Disruption of SARS-CoV-2 Virus Replication in the Presence of Potassium Ribosate
- 1. Biochemical Research A.E.I.E. Noreña, Spain
- 2. Virology Section of the Microbiology Laboratory Services of the Hospital, Universitario Central de Asturias, Spain.
- 3. Valsé Pantellini Foundation, Spain
- 4. Biochemical Research A.E.I.E. Noreña, Spain
- 5. Biochemical Research A.E.I.E. Noreña, Spain
- 6. Virology Section of the Microbiology Laboratory Services of the Hospital, Universitario Central de Asturias, Spain.
- 7. Virology Section of the Microbiology Laboratory Services of the Hospital, Universitario Central de Asturias, Spain
- 8. Virology Section of the Microbiology Laboratory Services of the Hospital, Universitario Central de Asturias, Spain
- 9. Virology Section of the Microbiology Laboratory Services of the Hospital, Universitario Central de Asturias, Spain
- 10. Biochemical Research A.E.I.E. Noreña, Spain
Abstract
Introduction: Finding products that control viral replication directly or indirectly on SARS-CoV-2 infection remains a challenge. Potassium Ribosate (RK), which has been shown to have an enhancer effect in limiting replication and viability in cancer cells, also appears promising for altering viral replication.
Methods: To determine its activity against SARS-CoV-2, an inhibition test was performed using 26 strains obtained from clinical samples of patients isolated on the Vero E6 cell line. For this, the strains were diluted in series and treated with 125mM of RK. Ribosate was administered in a single concentration (125 mM stock) in two ways: 24 hours before infection and in the culture medium after removal of the infectious inoculum. The culture medium was changed 3 days after inoculation. Seven days after inoculation, a visual reading of the cytopathic effect (CPE) and RT-qRCP was performed to calculate the viral load. The reduction of 3log (9Ct) between control and the Treaty was considered a very good response.
Results: Of the 26 evaluable strains, a reduction in viral replication was observed in 13 (50%) and 16 (61.5%) of the pretreatment and treatment study (p=0.29). This response was good or very good in 10 (38.4%) and 11 (42.3%) (p=0.4), respectively.
Conclusion: RK administration reduced the viral load of SARS-CoV-2 cultures in Vero E6 cells in more than half of the cases tested.
Keywords
• SARS-CoV-2
• Potassium
• Ribose
• Immune response
Citation
Sánchez-Vega MG, Martínez ZP, Riveiro JB, Paoli G, et al. (2025) Disruption of SARS-CoV-2 Virus Replication in the Presence of Potassium Ribosate. JSM Biol 7(1): 1022.
INTRODUCTION
One of the current challenges in SARS-CoV-2 infection is finding reagents/drugs/products that control viral replication directly (antivirals) or indirectly (immunomodulators). Numerous reviews of SARS-CoV-2 have been conducted in the scientific literature [1a,b,c]. All this knowledge is of relevance to the identification and understanding of this disease in the face of the different treatments that may continue to emerge. This background evidence shows that the immune response and possible early evasion play a crucial role in decreasing the viral load or infectious dose that governs disease severity and transmission [2a,b,c]. Potassium Ribosate (RK), is a compound that has been studied for several years by the Biochemical Research A.E.I.E., in collaboration with the Valsé Pantellini Foundation. These structures have been developing research projects based on Potassium salts, in particular Potassium Ascorbate and Potassium Ribosate, for several years on the basis of the studies of the Italian biochemist Gianfrancesco Valsé Pantellini in the second half of the last century and published between 1970 and 1999 [3-6]. The main objective of the above-mentioned projects was to assess the impact of these molecules to try to both prevent and combat degenerative pathologies, in particular oncological ones. Over the years, collaborations have been launched that have produced encouraging results both “in vitro” [7-15] and “in vivo” [16-19], developing a rational rationale for the use of these molecules [18]. We know that potassium is a very important metabolic regulator [20 22], but is particular characteristic of stabilising telomeres through the so-called G-quadruplexes seems less well known [11-14,23a]. It is this last feature that has allowed to focus attention on viral issues, with special attention to SARS CoV-2, assuming that Potassium may interfere with viral replication thanks to its action of maintenance and stabilisation of the genetic structure, inhibiting or limiting retrotranscription [23b]. The mechanism underlying this action is the physical process of resonance, within the biophysical paradigm of living systems [23c]. In this way,Potassium Ribosate could be used as a therapeutic method. It should be noted that preliminary tests in mice treated with RK showed no acute toxicity of the compound (Acute Toxicity Study, according to GLP regulations) [23d,e]. Therefore, a study has been developed to assess the influence of RK on SARS-CoV-2. The Virology section of the Microbiology laboratory Services of the Hospital Universitario Central de Asturias (HUCA) and the Foundation for Biosanitary Research of Asturias in conjunction with Biochemical Research A.E.I.E. developed an in vitro viral growth inhibition assay to monitor whether the active ingredient RK increased the resistance or defence capacity of the cell against SARS-CoV-2 infection.
