Molecular Docking study of the Complex between Novel ?-Amino acid Tripeptides Based Ligands and M-opioid Receptor
- 1. Department of Organic Chemistry, University of Salamanca, Spain
Abstract
The system of treating chronic pain needs to be changed as the opioid drugs used results in problems with potential and issues dependency. A large series of β-amino acids based tripeptides have been studied following the molecular docking strategy in order to find out how well they bind to the morphine’s receptor. As for the ligand-receptor complex properties, one tripeptide, whose results are comparable with morphine’s, has been highlighted. Although more studies are needed, this is a huge step in regards to the developing a new chronic pain treatment.
Citation
Manchado A, Salgado MM, Mateos J, García MJ, Díez D, Garrido NM (2017) Molecular Docking study of the Complex between Novel β-Amino acid Tripeptides Based Ligands and Μ-opioid Receptor. J Bioinform, Genomics, Proteomics 2(2): 1021.
Keywords
• β-Amino acid
• Chronic pain treatment
• Molecular docking
• Structure based drug design
• Morphine
ABBREVIATIONS
AMPc: Cyclic Adenosine Monophosphate; PKA: Protein Kinase A; SBDD: Structure Based Drug Design; Ph: Phenyl; Fur: 2-Furyl; Phoh: 4-Hydroxiphenyl; THF: 2-Tetrahydrofuryl; Dmphoh: 4-Hydroxy-3,5-Dimethylphenyl
INTRODUCTION
Chronic pain
Defined as pain which persist for months, years or even an entire lifetime, chronic pain can be caused by many factors, such as an ordinary injury or a severe illness. However, in many cases, there is no clear cause [1,2].
Activation of opioid receptors [3] µ, λ and δ is the human body’s endogen pathway to deal with the pain. Once a ligand binds with the receptor, adenylate cyclase’s activity is decreased, as well as both AMPc’s activity and PKA’s activity. Ca2+ channels’ closing and K+ channels’ opening are induced so pain sensation is reduced.
A well-known opioid drug is morphine, which is able to induce these changes. While morphine has a highly documented and well-demonstrated efficacy, its side effects are dangerous enough to reconsider its use in chronic pain treatments since, every year, more than 60.000 deaths and more than 15.000.000 dependency cases are registered because of these side effects [4].
The design of new drugs is needed, that are based on opioid receptor-ligand interaction, in order to improve chronic pain patients’ quality of life.
β-amino acids in pharmaceutical industry
Despite the fact that they are less common than α-amino acids in nature [5], β-amino acids are proving exceptionally useful in the pharmaceutical industry because of their properties [6-9]. In regards to biological activity, it is known that β-amino acid based peptides are able to establish their secondary structure with fewer amino acids than their relative α-amino acids based peptide [10-12].
MATERIALS AND METHODS
Structure based drug design
Once you know how the receptor, also known as macromolecule, is, and the way a ligand can bind to this receptor, you are able to design a huge battery of compounds whose characteristics enable them to bind property, as the 3D and topology characteristics of the macromolecule are known. SBDD is a cyclic process based on iterative calculations which consider ligand-receptor complex to quantify how good the ligand-receptor interaction is [13].
This study has taken the structure by Mosberg et al. [14,15], in order to make the in silico study of bindings. Determination of the structure was able to be discovered by the interactions between the receptor and some ligands and metal complex, as it is shown in the literature, and it is based on the X-ray structure of the rhodopsin.
Molecular docking
Molecular docking is a very commonly tool, used in SBDD, which allows us to know the most likely binding conformation of the ligand. In this case, Auto Dock is the software that makes all numeric calculations. Two steps are needed: the exploration of a large conformational space representing various potential binding modes and as accurate a prediction as possible, of the interaction of the energy associated with each of the predicted binding conformation [16].
Genetic algorithms [17] are used to know how genetic variables, based in biological evolution and genomic language, affect the macromolecule’s structure and interaction. They are mathematical schemes that consider the changes of variables such as torsional angles or atom positions. This software also uses a local search function to perform energy minimisation. The method which combines both strategies is known as Lamarckian Algorithm [18], the final technic that estimates the parameters we need to know [19].
