed a reduction in crystallinity of cellulose which was due to enzymatic hydrolysis of cellulose. The obtained glucose yield was 91% of theoretical maximum under experimental conditions. The enhancement in hydrolysis was as result of degradation of large molecules into well accessible substrate assisted by metal oxides [78]. Ahmad et al. [79], investigated adsorption of cellulase on PAA nanogel, having diameter around 150 nm and enriched with carboxyl groups. The surface charge of PAA nanogel depended on the pH of environment and affected the adsorption of cellulase. The immobilized cellulase showed enhanced heat stability, more active in acidic buffers and very high hydrolytic activity, above 75%. The PAA nanogel bound cellulase was easily recovered via centrifugation during its repeated uses. The cellulase was immobilized on two nanosupports; magnetic and silica NPs with immobilization efficiency of 85% and 76%, respectively. The nanobiocatalysts exhibited increase in Vmax , temperature optimum, pH and thermal stability as compared to soluble enzyme. The immobilized enzyme preparations were efficiently used repeatedly 5-times and were quite stable in 1-ethyl-3 methylimidazoliumacetate (EMIM)(Ac), an ionic liquid. These were used as green solvents to dissolve lignocellulosic biomass in order to obtain better saccharification. The cellulase immobilized on MNPs was used for in situ saccharification of (EMIM)(Ac) pretreated sugarcane bagasse and wheat straw for 2-cycles. The high hydrolysis yields (∼89%) obtained in this one-pot process coupled to ionic liquid stability and recycled use of immobilized cellulase, demonstrated its significance in biorefineries [80]. Manasa et al. [81], immobilized covalently cellulase on the activated ferrite NPs via GA. The enzymatic hydrolysis was performed on ultrasound-assisted alkaline pretreated sunn hemp biomass by soluble and immobilized cellulase. The immobilized cellulase showed optimum pH and temperature at pH 5 and 60°C and retained its full activity even after 3-repeated uses at 60°C. The immobilized enzyme exhibited 53% hydrolysis yield on pretreated sunn hemp biomass. Cellulase was immobilized directly and ultrasonic activated MNPs. The enzyme immobilized via ultrasonic activation showed 3.6 fold more catalytic activity compared to control. There were conformational changes in free cellulase and cellulase@MNPs before and after sonication that resulted into enhancement in its catalytic acivity [82].
Numerous commercial available were independently immobilized on distinct nanosupports using cellulases easy, efficient, safe and cheap adsorption/covalent attachment methods [62-64]. Most of the immobilized cellulases showed increased stability against various kinds of denaturants and on storage. These obtained preparations were repeatedly used successfully in batch processes as well as in continuous reactors. Thus, the immobilization process using nanosupports not only allows the efficient hydrolysis via creation of a cellulosome-like structure, but it also promotes enzyme recycling, which made it an extremely promising method from an industrial point of view [50,55,72]. The immobilized cellulases maintained higher enzyme activities using a wide range of substrates: amorphous cellulose, microcrystalline cellulose and lignocellulosic biomass [46,48,57,77]. The successful hydrolysis of such substrates strongly suggests that these immobilization methods can be applied to a wide range of cellulases.
Cellobiases/ β -Glucosidase
β-1,4 glucosidase (EC 3.2.1.21) catalyzes hydrolysis of β-1,4 bonds present between two glucose or glucose-substituted molecules i.e., the disaccharide cellobiose. It is also known as cellobiase. It is one of the components of cellulases [83,84]. β-Glucosidase can minimize the product inhibition of cellobiose in the cellulosic ethanol production by converting cellobiose into glucose [85]. Table 3 demonstrates cellobiases/β-glucosidases from various souces immobilized on different nanosupports and their improved properties/applications.
