Computational Assessment of Biological Effects of Methyl-, Ethyl-, Propyl- and Butyl-Parabens
- 1. Department of Biology-Chemistry and Advanced Environmental Research Laboratories, West University of Timisoara, Romania
ABSTRACT
Within this study we have used a computational approach to predict the ADME-Tox profiles, the pharmacokinetics properties and the biological effects of the most used parabens: methylparaben (MP), ethylparaben (EP), propylparaben (PP) and butylparaben (BP).Our results expose that all investigated parabens reveal good oral bioavailability and skin permeation meaning that they are rapidly absorbed and are able to reach the systemic circulation. They are also able to penetrate the blood brain barrier and consequently to affect the central nervous system. Investigated parabens reflect different degrees of toxicity, PP and BP revealing higher toxic effects. Our study also suggests that investigated parabens reveal no carcinogenicity and mutagenicity, a weak potential to inhibit the hERG channel and are able to inhibit human CYPs, BP having the higher inhibitory potential. CYPs inhibition by parabens affects the metabolism of numerous endogenous and exogenous compounds and may cause significant xenobiotics interactions.
KEYWORDS
• Parabens
• ADME-tox profiles
• Biological activities
• Toxicity
• Molecular docking
CITATION
Roman M, Roman DL, Ostafe V, Isvoran A (2018) Computational Assessment of Biological Effects of Methyl-, Ethyl-, Propyl- and Butyl-Parabens. J Bioinform, Genomics, Proteomics 3(1): 1029
ABBREVIATIONS
PB: Parabens; ADME-Tox: Absorption Distribution Metabolization Excretion and Toxicity; MP: Methylparaben; EP: Ethylparaben; PP: Propylparaben; BP: Butylparaben; IUPAC: International Union of Pure and Applied Chemistry; SMILES: Simplified Molecular-Input Line-Entry System; SDF: Structure-Data File; GI: Gastrointestinal Absorption; BBBP: Blood Brain Barrier Permeation; P-gp: P-glycoprotein; LogKp: Skin Permeation Coefficient; hERG: The Alpha Subunit of the Potassium Ion Channel.
INTRODUCTION
Parabens define a collective term for p-hydroxybenzoic acid esters being obtained by distinct chemical substitutions at the para position of the benzene ring. The substituent could be linear or branched, the chemical substitutions providing different properties of resulting parabens [1]
Parabens have antibacterial and antifungal properties and are broadly used as preservatives in cosmetics, pharmaceuticals, food and beverage processing due to their broad spectrum of activity, inertia and low cost. The popularity of parabens is due to their numerous beneficial properties such as: low sensitivity or irritation frequency, high chemical stability over wide ranges of pH and temperature, hydrolysis resistance, lack of changes in product consistency or coloration, biodegradability and low cost production [2,3].
There are literature data evidencing that parabens are present in some food and beverages, pharmaceuticals, cosmetics and personal care products [4-7]. Therefore, people which are using these products, especially those involved in their production and packaging, are exposed to parabens by ingestion, inhalation and/ or skin absorption. Specific literature emphasizes the presence of parabens in urine [8-12] and blood samples [13,14] of humans. These data advocate that paraben exposure is omnipresent and further evaluation of potential health risk of these chemicals is necessary.