MATERIAL AND METHODS
Material
All assays were performed in the biosafety laboratory of the Virology Section of the HUCA under appropriate safety conditions. In order to carry out the different inhibition assays and to quantify the effectiveness of aqueous Potassium Ribosate (Biochemical Research A.E.I.E., Spain) against the SARS-CoV-2 viral pathogen, a total of 29 strains were used, which were processed at three different times in the following way: 6 different strains in assay-1, 8 different strains in assay-2 and 15 strains, also different, in assay-3. These strains belonged to variant B.1.1.1.1.7 (α), all these strains were isolated from clinical samples in established Vero-E6 cell line, according to standardised protocols and stored in the viral section’s biobank. Classification of the variants corresponding to each of the strains was performed either by allelic discrimination techniques or by sequencing, according to the protocol of the Section.
Methods
For the inhibition assays, the viral titre of each strain was determined before or at the same time as the assay, depending on the needs of the moment (as determined later).Once the titre of each strain was determined, the inhibition study was carried out according to classical methods adapted in the laboratory [24].
a) Strain titration
Strains of all SARS-CoV-2 viruses were titrated for 50% CI according to inoculation assays in 96-well plates with confluent cell substrate (Vero-E6 cells). For this, starting from a viral culture, decreasing dilutions of the virus were made in base 10 and 200μl of the inoculum was inoculated into the wells of the plate. They were then incubated at 37°C for 1 hour in a 5% CO2 atmosphere. The inoculum was then removed and incubated for up to 7 days under the conditions described above.Viral growth was determined by DBS, Neutral Red and/ or PCR.
(a.1) Cytopathic effect (CPE)
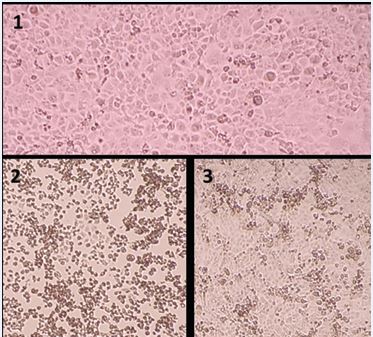
The Karber Method was performed by visualizing the cytopathic effect produced by the infection and calculating the IC 50% (Figure 1).
Figure 1 Images of cultures: (1) Uninfected Vero E6 cells; (2) CPE of titre -2 of a strain at 7 days; (3) CPE of titre -5 at 7 days.
(a.2) Vital dye (Neutral red)
To assess viral infection more accurately, the Neutral Red vital dye uptake method was sometimes applied: after 7 days of infection in the 96-well plate, 50μl of a buffered Neutral Red solution was added to each well. The plates were incubated for 45 minutes at 37°C and in a 5% CO2 atmosphere. Subsequently, the medium was removed, and the wells were washed twice with PBS. Finally, 150μl of ethanol phosphate buffer was added to elute the vital dye incorporated by the viable cells. The absorbance was read at 550nm (reference value of 620nm) on a PR 4100 spectrophotometer.
