Peroxynitrite-Mediated 9,10-Dihydroxylation of Deuterium-Labeled Oleic Acid in Human Hemolysate to Threo- Und Erythro-Dihydroxy-[9,102H2] Stearic Acid.
- 1. Centre of Pharmacology and Toxicology, Core Unit Proteomics, Germany
Abstract
Background: Peroxynitrite (ONOO−), the reaction product of nitric oxide (•NO) and superoxide anion (O2•–), is a potent oxidizing agent.
Methods: We investigated reactions of cis-[9,10-2H2]-oleic acid in hemolysate with commercially available sodium peroxynitrite (Na+ONOO−). Fatty acids were extracted with ethyl acetate and converted to their pentafluorobenzyl (PFB) ester trimethylsilyl (TMS) ether derivatives (PFB-TMS) for structural identification by gas chromatography-tandem mass spectrometry (GC-MS/MS) in the electron capture negative-ion chemical ionization mode.
Results: The expected anion for dihydroxy-[9,10-2H2]stearic acids [M – PFB]– with mass-to-charge ratio (m/z) of 461 was subjected to collision-induced dissociation. We observed two closely eluting, baseline-separated GC peaks of comparable size with the retention times of the PFB-TMS derivatives of synthetic threo- and erythro-9,10-dihydroxy-stearic acids. The product ion mass spectra obtained from both GC peaks were virtually identical and contained the anions m/z 371, 355, 299, 281, 263, 93, and 89. Except for m/z 89 ([TMSO]–) and 93 ([C6H5O]–), all other mass fragments were by 2 Da larger than of synthetic unlabelled threo- and erythro-9,10-dihydroxy-stearic acids, indicating the presence of two deuterium atoms in the fatty acid skeleton at C-9 and C-10. The underlying mechanism is likely to involve addition of the peroxy group of peroxynitrite on the 9,10-double bond of oleic acid. A mechanism is proposed for the formation of the product ion m/z 93 from m/z 461.
Keywords
Erythrocytes; Hydroxylation; Oleic acid; Peroxynitrite; Tandem mass spectrometry.
CITATION
Tsikas D (2017) Peroxynitrite-Mediated 9,10-Dihydroxylation of Deuterium-Labeled Oleic Acid in Human Hemolysate to Threo- Und Erythro-Dihydroxy-[9,10-2H2 ] Stearic Acid. JSM Biochem Mol Biol 4(2): 1027.
ABBREVIATIONS
BSTFA: N,O-bis(Trimethylsilyl)trifluoroacetamide; Collision-Induced Negative-Ion Dissociation; Chemical CID: ECNICI: Ionization; Electron-Capture cis-EpOA: cis-9,10 epoxystearic acid; GC-MS/MS: Gas Chromatography-Mass Spectrometry/Mass Spectrometry; M: Molecular mass; MUFAs: Monounsaturated Fatty Acids; m/z: mass-to-charge ratio; OA: Oleic acid; cis-d2-Oleic acid: cis-[9,10-2H2 ]-9-Octadecenoic Acid; PFB-Br: 2,3,4,5,6-Pentafluorobenzyl Bromide; PUFAs: Polyunsaturated Fatty Acids
INTRODUCTION
Oleic acid (OA) is the most abundant monounsaturated fatty acid in human blood. Major oxidized oleic acid metabolites in human plasma are cis-9,10-epoxystearic acid (cis-EpOA) and trans-9,10-epoxystearic acid (trans-EpOA). cis-EpOA concentrations in plasma of healthy and diseased human subjects are of the order of 10 – 40 nM [1-4]. In vitro experiments indicate that the hepatic cytochrome P450 (CYP450) system produces cis EpOA [5]. In vivo, circulating cis-EpOA and trans-EpOA in their free and esterified forms may also be formed by non-enzymatic epoxidation [4].