Both inhibition constant, biding energy and cluster selectivity are the AutoDock scores which show us how good the ligands theoretically bind to the receptor. The inhibition constant is calculated by AutoDock, which uses an expression where both complex ([C]), free receptor ([R]) and free ligand ([L]) concentrations are considered: ki=([C])/([R][L])
The cluster selectivity is calculated throw a mathematical expression which consider: ni as the cluster i population and nk as the biggest cluster population: σ=∑_i?ni/nk. All AutoDock conditions are shown in Table 1. We have previously demonstrated some morphan derivatives as good µ-receptor binding ligands [20,21], using this methodology.
Table 1: Autodock conditions.
| Number of Runs | 200 |
| PopulationSize | 500 |
| Max Number of Evaluations | 10.000.000 |
| Max Number of Generations | 15.000.000 |
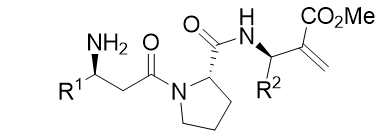
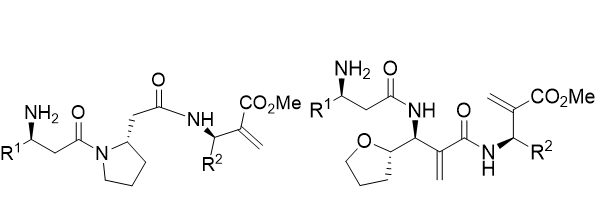
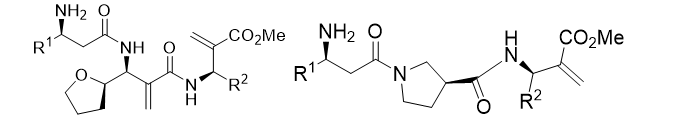
Tripeptides
In regards to opioid drugs design, Wang et al. [22,23], discovered α-amino acids based peptides which properly bound to the receptor. These tetrapeptides owned a double bond in the fourth side chain as well as an aromatic residue. Based on this, a series of different tripeptides were tested under Lamarckian Algorithm conditions (Table 2).
Table 2: Battery of tripeptides based in β-amino acids.
|
|
1 |
2 |
7 |
8 |
21 |
22 |
23 |
24 |
|||||
|
R1 |
Ph |
Ph |
Fur |
Fur |
Ph |
Ph |
Fur |
Fur |
|||||
|
R2 |
Fur |
Ph |
Fur |
Ph |
THF(S) |
THF(R) |
THF(S) |
THF(R) |
|||||
|
|
25 |
27 |
28 |
29 |
31 |
32 |
|||||||
|
R1 |
PhOH |
PhOH |
PhOH |
dmPhOH |
dmPhOH |
dmPhOH |
|||||||
|
R2 |
Ph |
THF(S) |
THF(R) |
Ph |
THF(S) |
THF(R) |
|||||||
|
|
3 |
4 |
9 |
10 |
26 |
30 |
|
R1 |
Ph |
Ph |
Fur |
Fur |
PhOH |
dmPhOH |
|
R2 |
Fur |
Ph |
Fur |
Ph |
Fur |
Fur |
|
|
13 |
15 |
17 |
19 |
|
R1 |
Ph |
Ph |
Fur |
Fur |
|
R2 |
Ph |
Fur |
Ph |
Fur |
|
|
14 |
tpt16 |
18 |
20 |
|
R1 |
Ph |
Ph |
Fur |
Fur |
|
R2 |
Ph |
Fur |
Ph |
Fur |
|
|
5 |
6 |
11 |
12 |
|
R1 |
Ph |
Ph |
Fur |
Fur |
|
R2 |
Fur |
Ph |
Fur |
Ph |
RESULTS AND DISCUSSION
Those with an aromatic residue in the first side chain seem to form π-π interaction with the receptor, and those with proline as the second amino acid, so peptide’s rigidity is increased, had the better results, as it is shown in Table 3.