|
Table 3: MNPs immobilized cellobiases/beta-glucosidases, their mode of immobilization and improved properties. |
||||
|
Name of enzyme |
Name of support |
Mode of immobilization |
Propert/properties Enhanced
|
Reference/s |
|
β-glucosidase |
Core shell magnetite NPs and SBA-15 |
Adsorption |
Stability at low pH & high temperature |
86 |
|
β-glucosidase |
Polymer nanofiber via GA |
Covalent binding |
36 times higher activity, high stability during shaking |
87 |
|
β1,4-glucosidase |
Functionalized SiO2NPs |
Covalent binding |
High temperature optima, specific activity, immobilization efficiency, stability & reusability |
88 |
|
β-glucosidase |
Magnetic Fe3O4 NPs |
Covalent binding |
Wider pH & temperature ranges of activation, better thermal & storage stability |
109 |
|
β-glucosidase |
Aminated starch-coated iron oxide MNPs via GA |
Covalent binding |
High activity & heat stability, preferable pharmacokinetics properties |
88 |
|
β-glucosidase |
Aminated magnetic iron oxide NPs via GA & PEGylated via NHS |
Covalent binding |
Very high stability in vivo, excellent pharmacokinetics properties |
99 |
|
β-glucosidase |
Functionalized MNPs |
Covalent binding |
Enhanced heat stability, high reusability |
90 |
|
β-glucosidase |
MNPs-ECH-IDA-Co2+ |
Adsorption |
Enhanced activity, thermal & operational stability, excellent reusability |
94 |
|
β-glucosidase |
SFNPs |
Adsorption |
High binding efficiency & operational stability |
92 |
|
β-glucosidase |
Functionalized magnetic carbon -cobalt NPs |
Adsorption |
High binding efficiency, stability and reusability |
91 |
|
β-glucosidase |
SiO2NPs |
Adsorption |
High binding efficiency, stability & reusability; effective treatment of sugar cane juice |
95,96 |
|
β-glucosidase |
Fe3O4/PMG/IDA-Ni2+NPs |
Covalent binding |
Excellent catalytic activity, stability & reusability |
100 |
|
β-glucosidase |
ZnONPs |
Adsorption |
High stability and reusability, better production of alkyl glucosides |
101 |
The effect of silica drived nano-supports; core shell magnetite NPs and SBA-15 on the activity and stability of cellobiase has been investigated. The enzyme immobilized on these nano-supports showed improved stability at the conditions of low pH and high temperature. SBA 15 provided better yield of immobilization than the core shell magnetite NPs, however it maintained lower specific activity compared to core shell magnetite NPs [86]. Lee et al. [87], demonstrated immobilization and stabilization of β-glucosidase in the form of enzyme coating on polymer nanofibers (NFs). The enzyme coating was made by crosslinking additional enzyme onto covalently attached β-glucosidase via GA. The initial activity of enzyme coated-β-glucosidase was 36-times higher than free enzyme. Polymer NFs coated enzyme retained remarkably very high activity on long incubation under shaking conditions and this enzyme preparation was easily collected magnetically during repeated uses. A crude preparation of β-1,4-glucosidase from Agaricus arvensis was covalently coupled to functionalized SiO2 NPs with an immobilization efficiency of 158%. The obtained apparent Vmax and kcat values for free and immobilized enzyme were 3,028 U mg-1 protein4,945 s-1 and 3,347 U mg-1 protein-1 5,466 s-1 respectively. The immobilized β-glucosidase showed a higher temperature-optima and improved thermostability compared to native enzyme. The t1/2 at 65°C showed a 288-fold improvement over the free enzyme. The immobilized enzyme maintained 95% activity after 25-repeated uses. Due to high specific activity, immobilization efficiency, stability and reusability, this immobiized enzyme preparation can be exploited in a number of industrial sectors [88]. T. reesei β-glucosidase was successfully immobilized on synthetic superparamagnetic magnetite Fe3 O4 NPs with a mean diameter of 10 nm, silanized and amino functionalized with 3-APTES. The concentration of enzyme immobilized on 1.0 g of silanized magnetite and funtionalized with GA was 0.10 g L-1. The immobilization retained 98% of the initial activity and this preparation was stable for at least 45 days. The immobilized β-glucosidase was employed to supplement cellulase in the enzymatic hydrolysis of three substrates: wheat straw pretreated by steam explosion, E. globulus pretreated by hydrothermolysis and pulp from hydrothermolysis followed by alkaline extraction. The hydrolysis yields by immobilized β-glucosidase and free cellulase for all pretreated materials were 76.1%, 83.6% and 75.6%, respectively. This obtained yield for hydrolysis was higher than those obtained only by using cellulase. These yields were 10% lower than the yields reported with cellulase supplemented with free β-glucosidase. The immobilized β-glucosidase was magnetically recovered and reused twice [89].