(a.3) Genomic detection and quantification: real time RT-PCR of SARS-Cov2
From each well, 50μl of supernatant was collected for subsequent viral quantification by genomic amplification (PCR, Figure 2).
Figure 2 Amplification of SARS-Cov2 in wells without Ribosate (red), with pre-treatment (violet) and without pre-treatment (green) in logarithmic (a) and linear (b) scale.
Viral replication in each of the cell culture dilutions was determined by averaging the viral load of all wells, by qPCR technique routinely used in the HUCA Virology Laboratory for virus diagnosis [25]. Briefly, once the viral genome was extracted with the Magnapure LC96 system (Roche Diagnostic, S.L., Switzerland), 5 μl was mixed with 10 μl of a reaction containing specific primers and MGB probes against the polymerase fragment and the N gene and with Fast-stepone 4x reagent (ThermoFisher, S.A. USA). The results obtained were expressed as a function of the Ct (PCR cycles) observed (the higher the Ct the lower the viral load). A Ct of 40 is assumed to mean no viral growth.
(b) Inhibition assay
In the trials carried out with different strains, two types of Potassium Ribosate administrations were performed: 24 hours prior to virus inoculation, which is referred pre treatment; or immediately after pathogen inoculation, which is referred treatment. At all times, the concentration at which Potassium Ribosate was administered was 125mM (Stock concentration as stated by Biochemical Research A.E.I.E., derived in vitro assays) [14-22,23e].To infect the cells, the medium was removed and 200 μl of each of the serial dilutions were added and incubated at 37°C for 1 hour in a 5% CO2 atmosphere. Subsequently, the inoculum was removed, and fresh maintenance medium was administered without and with RK. Three days after infection, the medium was renewed, and each culture was maintained under the same conditions as the original ones. In all cases, viral replication was measured at 7 days post inoculation. These were performed by visualization of the cytopathic effect and by PCR quantification (molecular readout). In the latter assay, analysis by Neutral Red staining was added in some cases. In order to assess the results obtained and quantify the degree of impact of Potassium Ribosate on viral replication of the different strains used in the assays, the following scale of criteria was developed:
(b.1) Biological readout response (DBS/NeutralRed):
- DBS: difference of more than 2 log between the titre with and without reagent.
- Neutral Red: less than 50% growth in the reading title.
(b.2) Molecular readout endpoints (RT-PCR):
- Less than 3 cycles difference: No response (NR)
- Between 3 and 6 cycles (or between 1 and 2 log): Response (R)
- Between 6 and 9 cycles (or between 2 and 3 log): Good Response (B)
- More than 9 cycles (or more than 3 log): Very Good Response (MB)
The final interpretation of the results was carried out according to the data obtained with the RT-PCR method as it better discriminates the difference in growth. The CPE and Neutral Red reading data (when used) were used to assess the evolution of the assay and to confirm the more accurate data from the RT-PCR method. This study was conducted in accordance with the Declaration of Helsinki of 1964 and its subsequent amendments (as revised in 2013). Due to the study design, local Ethical Committee approval was not required. No personal data were handled during the study.
EXPERIMENTAL RESULTS
Description and characteristics of each assay
As mentioned above (in the methods section), three trials were carried out at different time periods. In Assay-1, the pure control results and the cells from the plate detached from the plate of 6 strains were assessed. In Assay-2, 8 strains were assessed and in Assay-3, 15 strains were assessed. All data from these three trials are shown in Table 1.
Table 1: Experiment data; strains, titration, genomic amplification cycles (Ct) and VL (log/103 cell) in Pretreatment and Treatment.