Table 3: Results of molecular docking study.
| Ki (nM) | Eb (kcal/mol) | σ | |
| 1 | 28,51 | -10,29 | 1,96 |
| 2 | 5,53 | -11,21 | 1,83 |
| 3 | 10,25 | -10,90 | 2,94 |
| 4 | 6,74 | -11,15 | 4,81 |
| 5 | 47,97 | -9,98 | 4,65 |
| 6 | 6,41 | -11,18 | 11,11 |
| 7 | 94,90 | -9,58 | 1,24 |
| 8 | 34,06 | -10,19 | 1,23 |
| 9 | 59,41 | -9,89 | 1,90 |
| 10 | 19,52 | -10,52 | 2,94 |
| 11 | 228,81 | -9,06 | 3,12 |
| 12 | 157,96 | -9,28 | 10,00 |
| 13 | 62,07 | -9,83 | 12,5 |
| 14 | 33,17 | -10,2 | 14,28 |
| 15 | 388,21 | -8,75 | 11,11 |
| 16 | 57,10 | -9,88 | 14,28 |
| 17 | 159,50 | -9,27 | 11,76 |
| 18 | 209,91 | -9,11 | 12,50 |
| 19 | 184,06 | -9,19 | 14,28 |
| 20 | 80,74 | -9,68 | 12,50 |
| 21 | 13,36 | -10,38 | 2,90 |
| 22 | 20,52 | -10,49 | 1,56 |
| 23 | 138,06 | -9,36 | 1,60 |
| 24 | 130,15 | -9,39 | 1,23 |
| 25 | 9,27 | -10,96 | 4,16 |
| 26 | 47,22 | -9,99 | 4,65 |
| 27 | 32,68 | -10,21 | 3,28 |
| 28 | 53,43 | -9,92 | 6,25 |
| 29 | 9,41 | -10,95 | 4,76 |
| 30 | 75,92 | -9,71 | 4,76 |
| 31 | 35,94 | -10,16 | 5,40 |
| 32 | 29,53 | -10,27 | 4,35 |
| Abbreviations: Ki: Ligand-Receptor Complex Inhibition Constant; Eb : Ligand-Receptor Binding Energy; σ: Cluster Selectivity. | |||
While more studies are needed, the results shown in Wang et al studies and in previous literature [24,25] are very similar in terms of in silico affinity. Even most of the literature studies are made with tetrapeptides instead of tripeptides, we think that those compounds are good enough to be studied in this area, and more tripeptides will be assayed both in silico and in vivo way.
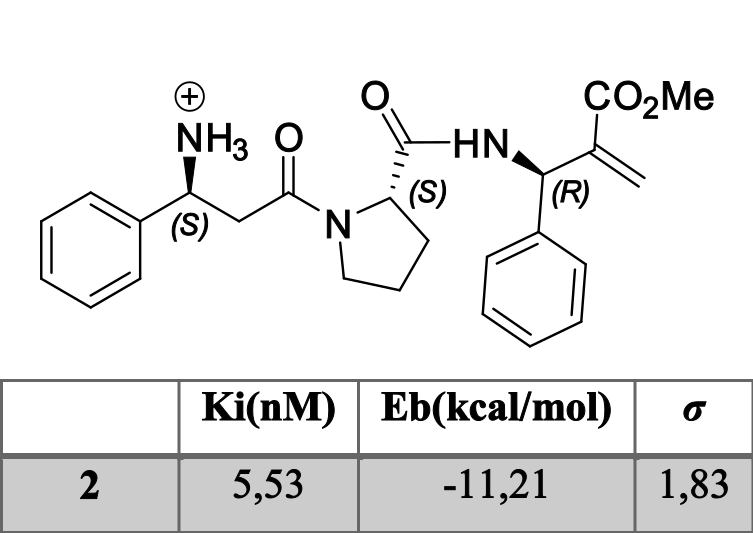
There was one of them which highlighted between the others. Tripeptide 2`s results were good enough to consider continuing its synthesis and it’s in vivo assays (Figure 1).
Figure 1: 2 and its results (calculated).