The thermostable Aspergillus niger β-glucosidase was covalently attached to the functionalized MNPs. MNPs bound enzyme showed 93% of immobilization yield, different pH optima and same temperature optima and very high heat stability at 70°C. The immobilized β-glucosidase maintained about 50% of its initial activity even after 16-reuses and this preparation was quite successful in the maximum glucose production from cellobiose in the time period of 16 h [90]. β-Glucosidase was covalently immobilized on both ferromagnetic and superparamagnetic NPs by using EDC or succinimidyl-4-(N maleimidomethyl) cyclohexane-1-carboxylate as coupling agents and used these preparations for sensing applications. These workers have examined the best conditions for the preparation of responsive bioactive magnetic system comparing various covalent bio-grafting methods [91].
The β-glucosidase was embedded into silk fibroin (SF) NPs, forming β-glucosidase-SFNPs with a diameter of 50-150 nm. The recovery of enzyme-SFNPs activity was 59.2 % compared to the free enzyme (specific activity was 1 U mg-1). SFNPs bound β-glucosidase showed significantly high operational stability and was successfully used repeatedly. These results confirmed that SFNPs were found good carriers as for the modification of enzymes with their application in the field of research and development. The modified β-glucosidase has a great future in food processing and production of flavour agents [92]. Carbon coated metallic NPs has shown its potential as support for immobilization of enzymes due to their large surface area, high magnetic saturation and manipulable surface chemistry. Functionalized magnetic carbon coated cobalt NPs used for covalent immobilization β-glucosidase, α-chymotrypsin, and lipase B. The enzyme-NCs retained their activity and stability after immobilization and were efficiently recycled from milliliter to liter scales in very short times [93]. Chen et al. [94], used MNPs-epichlorohydrin iminodiacetic acid-Co2+ support for β-glucosidase immobilization. The obtained support has enzyme adsorption capacity as 1.81 mg g-1 particles and maximum activity recovery of 117% g-1 protein during immobilization of β-glucosidase. Km and Vmax of the immobilized β-glucosidase were 0.904 mM and 0.057 μmol min-1, respectively and its activation energy was much lower than the soluble enzyme. The MNPs immobilized β-glucosidase achieved very high heat and operational stability. The immobilized enzyme retained about 90% of the activity after 15 repeated uses.
SiO2 NPs bound β-glucosidase showed 52% immobilization efficiency and 14.1% yield. SiO2 NPs bound enzyme exhibited temperature and pH optima at 60°C and 5.0, respectively. Immobilized enzyme was stable at 60-70°C. After immobilization, the Km value of β-glucosidase for p-nitrophenyl-β-d glucopyranoside (p-NPG) was increased from 0.9 to 1.074 mM and Vmax decreased from 3.5 to 1.513 U mg-1. The immobilized enzyme demonstrated increased storage stability at 4 and 25°C and was reusable up to 10 repeated uses with 70% remaining activity in p-NPG and 60% activity in sugarcane juice treatment. The treatment of sugar cane juice by immobilized β-glucosidase altered its density, viscosity and surface tension. β-Glucosidase treated sugarcane juice showed higher phenolics than untreated sugarcane juice. Caffeic acid which was absent in juice, was detected in β-glucosidase treated juice at a concentration of about 1 mg L-1 [95]. In a further study same group has carried out the immobilization of a purified β-glucosidase from Bacillus subtilis mutant PS-5CM-UM3 on SiO2 NPs with 52% efficiency and 14.1% yield. The immobilized enzyme showed improved storage stability and was reused for 10-times by retaining 70% activity. β-Glucosidase treatment in sugarcane juice increased the phenolics content to 2.6 folds and 2.4 folds increase in p-hydroxy benzoic acid and gallic acid, respectively. The findings of the work demonstrated that the immobilized enzyme is a novel green approach to improve sugarcane juice clarification [96].