|
|
|
Titration Strain |
Pretreatment with 2021-β |
Treatment with 2021-β |
||||||
|
Experiments |
Strains |
CPE |
Neutral red |
PCR Ct / VL1 |
CPE / (% Growth)2 |
PCR Ct/VL |
Valuation |
CPE (% Growth)2 |
PCR Ct / VL |
Valuation |
|
Test 1 |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
2 |
4 |
- |
22 / 6,7 |
- |
22 / 7,0 |
NR |
- |
35 / 3,13 |
VG |
|
|
3 |
4 |
- |
21 / 7,0 |
- |
24 / 6,4 |
R |
- |
31 / 4,32 |
VG |
|
|
4 |
2 |
- |
28 / 5,2 |
- |
37 / 2,5 |
VG |
- |
22 / 7,0 |
NR |
|
|
5 |
2 |
- |
21 / 6,7 |
- |
22 / 7,0 |
NR |
- |
21 / 7,3 |
NR |
|
|
6 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
|
Test 2 |
1 |
2,5 |
- |
25 / 6,1 |
- |
30 / 4,6 |
R |
- |
30 / 4,6 |
R |
|
2 |
2,8 |
- |
25 / 6,1 |
- |
28 / 5,2 |
R |
- |
30 / 4,6 |
R |
|
|
3 |
2,5 |
- |
27 / 5,5 |
- |
40 / 1,5 |
VG |
- |
40 / 1,5 |
VG |
|
|
4 |
2,5 |
- |
26/ 5,6 |
- |
37 / 2,5 |
VG |
- |
37 / 2,5 |
VG |
|
|
5 |
2,1 |
- |
26/ 5,8 |
- |
40 / 1,5 |
VG |
- |
30 / 4,6 |
R |
|
|
6 |
2,1 |
- |
26 / 5,8 |
- |
40 / 1,5 |
VG |
- |
35 / 3,1 |
G |
|
|
7 |
2,8 |
- |
27 / 5,5 |
- |
40 / 1,5 |
VG |
- |
40 / 1,5 |
VG |
|
|
8 |
2,1 |
- |
17 / 8,5 |
- |
28 / 5,2 |
VG |
- |
40 / 1,5 |
VG |
|
|
Test 3 |
1 |
3,5 |
4,55 |
29,3 / 4,83 |
+ / Not read |
25,6 / 5,93 |
NR |
+ / Not read |
25,8 / 5,87 |
NR |
|
2 |
3,5 |
4,00 |
23,7 / 6,49 |
+ / Not read |
25,6 / 5,93 |
NR |
+ / Not read |
26,5 / 5,66 |
R |
|
|
3 |
3,8 |
4,40 |
22,0 / 6,94 |
+ / Not read |
34,6 / 3,25 |
VG |
+ / Not read |
32,1 / 3,99 |
VG |
|
|
4 |
3,5 |
3,20 |
22,6 / 6,82 |
+ / Not read |
32,7 / 3,81 |
VG |
+ / Not read |
30,9 / 4,35 |
G |
|
|
5 |
4,8 |
4,00 |
25,4 / 5,99 |
+ / Not read |
25,7 / 5,9 |
NR |
+ / Not read |
29,8 / 4,68 |
R |
|
|
6 |
3,5 |
3,10 |
20,8 / 7,36 |
+ / 100 |
20,9 / 7,33 |
NR |
+ / 100 |
20,6 / 7,42 |
NR |
|
|
7 |
3,1 |
3,70 |
32,3 / 3,93 |
+ / 75 |
30,1 / 4,59 |
NR |
+ / 50,50 |
37,9 / 2,27 |
VG |
|
|
8 |
5,8 |
4,60 |
20,3 / 7,51 |
+ / 100 |
18,6 / 8,01 |
NR |
+ / 100 |
18,6 / 8,01 |
NR |
|
|
9 |
2,5 |
3,10 |
20,5 / 7,45 |
+ / 100 |
19,3 / 7,80 |
NR |
+ / 100 |
29,8 / 4,68 |
G |
|
|
10 |
3,1 |
3,70 |
35,0 / 3,13 |
- |
35,6 / 2,95 |
- |
- |
34,1 / 3,40 |
- |
|
|
11 |
3,5 |
4,50 |
24,3 / 6,31 |
+ / 91,40 |
22,8 / 6,97 |
NR |
+ / 86,40 |
22,5 / 6,85 |
NR |
|
|
12 |
3,5 |
3,20 |
22,6 / 6,82 |
¿? / 100 |
32,7 / 3,81 |
VG |
+ / 100 |
21,7 / 7,09 |
NR |
|
|
13 |
4,8 |
4,00 |
25,4 / 5,99 |
+ / 100 |
25,7 / 5,9 |
NR |
+ / 100 |
19,5 / 7,74 |
NR |
|
|
14 |
3,5 |
4,00 |
21,7 / 7,09 |
+ / 85,50 |
21,3 / 7,21 |
NR |
+ / 89,50 |
20,7 / 7,39 |
NR |
|
|
15 |
3,8 |
4,40 |
20,6 / 7,42 |
+ / 100 |
19,5 / 7,74 |
NR |
+ / 79,30 |
18,5 / 8,04 |
NR |
|
Ct: genomic amplification cycle, VL: viral load, NR: no response, R: normal response, G: good response, VG: very good response.