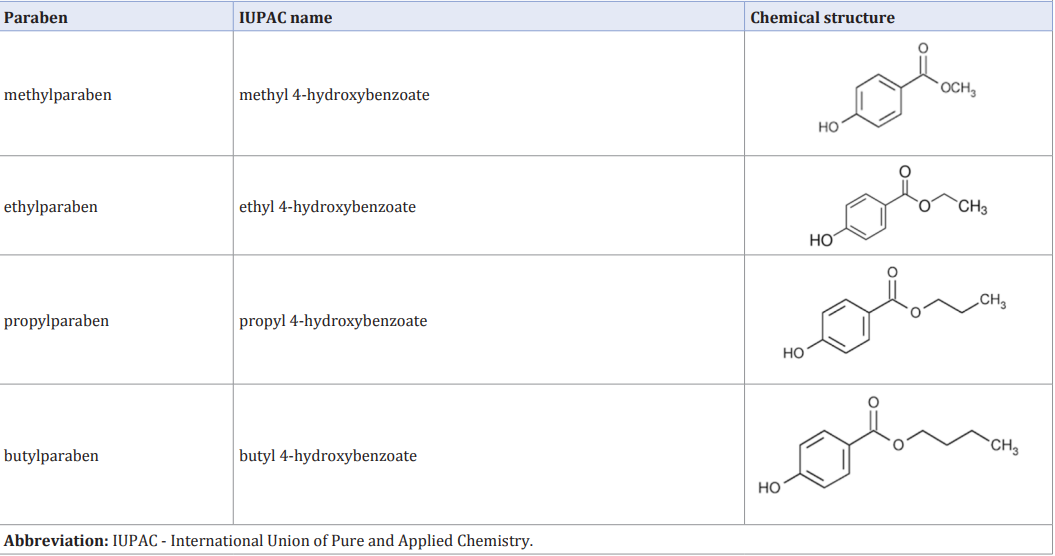
It has been revealed that most parabens are rapidly metabolized into p-hydroxybenzoic acid by esterases and are excreted in the form of salt and other metabolites [15]. However, part of parabens may not undergo hydrolysis and a definite quantity of unmetabolized compounds may remain systemically available [7,12,14].Within this study we only consider the non-metabolized parabens and do not take into account their biotransformation and the hydrolyzed/conjugated forms: methylparaben (MP), ethylparaben (EP), propylparaben (PP) and butylparaben (BP). These compounds are presented in Table (1).
Table 1: Parabens considered in this study.
Investigated parabens are relatively inexpensive and believed to have low levels of toxicity, irritation and sensitizing potential [3]. Official reports published for MP, EP, PP and BP conducted to conclusion that these compounds are safe as cosmetic ingredients in specified quantities and practices of use [16]. Even if the parabens are generally considered safe, there are published data revealing controversial results. Literature data emphasize that some parabens may stimulate tumor cell proliferation in humans [17,18], may cause respiratory allergies, toxicity and hormonal disorders [19], premature aging of the skin and destruction of DNA of the epithelial cells [20]. Other studies reveal that parabens and their metabolites have endocrine disruptor potential [21- 25]. An in vitro study on the human lymphocyte cells revealed parabens may expose genotoxic potential [26]. The health effects of parabens in humans are not clear, available data resulted from studies in animals and/or cell cultures and conduct to doubtful conclusions. As it is difficult to find subjects for such studies, we have used a computational approach to predict the biological effects of the most commonly used parabens.
The aim of the present study is to predict the absorption, distribution, metabolization, excretion and toxicity (ADME-Tox) profiles, pharmacokinetic characteristics, the biological activity spectra and the toxicological and/or side effects of the investigated parabens, to assess the predicted interactions by molecular docking and to correlate these predictions with available literature data.
MATERIALS AND METHODS
Structural information concerning the investigated parabens
Structural information concerning the considered compounds that is needed for the further computational analysis has been extracted from ZINC database [27]. The structure of every chemical compound may be represented in different formats and every computational tool use one of these formats. Consequently, distinct formats of the structural data of investigated parabens have been extracted: the simplified molecular-input line-entry system (SMILES), structure-data file (SDF) and MOL2, respectively.
Prediction of ADME-Tox profiles, pharmacokinetics properties and biological effects
FAF-Drugs4tool [28] has been used to assess the achievement of: (i) rules addressing the oral availability: Lipinski’s rule [29], Veber’s rule [30], Egan’s rule [31] and Bayer Oral Physchem Score [32]; (ii) safety profile: GSK rule [33], Pfizer’s rule [34], phospholipidosis inducer [35] and Lilly Med Chem rules [36] for each considered paraben.