Zhou et al. [97], developed a novel and efficient immobilized β-glucosidase using magnetic Fe3 O4 NPs as a carrier. The immobilized β-glucosidase showed wider pH and temperature ranges of activation, higher accessibility of substrate, better thermal and storage stability compared to native enzyme. The magnet directed enzyme/prodrug therapy was developed by coupling β-glucosidase to aminated starch-coated iron oxide MNPs using Glu. MNPs bound enzyme showed 85.54% ± 6.9% activity and remarkably high thermostability. The animal study exhibited that β-glucosidase-MNPs revealed preferable pharmacokinetics properties in relation to MNPs. MNPs bound β-glucosidase was specifically delivered into a subcutaneous tumor of a glioma-bearing mouse in a big amount. The activity of delivered β-glucosidase in tumor lesions was noticed as high as 20.123 ± 5.022 mU g-1 tissue with 2.14 of tumor/non-tumor β-glucosidase activity [98]. Similar investigators have used aminated magnetic iron oxide NPs for the immobilization of β-glucosidase and this preparation was crosslinked by using GA and further PEGylated via N-hydroxysuccinimide (NHS). β-Glucosidase-MNPs and polyethleneglycol (PEG)-β-glucosidase MNP retained 73.0% and 65.4% of its activity, respectively. PEG glucosidase-MNP in vitro/in vivo demonstrated higher stability compared to β-glucosidase-MNP as probed by magnetophoretic mobility and pharmacokinetics studies. Magnetic targeting of PEG-β-glucosidase-MNP in vivo was performed by magnetic resonance imaging and electron spin resonance analysis in a mouse model of subcutaneous 9L-glioma. The accumulation of PEG-β-glucosidase-MNP in tumor tissue was successfully evaluated, with an iron content of 627 ± 45 nmol Fe g-1 tissue and enzyme activity of 32.2 ± 8.0 mU g-1 tissue [99].
Fe3 O4 /PMG/IDA-Ni2+ NPs were efficiently and conveniently used to purify and immobilize his-tagged β-glucosidase. The results showed that Fe3 O4 /PMG/IDA-Ni2+ NPs appeared as an excellent purification material. β-glucosidase was immobilized on the surface of Fe3 O4 /PMG/IDA-Ni2+ NPs to form Fe3 O4 / PMG/IDA-NPs β-glucosidase by means of covalent coupling with imidazolyl and Ni2+. The immobilized enzyme exhibited excellent catalytic activity and stability compared to free enzyme. Moreover, immobilized enzyme was reused several times and maintained over 65% of its initial activity. The obtained support has demonstrated remarkable potential in the purification and immobilization of β-glucosidase [100]. A novel β-glucosidase from Streptomyces griseus was successfully immobilized onto ZnONPs via simple adsorption. It remained tightly bound even after extensive washing and was repeatedly used 10-times without any loss in its initial activity. The immobilised β-glucosidase exhibited a higher optimum temperature and heat stability compared to native enzyme. The immobilized β-glucosidase catalyzed more favourably synthesis of alkyl glucosides [101].
Coimmobilized Cellulolytic Enzymes
The co-immobilization of multiple cellulase complexes has attracted the attention of biotechnologists due to their use in the efficient hydrolysis of celluloses. Table 4 shows the list of coimmobilized cellulolytic enzymes on various nanomatrices, their substrates and products.
|
Table 4: Depicts coimmobilized cellulolytic and other enzymes on various nanomatrices, their substrate and product. |
||||
|
Enzymes |
Nanocarrier |
Substrate |
Product |
Reference/s |
|
Cellulase, β-glucosidase |
CNT –polyurethene foam |
Cellulose, biomass |
Glucose |
99 |
|
An endoglucanase, an exoglucanase |
CdSeZnS core shell QDs |
Cellulose |
Glucose |
103 |
|
β-glucanase, & glucose oxidase |
PB-CS & AuNPs-CS composites |
Cellulose |
β-glucan |
104 |
|
Cellulase, β-glucosidase |
PAA-silica NPs |
paper & Solka-Floc |
Cellobiose &glucose |
105,106 |
|
Endoglucanase, β-glucosidase |
MNPs |
Insoluble cellulose & CMC |
Glucose |
107 |
|
β-glucosidase A, cellobiohydrolase D |
Superpara-magnetic NPs |
CMC |
Glucose |
108 |
|
Cellulase, pectinase |
MNPs, APTES & GA |
Mandarin orange peel |
Extraction of carotenoidic pigments |
109 |
|
α-Amylase, pectinase, cellulase |
Amine-functionalized MNPs & GA |
Juices of apple, grapes & pineapple |
Clarification of apple, grapes & pineapple juices |
114 |
|
Pectinase, xylanase & Cellulase |
APTES-MNPs |
Fruit juices & wheat straw |
Clarification of pine apple & orange juices; production of bioethanol |
115 |