1In trials 1, 2, 4 and 5, values separated by | refer to the result of infected cultures without 2021-β for the pre-treatment (left) and treatment (right) trials.2percentage of growth calculated with respect to uninfected cells adjacent to the dilution
Of the 29 strains tested, evaluable results were obtained in 26. Overall, a response was observed in 13 (50%) pre treated and 16 (61.5%) treated strains at inoculation (p=0.29). This response was good or very good in 10 (38.4%) of the pretreated and 11 (42.3%) of the treated strains (p=0.4).
DISCUSSION
SARS-CoV-2 generates a wide range of clinical manifestations ranging from infection with mild symptoms to severe disease. Studies show that the total viral load to which the individual is exposed is relevant. Damm et al. have found that there is a dose-dependent relationship between viral load and disease severity, so it is important to assess the change in viral load over time, defined as viral kinetics [26a]. The systematic review by Billah et al., shows that assessing this parameter could provide insight into the possibility of an increase in CoV infections [26b]. The basis for this study lies in the immune response and early evasion of the virus. In this respect, many studies have been carried out with different products tested with varying degrees of success [27]. It should be emphasized that the compound under test, Potassium Ribosate, is not an antiviral, so it is necessary to interpret its ability, highlighted in some strains, to strongly reduce viral replication with a significant reduction of viral load. As mentioned in the introductory part, the working hypothesis formulated by Biochemical Research A.E.I.E., together with the Valsé Pantellini Foundation, is that the Potassium present in the compound acts on the G-quadruplexes (G4) not only of human DNA, for their formation and stabilisation [28a], but also and above all on the mRNA of the SARS-CoV-2 coronavirus [28b]. Only the stabilisation of these structures by a compound or, more specifically, an element, such as the cation K+, which interacts with G4, can reduce viral RNA replication and inhibit protein translation [28c]. In this work, a reagent has been tested which, on the one hand, aims to improve cellular defence against alpha variants of SARS-Cov2 and, on the other hand, could act on the virus itself (although its mechanism of action is not known). Potassium Ribosate was able to alter viral replication in more than 60% of the strains. And this response was good or very good in 40% of these strains, suggesting that it is a good drug to alter viral replication and fight infection [29]. These results are in line with other recently tested reagents, which, although they do not reach the 80% efficiency values of direct antivirals, do present values above 60%, similar to those presented in this work [30]. To evaluate its activity, three trials were carried out, in all of which pretreatment and treatment were evaluated (as detailed in Material and Methods), in different periods due to the workload involved. And we worked with the alpha variant, which was the one available in the laboratory at the time. During the evaluation, the SARS-Cov2 typology was not modified so as not to introduce new variables that could alter the final result. The results obtained at the end of each trial for both Pretreatment and Treatment revealed viral load reductions of 50% and 61.5%; this response was Good or Very Good in 10 (38.4%) and 11 (42.3%) of the strains, respectively. In all cases that resulted in a “Very Good Response” the cytopathic effect was negative, showing the weakness of the virus to continue to affect these cultures.