Swiss ADME tool [37] has been used to predict the passive human gastrointestinal absorption (GI), penetration of the blood-brain barrier (BBBP), skin penetration and inhibition of the human cytochromes (CYPs) mostly involved in the metabolism of xenobiotics: CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4. The same pharmacokinetic properties have been predicted using admetSAR, a large database providing lot of estimation by ADME-Tox profiles of different compounds [38]. The results obtained using Swiss ADME and admetSAR were compared. AdmetSAR also envisages toxicological endpoints such as carcinogenicity, mutagenicity and hERG channel-blocking potential [38]. Toxtree software [39] has been used for accomplishing predictions concerning carcinogenicity and mutagenicity. The biological activity spectra and side effects of investigated compounds are predicted using PASS online tool [40].
Assessment of the predicted molecular interactions
In order to further evaluate the inhibition of CYPs by investigated parabens, molecular docking approach has been used to assess their interactions with the human CYPs. Structures of parabens are extracted from ZINC database [27] and structural files of the cytochromes are extracted from Protein Data Bank [41]: 2HI4 for CYP1A2, 4NZ2 and 5A5I for CYP2C9, 4GQS for CYP2C19, 4XRZ and 4XXSfor CYP2D6, 2J0D and4D6Z for CYP3A4. Only the A chain is considered when more than one chain is present in the crystallographic structure. Chimera software [42] has been used to prepare the structural files for molecular docking (ligands excepting hem are removed, hydrogens and charges are added) and to visualize and analyze the molecular docking results. SwissDock computational utility [43] that is based on EADock algorithm [44], has been used to implement the molecular docking study. A blind, rigid and accurate docking has been considered. From this reason, in order to take into account the enzyme flexibility, in the molecular docking calculations we have considered more than one crystallographic structure for the same cytochrome when available.
Specific literature is abundant in computational tools that may be used to predict bioavailability, safety and biological effects of chemical compounds. We have used the specified tools taking into consideration their accuracy for prediction (higher than 70%) and their user-friendly interfaces.
RESULTS AND DISCUSSION
Results concerning the ADME-Tox profiles of investigated parabens obtained using FAFDrugs4 tool illustrate that all rules regarding the oral bioavailability are respected, but Pfizer’s rule addressing the safety of compounds is partly respected by MP and EP and violated by PP and BP (Table 2). Taking into account all results presented in Table (2), investigated parabens comply with almost all the rules imposed by filtering and do not contain high risk toxic groups. The EU Scientific Committee on Consumer Safety established that MP, EP, PP and BP are safe at the maximum authorized concentrations [45] Table (2).
The outcomes of SwissADME and admetSAR predictions are summarized in Tables (3,4). They coincide in respect to GI, BBBP and P-gp substrate, but there are some divergences between the predictions considering the inhibition of CYPs. All considered parabens are predicted to have high gastrointestinal absorption, to be able to penetrate the blood brain barrier and not being substrates of P-gp protein (Table 3), meaning that their systemic exposure is low. These predictions are in agreement with few available studies concerning human oral administration of MP and PP conducting to fast absorption and fast metabolism [7, 46] Table (3).
The skin permeability coefficients (logKp, illustrating the transport of compounds through mammalian epidermis) of investigated parabens are comparable with that of diclofenac (logKp=-4.96 cm/s) [37] and it illustrates their capacity to penetrate skin (Table 3). It is an important observation as they are mostly used in cosmetic products. It must be noticed that we did not consider that parabens are partially metabolized in the skin layers and we did not filter their hydrolyzed/conjugated forms. Our findings are in good agreement with literature data revealing that parabens contained into certain skin-care products are rapidly absorbed through the skin into the human body [7, 47-49].
The predictions considering the inhibition of CYPs by parabens obtained using SwissADME and admetSAR respectively, are not similar. Both SwissADME and admetSAR tools predict that MP and EP do not inhibit human CYPs. SwissADME predicts that BPis able to inhibit CYP1A2 and admetSAR predicts that PP and BP are able to inhibit both CYP1A2 and CYP2C19 (Table 4). Taking into account the inconsistency of the predictions made by SwissADME and admetSAR, we have assessed the interactions of all investigated parabens with CYPs using the molecular docking approach implemented under SwissDock server [43]. The results are shown in Table (4).
