Prepubertal Exposure to Pharmacological Stress Induces Structural Alterations in the Prostate of Rats
- 1. Urogenital Research Unit, Rio de Janeiro State University, Brazil
- 2. Department of Applied Mathematics, Rio de Janeiro State University, Brazil
Abstract
Here, we report the results obtained in a study on the late changes in ventral prostate of rats, especially in acini, induced by pre pubertal exposure to pharmacological stress with by high dose of Corticosterone, excluding the testosterone influence. Twenty five healthy 5 days aged male rats of the Wistar strain were randomly assigned to Control (n=7) and CORT (n=16) groups, respectively. CORT received diary intraperitoneal injections of Corticosterone (2 mg/100g weight) from 7th to 29th day of life, in morning (between 8:00 and 10:00). This corresponds to the pre pubertal stage of sexual development in rats, including the infantile period (8th to 2st day of postnatal life) and first week of the juvenile period (22nd to 35th day of life). Treatments were interrupted with observation of testicular descent, proceeding to the first significant increase of plasma testosterone, which normally happens between 40 and 50 days of age, significant difference in the body weight gain between two groups was found in the first two weeks. No significant difference was observed between prostatic/body weight ratio. CORT was able to retard the prostatic acini growth and the epithelial development. A significant increase in the ratio of medium acini (0.01 < area ≤ 0.1 mm2 ) and reduction of small acini (area ≤ 0.01 mm2 ) were observed in CORT, and no significant difference was found in the ratio of large acini (area > 0.1 m2 ). About 98.0% of prostatic acini of the Control group could reached areas ≥ 0.01 mm2 , and for CORT group, only 78.8%.
Keywords
Prostate, Pharmacological stress , Corticosterone, Prepubertal , Rat , Morphometry
Citation
Morone Pinto FC, Silva D, Silva CS, Silva PC, de Souza DB, et al. (2016) Prepubertal Exposure to Pharmacological Stress Induces Structural Alterations in the Prostate of Rats. JSM Biol 1(1): 1001.
ABBREVIATIONS
GC: Glucocorticoid; CORT: Corticosterone
INTRODUCTION
It is known that long-term treatments (more than 5 days) with high daily doses of glucocorticoid (GC) are able to simulate chronic stress conditions, since adrenal GCs are found at high levels during the stress state. During the stress state, GCs prepare the organism to meet the growing energetic demand and increase the overall defense mechanisms to adapt it to the stressor [1-3]. Despite the important functions in eukaryotic cells and tissues, stress and excess of GCs inhibit several body activities; influence negatively the cellular proliferation and cellular differentiation in several tissues [4]. Early exposure to GC can accelerate or delay the functional maturation of organs, depending on the dose and exposure time [5].
In previous works, we reported important morphological alterations in cervical ganglion neurons [6], and in the bladder [7], of rats submitted to chronic pharmacological stress before puberty by high doses of cortisol and Corticosterone (CORT), respectively. In bladder, treatment with CORT during 22 days before puberty promoted an important alteration in the collagen organization, and an increase in elastic system fibers. In addition, the smooth muscle fibers density was increased 19% (p < 0.05) comparing to control [7]. We also observed that pre pubertal rats submitted to daily restraint stress for 2 hours (from 4th to 9th week of life) presented significant reduction in the number of nephrons and important alterations on the penile corpus cavernosum, suggesting a possible predisposing to diseases later [1,8,9].
It is important remember that before puberty body growth, as well as growth of organs, are mainly due to GH and GC, which stimulate specific growth factors in all tissues. In pubertal phase, gonadal hormones start to play a key role in body and behavioral developments.
The prostate is an accessory gland of male reproductive system that has been the subject of interest in the biomedical sciences, due to its architecture and the nature of secretions in the prostatic follicles [10], as well as the high incidence of pathologies, including benign hyperplasia and prostate cancer [11]. The prostate is an androgen-dependent gland, but other hormones also have an important function in prostatic physiology, including insulin and GCs [12-14].
GCs have been widely used in the treatment of prostate cancer, because they have inhibitory effect on tumour growth, decreasing prostate-specific antigen levels and improving symptoms [15,16]. However, very little is known on mechanisms responsible by such effects. For Plowman [17], it could be result of the adrenal androgen suppression caused by GCs administration. Otherwise, Smith et al [18] showed that GCs inhibit proliferation of prostatic adenocarcinoma in rat cells by inhibiting the release of growth factors, but that this event is not dependent on the increase inhibition of the number of androgen receptor.