CONCLUSION
In summary, administration of Potassium Ribosate reduces the viral load and cytopathic effect of SARS CoV-2 cultured in Vero E6 cells in more than half of the cases and can be considered as a possible therapeutic/ immunomodulatory method aiming to minimize viral replication in the early undetectable stages and its infectious capacity. Of course, the subject is complex, but the working hypothesis formulated may shed new light on the inhibition of viral replication using a simple and physiological molecule such as Potassium Ribosate and may open the door to further studies to better understand the mechanisms involved.
REFERENCES
- a) Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nature Rev Microbiol. 2021; 19: 141-1 54.
b) Suhail S, Zajac J, Fossum C, Lowater H, McCracken C, Severson N, et al. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020; 39: 644-656.
c) Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nature Rev Microbiol. 2022; 20: 270-284.
2. a) Oliveira BA, Oliveira LCD, Sabino EC, Okay TS. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Revista do Instituto de Medicina Tropical de São Paulo. 2020; 62: e44.
b) Forero-Argüello H, AH Martínez, DL Moncada, DFG Bohórquez, JEF Rivera. Caracterización y fisiopatología del Sars-Cov-2, Revisión de literatura actual. Infectopatología.2021; 61-75.
c) Cevik MKK. Virology, transmission, and pathogenesis of SARS- CoV-2. BMJ. 2020; 371:m3862.
3. Valsé Pantellini G. Breve cenno sulla genesi dei tumori e sopra una eventuale terapia dei medesimi con sali di potassio e in particolare con ascorbato di potassio. Rivista di Patologia e Clinica. 1970: 219- 225.
4. Valsé Pantellini G. Legami idrogeno (H) e salificazione degli stessi da parte del potassio (K) nella strutturazione della materia vivente. Rivista di Patologia e Clinica. 1974: 193-198.
5. Valsé Pantellini G. I nuovi orientamenti sulla terapia dei tumori dal punto di vista biochimico e immunologico. In Atti del Quarto Convegno Nazionale “L’Uomo tra Microcosmo e Macrocosmo”. A cura di Marinucci G. Urbino. 1984; 171-176.
6. Valsé Pantellini G, Paoli G. Meccanismo d’azione dell’ascorbato di potassio nei sistemi biologici. In LXXXV Congresso Nazionale della Società Italiana di Fisica. Pavia. 1999; 108.
7. Croci S, Pedrazzi G, Paoli G, Monetti D, Bronzetti G, Ortalli I: Potassium ascorbate as protective agent in oxidation of red cells. Anticancer Research, 21. Abstract of the International Conference on Antioxidant in Cancer Prevention and Therapy. 2001; 1571-1572.
8. Croci S, Pedrazzi G, Paoli G, Monetti D, Ortalli I. Potassium ascorbate as protective agent in the oxidation of the red blood cells. Hyperfine Interactions (C). Proceedings of the International Conference on the Applications of the Mössbauer effect (ICAME 2001). Thomas MF, Williams JM, Gibb TC Ed.(s), Kluwer Academic Publishers. 2002; 241- 244.
9. Paoli G: The biomagnetic nature of cancer and the role of potassium ascorbate and ribose against cellular degeneration. J New Energy. 2003; 7: 114-211.
10. Croci S, Bruni L, Bussolanti S, Castaldo M, Dondi M. Potassium bicarbonato and D-ribose effects on A72 canine and HTB-126 human cancer cell line proliferation in vitro. Cancer Cell Int. 2011; 11:30.
11. Bruni L, Babarinde AA, Ortalli I, Croci S. K-D: rib dampens Hs 578T cancer cell chemoinvasion and proliferation. Cancer Cell Int. 2014; 14:77.
12. Bruni L, Croci S. K-D: rib cancer cell proliferation inhibitor and DNAzyme folding promoter. J Biol Res. 2014; 87: 2135.
13. Bruni L. Antitumorigenicità del D-ribosio e KHCO3 sulla linea di carcinoma mammario Hs 578T ed effetti sulla linea d’epitelio mammario umano non tumorale Hs 578BST. Tesi di Dottorato di Ricerca in Biotecnologie.Università degli Studi di Parma. 2014; Ciclo XXVI.