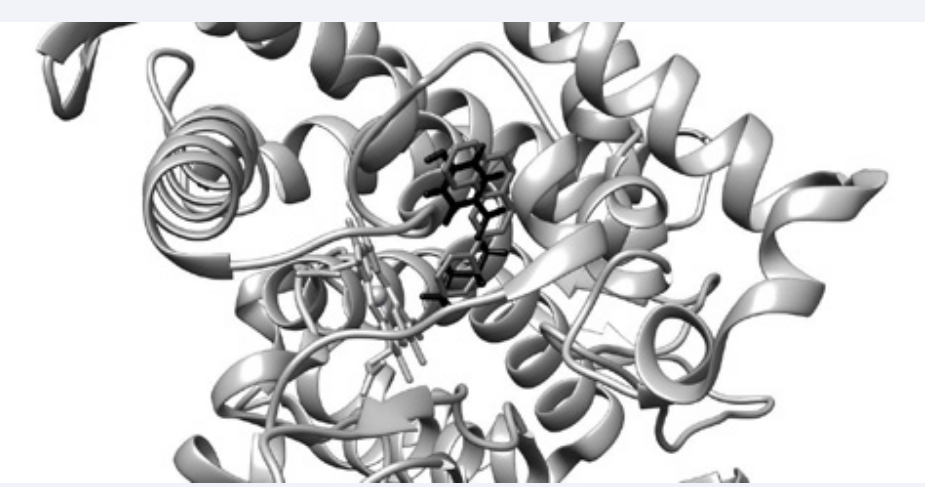
Molecular docking study emphasizes that all investigated parabens are predicted to bind to the active sites of the human CYPs, the most favorable interactions being obtained for CYP1A2. It reflects that parabens could have inhibitory effects on these enzymes, BP being a more potent CYPs inhibitorthan other studied compounds. Figure (1) illustrates the result provided by SwissDock concerning the evaluation of the interaction of BP (black sticks) with CYP1A2 (grey cartoon).
Figure 1: Illustration of the molecular docking result concerning the interaction of butylparaben (black sticks) with CYP1A2 (light grey cartoon): BP binds to the active site of CYP1A2 in the same position as alpha-naphthoflavone (dim grey sticks), the inhibitor that is present in crystallographic structure of CYP1A2.
It reveals that BP is able to bind to the active site of CYP1A2. BP binds to CYP1A2 in the same position as alpha-naphthoflavone (light grey sticks), the inhibitor that is present in the crystallographic structure of the enzyme Figure (1).
The inhibitory potential of parabens on CYP isoforms has been observed in an in vitro study using rat liver microsomes as the enzyme source [50]. The interactions of parabens with human CYPs may affect the binding of other endogenous (hormones) or exogenous compounds (especially drugs) to CYPs influencing the drug metabolism and increasing the side effects of drugs.
Both Toxtree and admetSAR computational tools revealed that all investigated parabens provided not nongenotoxic carcinogenicity, genotoxic carcinogenicity and mutagenicity and they reflect a weak potential to inhibit the hERG potassium channels that are essential for normal electrical activity in the heart (data not shown). In vitro and animal studies also reflected non carcinogenic and non-mutagenic effects of parabens [46,51,52]. It must be specified that we did not take into account the accumulation of parabens and the possible synergic effects of the presence of more than one paraben (or other exogenous chemical compounds) in the human body.
The outcomes of the PASS Online tool show that investigated parabens reflect a broad spectrum of biological activities being possible inhibitors for many enzymes and having few side effects: ulcer, muscle weakness, eye and skin irritation and apnea (data not shown). Skin irritation has been observed for MP in vivo [53] and an in vitro study revealed that the rate of allergic contact dermatitis produced by MP in humans is low [54]. Furthermore, other studies confirmed the inhibitory effects of parabens on some enzymes [46,55].