Novel nano-biocarriers were developed by layer-by-layer deposition of carbon nanotube (CNT) on the foam structures, and their efficiency for cellulase and β-glucosidase immobilization was evaluated. Cellulase immobilized on CNT coated polyurethane foam demonstrated 3-fold enhancement in its activity. Moreover, both cellulase and β-glucosidase immobilized on the CNT-foam showed remarkably high storage and operational stability than the enzyme immobilized on the commercial Celite. The obtained enzyme nanobiocomposites have appeared potential candidates for the efficient saccharification of biomass and in the reduction of cost of the equipment [102]. Tsai et al. [103], described the effect of NPs size on the hydrolytic activity of artificial cellulosomes. A simple method based on metal affinity coordination was developed to directly conjugate two enzymes, an endoglucanase and an exoglucanase, onto CdSe-ZnS core-shell quantum dots (QDs) without the use of any chemical modification or linker molecules such as streptavidin. Artificial cellulosomes were developed by attaching the enzymes onto two separate QDs (5 and 10 nm) in order to understand the influence of particle size and QDs to enzyme ratio on the enhancement in cellulose hydrolysis. These findings have revealed that enzyme proximity is the most important factor for activity enhancement while the effect of particle size is relatively modest. This nanocomposite of endoglucanase and exoglucanase has a great scope in field of biofuel production, bioremediation and drug design. Wang et al. [104], constructed a biosensor by immobilizing β-glucanase and glucose oxidase on nano-Prussian blue (PB)-CS (PB-CS) and AuNPs-CS composites and the biosensor was used for the determination of β-glucan concentration. Both the PB-CS and AuNPs-CS film were directly electrodeposited on the surface of gold electrode. It was found that PB-CS NCs exhibited an excellent electrocatalytic reduction towards H2 O2 at a low applied potential window. The synergistic effect of AuNPs-CS/PB-CS NCs had remarkably improved the performances of biosensor. The biosensor exhibited a broad linear range of 6.25-93.75 μM, with a correlation coefficient of 0.9991 under standard experimental conditions. The sensitivity at an applied potential of 0.0 V was 100 nA μM-1 cm-2, with a detection limit of 1.56 μM. The apparent Km was found to be 1.0 mM, it showed a high affinity of the immobilized enzyme for β-glucan. The biosensor showed a fast response within 10 s toward β-glucan, with a good selectivity and stability. Cellulase and β-glucosidase were adsorbed on a polyacrylic acid (PAA) polymer brush grafted on silica NPs to produce enzymogels as a form of enzyme immobilization. Enzyme loading on the enzymogels was increased to a saturation level of 110 μg protein mg-1 particle for each enzyme. Enzymogels with varied enzyme loadings were then used to determine the impact on hydrolysis rate and enzyme recovery. Soluble sugar concentrations during the hydrolysis of cellulose by cellulase and β-glucosidase were adsorbed on a PAA polymer brush grafted on silica NPs filter paper and Solka-Floc with the enzymogels were 45 and 53%, respectively, of concentrations when using free cellulase. β-Glucosidase enzymogels showed lower performance; hydrolyzate glucose concentrations were just 38% of those using free enzymes. Increasing enzyme loading on the enzymogels did not reduce net efficacy for cellulase and improved efficacy for β-glucosidase. The use of free cellulases and cellulase enzymogels resulted in hydrolyzates with different proportions of cellobiose and glucose, demonstrating differential efficacy of endoglucanases, exoglucanases and β-glucosidases present in cellulase mixtures. When loading β-glucosidase individually, higher enzyme loadings on the enzymogels produced higher hydrolyzate glucose. Approximately 96% of cellulase and 66% of β-glucosidase were recovered on the enzymogels [105]. Moreover, some workers have developed a RSM model to evaluate the effects of pH and temperature on hydrolysis and recovery of free and attached cellulase (NS50013) and β-glucosidase (Novozyme 188) on enzymogels made of PAA-silica NPs. Hydrolysis yields using both enzymogels and free cellulase and β-glucosidase were highest at 50°C. The optimal pH for cellulase enzymogels and free enzyme was 5.0 and 4.4, respectively, while both for free β-glucosidase and enzymogels had an optimal pH near 4.4. Both cellulase and β-glucosidase enzymogels produced 69 and 53% hydrolyzed sugar, respectively. Enzyme recovery using enzymogels decreased with increasing pH, but cellulase recovery remained greater than 88% below pH 5.0 and it was greater than 95% at below pH 4.3. The recovery of β-glucosidase enzymogels was not affected by temperature and it was not affecting cellulase recovery [106]. Endoglucanase and β-glucosidase were simultaneously immobilized on MNPs in order to prepare endoglucanace/β-glucosidase-MNPs NCs. The obtained enzymes magnetic NCs were employed for the hydrolysis of celluloses and the rapid hydrolysis of CMC was recorded. Moreover, the fusion of CBD to the NCs improved the hydrolysis of insoluble cellulose. Thus these workers have proved that the magnetosome display system can expand the possibilities of mimicking natural cellulosome organization on MNPs [107].