14. Frajese GV, Benvenuto M. Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines “in vitro”. Oncology Lett. 2016; 11: 4224-4234.
15. Cavicchio C. Potassium Ascorbate with Ribose: Promising Therapeutic Approach for Melanoma Treatment. Oxidative Medicine and Cellular Longevity. 2017.
16. Anichini C. Beckwith-Wiedemann Syndrome: Potassium Ascorbate with ribose Therapy in a Syndrome with High Neoplastic Risk. Anticancer Res. 2011: 31: 3973-3976.
17. Anichini C. Antioxidant effects of potassium ascorbate with ribose therapy in a case of Prader Willi Syndrome. Dis Markers. 2012; 33: 179-183
18. Anichini C. Antioxidant Effects of Potassium Ascorbate with Ribose in Costello Syndrome. Anticancer Res. 2013; 33: 691-696
19. Anichini C. Antioxidant strategies in genetic syndromes with high neoplastic risk in infant. Tumori. 2014; 100: 590-599.
20. Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015; 10: 1050-1060.
21. Gumz ML, Rabinowitz L, Wingo CS. An Integrated View of Potassium Homeostasis. N Engl J Med. 2015; 373: 60-72
22. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019; 74: 682-695.
23. a) Paoli G. Ascorbato di potassio. La molecola intelligente per regolare le difese dell’organismo. Aam Terra Nuova Ed. Firenze. 2020.
b) Paoli G. Ribosate di potassio e SARS-CoV-2. Le connessioni inattese fra virus e molecole semplici. Natura Docet: la Natura insegna – ND, Anno III. 2022; 9: 18-22.
c) Paoli G. Can a molecule be “intelligent”? Unexpected connections between Physics and Biology. Open J Biophys. 2022; 12: 234-244.
d) S.L., V. R. STUDY PLAN B-02959 Toxicity Evaluation of the Test Itemvia Intramuscular Administration in Female Sprague Dawley Rats by the Acute Toxicity-Up-and-Down Procedure. Spain: Nanoimunotech. 2019.
e) A.E.I.E., B. R. (junio de 2021). Biochemical Research A.E.I.E. Obtenido de Investigación-Estudios:
24. a) de la Iglesia P, Melón García S, López B, Rodríguez M, Blanco MI, Mellado P, et al. Rapid screening tests for determining in vitro susceptibility of herpes simplex virus clinical isolates. J Clin Microbiol. 1998; 36: 2389-2391.
b) Álvarez ÁL, Habtemariam S, Abdel Moneim AE, Melón S, Dalton KP, Parra F. A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in Vero cells. Antiviral Res. 2015; 119: 8-18.
c) Alvarez AL, Dalton KP, Nicieza I, Diñeiro Y, Picinelli A, Melón S, et al. Bioactivity-guided fractionation of Phyllanthus orbicularis and identification of the principal anti HSV-2 compounds. Phytother Res. 2012; 26: 1513-1520.
d) Pérez Martínez Z. “Efecto de la melatonina en células infectadas con el Herpes Simplex Tipo 1”. Universidad de Oviedo. Asturias- España. 2021.
25. a) Martín G, Rojo-Alba S, Castello-Abietar C, Abreu-Salinas F, Costales González I, Boga JA, et al. Comparison of in-house SARS-CoV-2 genome extraction procedures. A need for COVID-19 pandemic. Research Square preprint. 2021.
b) Sandoval Torriente M, Castello-Abieta C, Boga Riveiro J, Álvarez- Argüelles ME, Rojo-Alba S, Abreu-Salinas F, et al. A novel single nucleotide polymorphism assay for the detection of N501Y SARS- CoV-2 variants. J Virol Methods. 2021; 294: 114143.
26. a) Van Damme WDR. COVID-19: Does the infectious inoculum dose- response relationship contribute to undertanding heterogeneity in disease severity and transmission dynamics? Med Hypotheses. 2021; 146.
b) Billah MA. MM Reproductive number of coronavirus: A systematuc review and meta-analysis based on global level evidence. PLos ONE. 2020; 15: e0242128.