Peroxynitrite (ONOO–; pKa ≈ 6.8 for ONOOH) is the reaction product of nitric oxide (•NO) and superoxide anion (O2 •–) and possesses a high oxidative and moderate nitrative potential against various biomolecules including thiols and tyrosine. Peroxynitrite-mediated oxidation reactions are due to its peroxy group and may include its decomposition products hydrogen peroxide (H2 O2 ) and atomic oxygen. Remarkably, reactions of peroxynitrite and other reactive nitrogen species such as •NO2 [6,7] with oleic acid and other fatty acids have not attracted much attention thus far, unlike PUFAs, notably linoleic acid. Also, available information about the extent of nitration and oxidation of oleic acid by peroxynitrite is limited. Previously, we investigated peroxynitrite-induced oxidative modifications of cis-[9,10-2H2 ]-9-octadecenoic acid (cis-d2-oleic acid) by gas chromatography-mass spectrometry/mass spectrometry (GC MS/MS) and observed formation of deuterium-labelled cis-9,10 epoxystearic acid and several monohydroxy-stearic acids in low yield. In the present work, we investigated peroxynitrite-induced dihydroxylation of cis-d2- oleic acid in human hemolysate by GC MS/MS. We identified formation of two 9,10-dihydroxy-[9,10 2H2 ] stearic acid isomers in comparable amounts.
MATERIALS AND METHODS
Chemicals
All chemicals used in this study were of the highest purity available (≥ 98 %). cis-[9,10-2H2 ]-9-Octadecenoic acid (cis-d2 oleic acid; declared isotopic purity 98 atom% 2H) was obtained from Isotec Inc. (Miamisburg, Ohio, USA). cis-d2-Oleic acid was analyzed by GC-MS [3,6] prior to use and found to be free of oxidized species. Peroxynitrite was purchased as a solution of Na+ONOO− in 400 mM NaOH with a declared concentration of 44 mM (Cayman Chemicals, Ann Arbor, Mi, USA). The alkaline Na+ONOO− solution was stored at -80°C in 100-µL aliquots. 2,3,4,5,6-Pentafluorobenzyl bromide (PFB-Br, 99%) and N,O bis(trimethylsilyl)trifluoroacetamide (BSTFA, 99%) were obtained from Pierce (Rockford, Ill, USA).
Reaction of peroxynitrite with cis-d2-oleic acid in hemolysate
Antecubital venous blood anticoagulated with EDTA was placed immediately upon drawing in an ice bath. Erythrocytes were separated from plasma by centrifugation (800×g, 5 min, 4°C) followed by complete plasma decantation. Erythrocytes were lyzed without washing by freezing for 30 min at -70°C, slow thawing on ice and by vortex-mixing for 1 min with the same volume of ice-cold distilled water. To 1-mL aliquots of hemolysate, 10-µL aliquots of a freshly prepared ethanolic solution of cis-d2-oleic acid (10 mM) were added at a final concentration of 100 µM. Na+ONOO− was then added in portions at a total added concentration of 4 mM. The final pH value of the treated hemolysate was about 8. The incubation time was 20 min at 37°C. Reaction products were extracted by vortex-mixing with 1-mL ethyl acetate for 2 min. After centrifugation (4,500×g, 2 min, 4°C), the organic phase was decanted and dried over anhydrous Na2 SO4 . The solvent was evaporated to dryness under a stream of nitrogen and the residue was derivatized first with PFB-Br and then with BSTFA [4].
Mass spectrometry analyses
GC-MS/MS analyses were performed on a Thermoquest TSQ 7000 triple-stage quadrupole mass spectrometer interfaced with a Thermoquest gas chromatograph model Trace 2000. GC-MS and GC-MS/MS conditions were essentially as described previously [5]. Interface and ion source were kept at 290 and 180°C, respectively. Samples (1 µL of BSTFA solutions) were injected in the splitless mode. For electron-capture negative-ion chemical ionization (ECNICI) methane was used as a reagent gas (65 Pa). Argon (0.15 Pa) was used for collision-induced dissociation (CID). Collision energy, electron energy and emission current were 25 eV, 200 eV and 600 μA. Carboxylate anions [M – PFB]− were subjected to CID and product ion mass spectra were obtained by scanning (1 scan/s) in the m/z-range 40 - 500.
RESULTS AND DISCUSSION
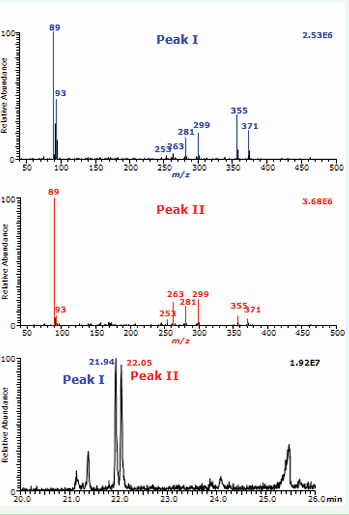
GC-MS/MS analysis of hydroxylated fatty acids in the ECNICI mode requires preceding conversion into their PFB ester TMS ether derivatives [4,8]. Targeted GC-MS/MS analysis of the PFB TMS derivatives of the reaction products of cis-d2-oleic acid and peroxynitrite in hemolysate for dihydroxy derivates was performed by generating product ion spectra from m/z 461 ([M – PFB]–). We observed two equal baseline-separated GC peaks (Peak I and Peak II; Figure 1, lower panel) with retention times almost identical to the PFB-TMS derivatives of synthetic threo- and erythro-9,10-dihydroxy-stearic acids, respectively, by generating product ion spectra from CID of the anion m/z 459 ([M – PFB]–).