It has been showed that GCs also are involved in important morphological changes in the stromal prostate cell population, and that GC-receptor plays an important role in regulation of prostate development and proliferation [4,16,19]. A considerable number of papers reporting acute GC effects can be found in literature, but there is little information available regarding the chronic effects of GCs on prostatic morphology during the first phases of development until the puberty.
This paper reports the results obtained in a study on the structural changes of rat prostate induced by pharmacological stress with high doses of CORT during pre pubertal phase (from 7th to 29th day of life), using stereological and morphometric methods. In rodents and others animals, CORT is the main GC involved in homeostasis, as well as in immune and stress responses. The stereological and morphometric of analysis has been used to study the ordinary growth kinetics of genital rat structures [11,20].
MATERIAL AND METHODS
Twenty five healthy 5 days aged male rats of the Wistar strain were included in this study. The animals were kept with their mothers during all treatment period, in a well-ventilated and humidity-controlled animal with controlled temperature (25 ± 1 ºC) and artificial dark-light cycle (lights on from 7:00 am to 7:00 pm). They were fed standard rat food and water ad lib. The rats were weighed daily until the day of death. All the experiment was performed in accordance with Brazilian law for scientific use of animals, and the study was formally approved by the local ethical authority.
The rats were randomly assigned into two groups, Control (n=7) and CORT (n=16). The groups were submitted to following procedures: CORT rats received daily intraperitoneal injections of CORT (C2505 Sigma®), 2 mg/100 g of body weight (0.1 mL of solution), from the 7th day to 29th day of postnatal life (22 days of treatment); and Control rats received injections of 0.1 mL of saline. The procedures were performed in the morning, always between 8:00 and 10:00 am, according to previously established protocols. CORT and saline treatments were interrupted (29th postnatal day) with observation of testicular descent, but the evolution of animal body weight was monitored daily until sacrifice.
After 36 days from the treatment interruption, all rats were sacrificed on 65th postnatal day by forced inhalation of carbon dioxide (CO2 ), and their ventral lobes of the prostates immediately were isolated from the prostatic complex under stereoscopic microscope. Lobes were weighted and then immersed in formaldehyde solution (1.27 mol/L formaldehyde in 0.1M phosphate buffer, pH 7.4). The material was processed for paraplast embedding, and serial 5 μm sections of the entire lobe were stained by hematoxilyn-eosyn. For the morphometric analysis of collagen density, sections of 5 μm thickness were stained by Picro Sirius Red.
For quantitative analysis was used Zeiss-Jenamed light microscope (Jena, Germany) coupled to an image analysis system (Image-pro-Express® (1993-2004 Media Cybernetics, Inc., version 5.0 for Windows software Md., USA) and processed using Adobe Photoshop 6.0® - Technique Computed Histofotometria [21]. A 100-point grid was superimposed over the images using the Image J® program (Image Processing and Analysis in Java, version 1:37, National Institutes of Health, USA) for the analysis of the captured images. Five different fields were selected from five non adjacent slices, for a total of 25 images, or 2500 points, counted for each animal. The following morphometric parameters were analyzed: number of acini and height of the acinar epithelium. For the area measurement, the acini were classified according to size using following criteria: Small, area ≤ 0.01 mm2 ; Medium, 0.01 < area ≤ 0.1 mm2 ; and Large, area > 0.1 mm2 .
Student t-test was used to adjust data to the normal curve. The Mann-Whitney test was applied to analyze non-parametric data, and Chi-square test was performed to determine proportions and percentages. All differences were considered statistically significant for p < 0.05. Statistical analyzes were performed using Graph Pad Prism® program (Prism 5 for Windows, version 5.00, March, 2007).
RESULTS AND DISCUSSION
Prepubertal rats were submitted to pharmacological stress with high CORT doses. A long-term treatment of rats with CORT is considered a good model to simulate physiological stress, since this GC isfor rats as cortisol for humans, taking a central role in stress state. Treatment was carried out during infantile period (8th to 2nd day of postnatal life) and first week of the juvenile period (22nd to 35th day of life) [22].