27. a) COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health.
b) Hodgson SH, Mansatta K, Mallet G, Harris V, Emary K, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS- CoV-2. The Lancet. Infectious diseases. 2021; 21: e26-e35.
c) Osamu Kanauchi, Zhao Xuan Low, Kenta Jounai, Ryohei Tsuji, Sazaly AbuBakar.. Overview of anti-viral effects of probiotics via immune cells in pre-, mid- and post-SARS-CoV2 era. Front Immunol. 2023: 14: 1280680.
d) Dhuli K, Micheletti C, Maltese PE, Tanzi B, Benedetti S, Tezzele S , etal. The Role of Olive Tree Polyphenols in The Prevention of COVID-19: A Scoping Review Part 2. Clin Ter. 2023; 174: 149-153.
e) Jorge Calderón-Parra, Andrea Gutiérrez-Villanueva, Gerard Ronda-Roca, Maria Luisa Martín Jimenez, Helena de la Torre , María Ródenas-Baquero, et al. Efficacy and safety of antiviral plus anti-spike monoclonal antibody combination therapy vs. monotherapy for high- risk immunocompromised patients with mild-to-moderate SARS- CoV2 infection during the Omicron era. A prospective cohort study. Int J Antimicrob Agents. 2024; 63: 107095.
f) Saad Alhumaid, Abbas Al Mutair, Jalal Alali, Nourah Al Dossary, Sami Hussain Albattat, Sarah Mahmoud Al HajjiMohammed, et al. Efficacy and Safety of Tixagevimab/Cilgavimab to Prevent COVID-19 (Pre- Exposure Prophylaxis): A Systematic Review and Meta-Analysis Diseases. 2022; 10: 118.
28. a) Soldà P. Ligands-mediated modulation of G-quadruplex structures within the HIV-1 genome during lytic and latent state of infection. Tesi di Dottorato di Ricerca in Biomedicina. Università degli Studi di Padova, Ciclo XXXII (2019).
b) Tan J. The SARS-Unique Domain (SUD) of SARS Coronavirus contains two macrodomains that bind G-quadruplex. PLoS Pathog. 2009; 5: e1000428.
c) Brian WX. Binding of cellular nucleolin with the viral core RNA G-quadruplex structure suppresses HVL replication. Nucleic Acids Res. 2019; 47: 56-68.
29. a) Marta Gargantilla, Clara Francés, Anmol Adhav, Alicia Forcada- Nadal, Belén Martínez-Gualda, Olaia Martí-Marí, et al. C-2 Thiophenyl Tryptophan Trimers Inhibit Cellular Entry of SARS-CoV-2 through Interaction with the Viral Spike (S) Protein. J Med Química. 2023; 66: 10432-10457.
b) Dustin Siegel, Hon C. Hui, Edward Doerffler, Michael O. Clarke, Kwon Chun, Lijun Zhang, et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C- Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses J Med Química. 2017; 5: 1648-1661.
c) Beigel JH, Beigel KM, Tomashek LE, Dodd AK, Mehta BS, Zingman AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. J Med. 2020; 383: 1813-1826.
d) Painter GR, Natchus MG, Cohen O, Holman W, Painter WP. Developing a Direct Acting, Orally Available Antiviral Agent in a Pandemic: The Evolution of Molnupiravir as a Potential Treatment for COVID-19. Curr Opin Virol. 2021; 50: 17-22.
e) Hancioglu BSD. A dynamical model of human immune response to influenza A virus infection. J Theor Biol. 2007; 246: 70-86.
30. Abdou K Allayeh, Aliaa H El-Boghdady, Mohamed A Said , Mahmoud GA Saleh, Mohammed T Abdel-Aal , Mohamed G Abouelenein. Discovery of Pyrano[2,3- c]pyrazole Derivatives as Novel Potential Human Coronavirus Inhibitors: Design, Synthesis, In Silico, In Vitro, and ADME Studies. Pharmaceuticals (Basel). 2024; 17: 198.