Table 2: ADME-Tox profiles of considered parabens obtained using FAFDrugs4 tool: light grey boxes illustrate that corresponding rules are respected,dark grey boxes denote rules that are partially respected and black boxes illustrate rules that are entirely broken.
| Parabens name | ADME-ToxProfile | |||||||
| Oral Bioavailability | Safety Profiling | |||||||
| Lipinski Rule | Veber Rule | Egan Rule | Bayer Oral Physchem Score | GSK 4/400 Rule | Pfizer 3/75 Rule | Phospholipidosis Inducer |
Lilly Med Chem Rules | |
| Methylparaben | ||||||||
| Ethylparaben | ||||||||
| Propylparaben | ||||||||
| Butylparaben | ||||||||
| Abbreviations: ADME-Tox: Absorbition, Distribtion, Metabolization, Excretion, Toxicity | ||||||||
Table 3: Pharmacokinetics of considered parabens predicted using SwissADME and admetSAR computational tools.
| Paraben name | GI | BBBP | P-gp substrate | Swiss ADME LogKp (cm/s) | |||
| Swiss ADME | admet SAR | Swiss ADME | admet SAR | Swiss ADME | admet SAR | ||
| Methylparaben | Yes | Yes | Yes | Yes | No | No | -4.95 |
| Ethylparaben | Yes | Yes | Yes | Yes | No | No | -5.24 |
| Propylparaben | Yes | Yes | Yes | Yes | No | No | -5.56 |
| Butylparaben | Yes | Yes | Yes | Yes | No | No | -5.84 |
| Abbreviations: GI: Gastrointestinal Absorption; BBBP: Blood-Brain Barrier Permeation; P-gp: P-glycoprotein; Log Kp: skin permeation coefficient. | |||||||
Table 4: Predictions concerning the inhibition of the cytochromes mainly involved in the metabolism of xenobiotics by considered parabens.
| Parabens/ CYP inhibition | Methylparaben | Ethylparaben | Propylparaben | Butylparaben | |
| CYP1A2 inhibitor | SwissADME | No | No | No | No |
| admetSAR | No | No | No | No | |
| SwissDock (ΔG kcal/mol) | -7.00 | -7.31 | -7.41 | -7.85 | |
| CYP2C9 inhibitor | SwissADME | No | No | No | No |
| admetSAR | No | No | No | No | |
| SwissDock (ΔG kcal/mol) | -6.24 | -6.11 | -6.70 | -6.87 | |
| CYP2C19 inhibitor | SwissADME | No | No | No | No |
| admetSAR | No | No | No | No | |
| SwissDock (ΔG kcal/mol) | -6.42 | -6.70 | -7.08 | -7.31 | |
| CYP2D6 inhibitor | SwissADME | No | No | No | No |
| admetSAR | No | No | No | No | |
| SwissDock (ΔG kcal/mol) | -5.66 | -5.48 | -6.01 | -6.45 | |
| CYP3A4 inhibitor | SwissADME | No | No | No | No |
| admetSAR | No | No | No | No | |
| SwissDock (ΔG kcal/mol) | -6.48 | -6.73 | -6.49 | -7.09 | |
| Abbreviations: CYP: Human Cytochrome. | |||||
CONCLUSION
Within this study we have predicted the biological activities and side effects of parabens in humans. Our study confirmed that investigated parabens reveal good oral bioavailability and good skin permeation illustrating that they are rapidly absorbed into the human body and reach the systemic circulation. Parabens are predicted as being able to penetrate the blood brain barrier and, consequently, they may affect the central nervous system.
Investigated parabens reveal no carcinogenicity and mutagenicity and a weak potential to inhibit the hERG channel. On the contrary, they are able to inhibit the human CYPs, BP having the higher inhibitory potential. CYPs inhibition by parabens affects the metabolism of numerous endogenous and exogenous compounds and causes significant xenobiotics interactions. As humans are exposed to many types of xenobiotics (phthalates, food additives, pesticides, etc.), their interactions with CYPs contribute to a broad range of health problems in humans.
The great majority of the results that we have obtained in this study are in good agreement with published data obtained through in vitro and/or on animal experiments. All these results are important for people awareness, especially for those that are professionally exposed to high amount of parabens. The results obtained by computational tools can complete the in vivo toxicity tests to improve predictive toxicity and safety assessment of parabens.
REFERENCES
1. Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004; 17: 251-263.
26. Güzel Bayülken D, Ayaz Tüylü B, Sinan H, Sivas H. Investigation of genotoxic effects of paraben in cultured human lymphocytes. Drug Chem Toxicol. 2017: 1-8.