Figure 1: GC-MS/MS analysis of reaction products formed from the reaction of cis-d2-oleic acid (100 µM) and synthetic peroxynitrite (4 mM) in human hemolysate at 37 °C after a 20-min incubation. Product ion mass spectra obtained from CID of m/z 461 of the PFB-TMS derivatives of peak I (upper panel), peak II (middle panel) which were separated by GC (lower panel). Blood was donated by the author.
The product ion mass spectra of Peak I (Figure 1, upper panel) and Peak II (Figure 1, middle panel) contained the mass fragments m/z 371 ([M – PFB – TMSOH]–), 355 ([M – PFB – TMSOH – O]–), 299, 281, 263, 93, and 89 ([TMSO]–). Except for m/z 89 and 93, all other mass fragments were by 2 Da larger than the product ions of m/z 459 ([M – PFB]–) of the synthetic threo- and erythro-9,10-dihydroxy-stearic acids. Proposed structures and a mechanism for the formation of the product ions are depicted in Figure 2.
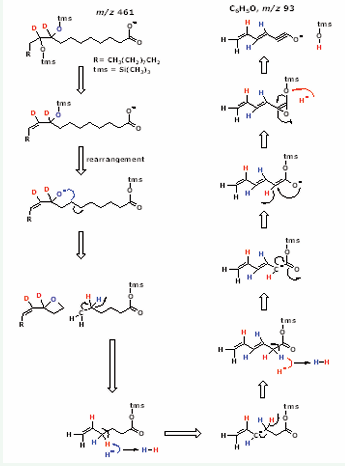
Figure 2: Proposed mechanism for the formation of the product ion m/z 93 of the PFB-TMS derivative of peak I (see Figure 1) obtained by CID of m/z 461 [M – PFB]−. This mechanism is based on an ether to-ester rearrangement of the trimethylsilanolate group from carbon atom 9 to the oxygen atom of the carboxyl group previously reported for hydroxylated eicosanoids [6]. tms, trimethylsilyl.
This mechanism is based on anether/ ester rearrangement of the trimethylsilanolate group which seems to be common in PFB-TMS derivatives of hydroxylated fatty acids and eicosanoids [6]. These observations indicate that the uncommon product ion of m/z 93 of Peak I is specific for threo-9,10-dihydroxy-[9,10-2H2 ]stearic acid. threo- and threo 9,10-Dihydroxy-[9,10-2H2 ]stearic acids carry the original two deuterium atoms of cis-d2-oleic acid in the fatty acid skeleton on the C atoms 9 and 10. The latter finding suggests that the reaction of peroxynitrite with oleic acid is an addition reaction by which the peroxy group of peroxynitrite is added to the 9,10-double bond of cis-d2-oleic acid without loss of the two olefinic 2H atoms. Alternative mechanisms could involve H2 O2 which can be formed from decomposed peroxynitrite. Yet, H2 O2 is unlikely to produce 9,10-dihydroxy-stearic acids because it is formed in low yield, is much less reactive toward oleic acid and due to the presence of catalase in the hemolysate.
CONCLUSION
In human hemolysate, cis-d2-oleic acid was found by GC MS/MS to be dihydroxylated by synthetic peroxynitrite to two isomeric dihydroxy fatty acids, most likely the threo- and erythro 9,10-dihydroxy-[9,10-2H2 ]stearic acids. The PFB-TMS derivatives are separated by GC. The product ion mass spectra of these reaction products differ only in the anion m/z 93 (C6 H5 O) which seems to be characteristic for threo-9,10-dihydroxystearic acid. In the rat, dietary dihydroxy-stearic acids have been reported to induce vitamin K deficiency-like syndromes [9]. The physiological occurrence of threo- and erythro-9,10-dihydroxystearic acids and their biological significance in humans remain to be investigated.
ACKNOWLEDGEMENTS
I thank Drs. Anke Böhmer and Arne Trettin for their contribution to this work.