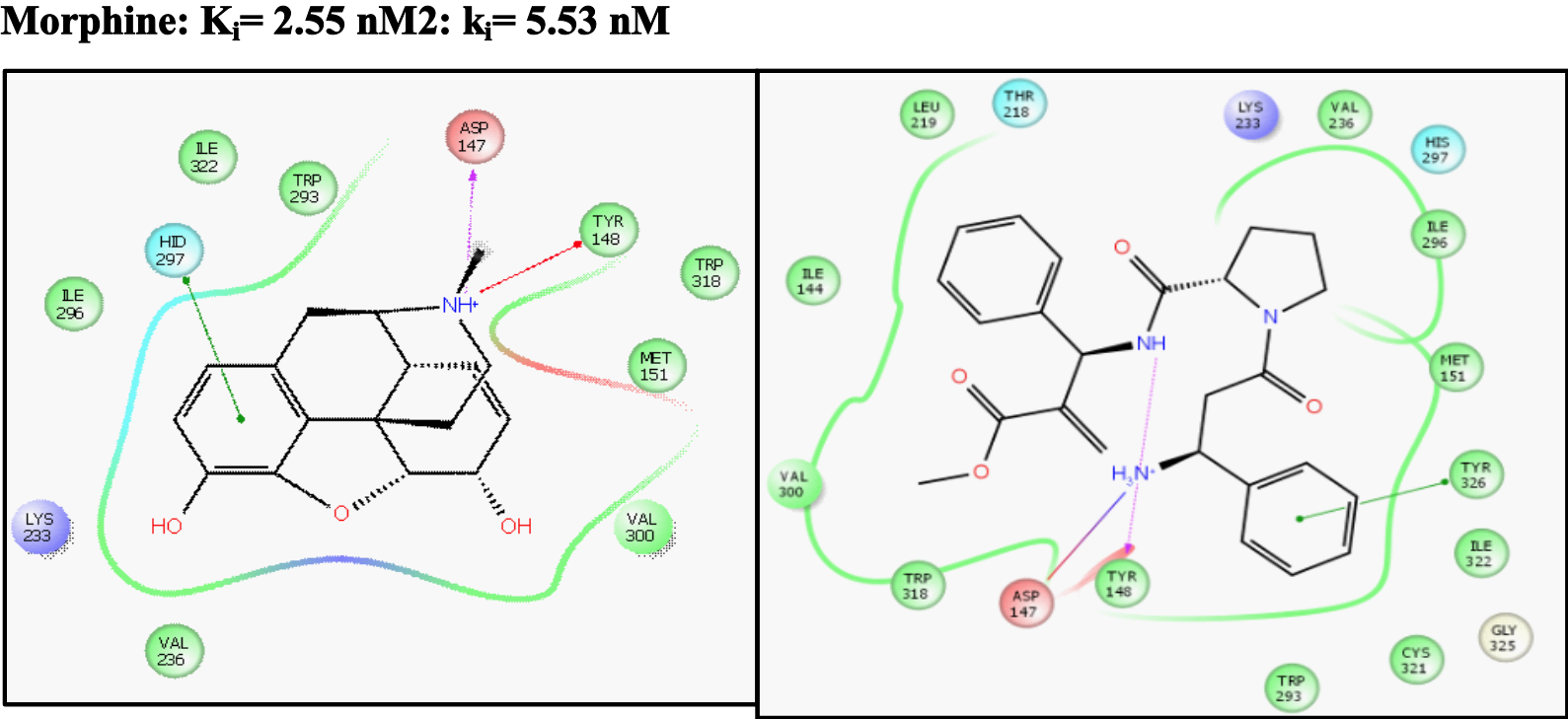
Both results and ligand-receptor interaction are comparable with morphine’s, as it is shown in Figure 2,
Figure 2: Morphine-receptor interactions and 2-receptor interaction (calculated).