More recently some investigators have reported co immobilization of two types of cellulases, β-glucosidase A and cellobiohydrolase D on sub-20 nm superparamagnetic NPs. The NPs immobilized 100% enzyme activity for both investigated enzymes. The immobilized β-glucosidase and cellobiohydrolase D retained 67.1% and 41.5% of their original activities, respectively. The immobilized β-glucosidase and cellobiohydrolase D maintained about 85% and 43% of their initial activities after 3 and 10 repeated uses, respectively. Coimmobilization of β-glucosidase and cellobiohydrolase D on MNPs is a promising strategy to promote synergistic action of cellulases in order to decrease enzyme use [108]. Kumar et al. [109], evaluated coimmobilization of cellulase and pectinase on MNPs with average diameter of 40-90 nm via APTES and GA. Nagpur mandarin orange (Citrus reticulata) peels were treated with MNPs bound enzymes and this treatment followed by extraction into organic solvents resulted in 8-9 fold increase in extraction of carotenoidic pigments compared to free enzymes. The obtained optimum pH and temperature for this process were pH 5.0 and 50°C, respectively. The immobilized enzymes preparation was reused 3-times with a marginal loss of only 15% activity.
The biggest hurdle in fruit juice quality improvement is the presence of polysaccharides components in the form of disrupted fruit cell wall and cell materials [110]. Therefore, it requires the breakdown of cellulose together with pectin and starch for the purpose of juice processing [111]. These days enzymatic degradation of fruits pulp is getting popularity for the extraction and clarification of juice. Enzymatic treatment of the cell walls enhances the extraction yield, reducing sugars and soluble dry matter contents. Currently, pectinases, cellulases and amylases are employed for improvement in pressing, extraction and clarification of fruit and vegetable juices [112,113]. For this purpose magnetic nanobiocatalyst was prepared by simultaneously co-immobilizing three enzymes; α-amylase, pectinase and cellulase on amino-MNPs via crosslinking with GA in a single pot juice clarification. The heat (50-70°C) and pH (3-6) stability studies demonstrated more than 2-folds enhancement in half-life and increased resistance to lower pH. The immobilized enzymes preserved about 75% of their activity even after 8 repeated uses. This three-enzyme nanobiocatalyst was successful in the clarification of apple, grapes and pineapple juices to 41%, 46% and 53% reduction in turbidity within 2 5 h, respectively [114]. Perwez al. [115], prepared a multipurpose magnetic nanobiocatalyst by covalently coupling Pectinex 3XL, a commercial preparation including pectinase, xylanase and cellulase on 3-APTES-MNPs. The bound enzyme showed 87%, 69% and 58% activity of pectinase, xylanase and 58% cellulase, respectively. The immobilized enzyme preparation was found quite stable at 70°C, in organic solvents and chemical reagents, and this preparation was successfully reused 5-times without much loss in its activity. This immobilized enzyme system has successfully been applied for the clarification of pine apple and orange juice, and for the production of bioethanol. Immobilized enzyme system hydrolyzed higher amount of pretreated wheat straw and produced higher quantity of bioethanol compared to native Pectinex 3xL.
CONCLUSION
The immobilization of cellulolytic enzymes by using distinct kinds of NMs has provided very high yield and efficiency of enzyme immobilization. It is evident from the findings of the work reviewed in the this article that in majority of the NMs bound enzymes, the stability of the enzymes enhanced against various types of physical and chemical denaturants. Nanosupport bound cellobiases were found quite resistant to be inhibited by its own product, glucose. Cellulolytic enzymes immobilized on/within NMs exhibited high operational stability and reusability. Nano enzyme conjugates have successfully been employed in various batches as well in continuous reactors for the conversion of cellulosic material. Various coimmobilized cellulolytic and other enzymes immobilized on/in nanocarriers have also shown their potential in the effective hydrolysis of cellulose into glucose and for further conversion of glucose into other useful compounds. These coimmobilized preparations have also demonstrated their potential in fruit juice clarification.
ACKNOWLEDGEMENT
Department of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, India is gratefully acknowledged for all kinds of help during writing of this manuscript.