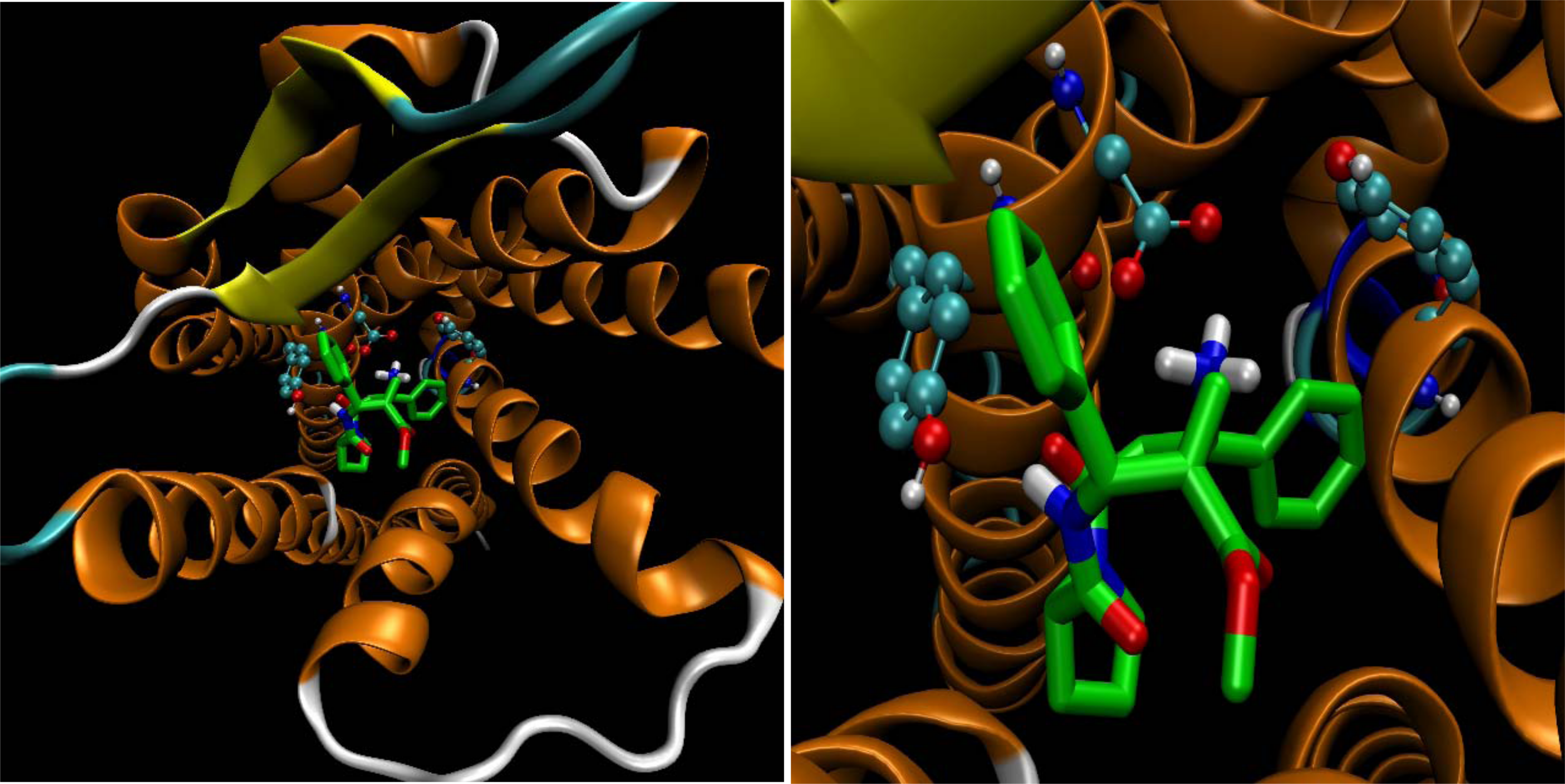
which was made also by AutoDock their docking score were calculated by AutoDock. Also, a 3D representation was made, with VMD software, in order to prove the data obtained (Figure 3).
Figure 3: 3D representation of 2-receptor complex.
CONCLUSION
As for the molecular docking results, these conclusions are suggested:
-First side chain: the more aromatic the radical is; the better interaction the peptide has. On the other hand, residues such us –PhOH and –dmPhOH do not allow the nitrogen at the first side chain to form the H-bond needed in order to have a stable complex.
-Second side chain: proline had the better results, showing us that the more rigid the tripeptide is the better.
-Third side chain: no preferences were shown, as soon as they have Wang et al studies based amino acids, they are good enough to be placed in the tripeptide.
ACKNOWLEDGEMENTS
We thank to MINECO CTQ2015-68175-R, FEDER Junta de Castilla y León (UIC21)