It is known that the most pronounced GC effect is the increase of blood glucose from hepatic gluconeogenesis, but GC also promotes important effects on the cardiovascular, nervous and endocrine systems, as well as inflammatory and immunological responses. The GC-induced activation of hepatic gluconeogenesis results from the increase of circulating amino acids (from increased protein catabolism and decreased protein synthesis) and of enzymes to convert amino acids into glucose [23]. GC together to leptin influences food intake and the weight control [24], it activates the lipolysis and inhibits the lipogenesis in fatty tissue, and plasma free fatty acid is available for liver. All these mechanisms (gluconeogenesis, lipolysis etc.) together protect the organism from hypoglycemia, but long-term stress may result in important organ damages [5,7-9].
Table (1) shows the means of body weights (g) and weight gains (%) for groups CORT and Control at 12th and 22nd days of postnatal life (after 5 and 15 days of treatment, respectively), and at 65th postnatal day. In this day, the rats were sacrificed, after 36 days from the treatment interruption.
The analysis of body weights of the animals at 65th day of postnatal life revealed no statistically significant difference (p > 0.05) between the means of body weight gain of the two groups, CORT (1909 ± 256%) and Control (1850 ± 379%). Nonetheless, the differences were significant at least during the first two weeks of pharmacological stress. After 5 days of treatment, the means of body weight gains were 35.1 ± 9.2% for CORT and 44.7 ± 8.8% for Control (p < 0.5). At 15th day, the mean values were 81.5 ± 12.2% for CORT and 95.8 ± 20.6%, for Control (p < 0.5). No statistical significance was also found between the two groups to ventral prostate weights and the prostate/body weight ratio (p > 0.05). The means of prostate weights (Table 1) were 0.92 ± 0.09% for CORT and 0.99 ± 0.20% for Control, and the means of prostate/body weight ratios were 0.35 ± 0.03% and 0.36 ± 0.09%, respectively
Thus, results show that the effect of pharmacological stress on the body weight variation of rat is dependent on the observed period. In a previous work [25], the comparison of weight gain between the rats treated with GC and control rats considering the entire treatment period (from the 5th to the 25th day) indicated no statistical significance. However, present analysis of the first two weeks of treatment showed significant difference in weight gain between two groups for every day. We still observed that after the 15th day, the difference decreased gradually, evidencing the animal adaptation to pharmacological stress.
Our results are in accordance to the observations of Mukerjee and Rajan [26], who found significant retard in body weight gain in rats during long physiological stress period (5th to 70th postnatal days). First, the animals were exposed to the maternal deprivation (5th to 20th postnatal day), and after they were submitted to sessions of foot-shock (21th to 70th postnatal day). In the post-stress period (after 70th postnatal day), the authors observed that, despite the rate of weight gain faster than control rats, the mean body weight of the stressed rats does not achieve the level of control. However they demonstrated that, different from our observation for pharmacological stress, prolonged physiological stress significantly retard the prostate weight increase during stress and in the post-stress period (between 42th and 98th day).
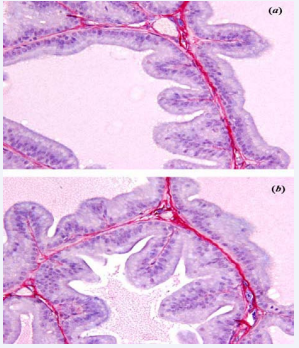
Figure 1 Photomicrography of prostatic acinus of (a) group Control and (b) group CORT
The analysis of the prostatic tissue (65th postnatalday) evidenced changes in the number of acini by size (%), height of the epithelium (μm) and collagen distribution by area (%), some were statistically significant and other not, as shown (Table 2). Figures 1(a) and (b) show photomicrography of prostatic acini for Control and CORT, respectively. In these, we can observe secretary epithelia showing many folds, with tall cells. The luminal contend indicates preserved secretary activity for the two groups, although it seems slightly decreased in CORT.
Table 1: Means of body and prostate weights, weight gain and prostate/ body weight ratio.
| tPeriod / parameter (mean) | CORT | Control | p |
|---|---|---|---|
| 5th day of treatment: | < 0.04 | ||
| Body weight (g) Body weight gain (%) |
18.4 ± 2.2 35.1 ± 9.2 |
20.4 ± 1.7 44.7 ± 8.8 |
|
| 15th day of treatment: | < 0.05 | ||
| Body weight (g) Body weight gain (%) |
24.7 ± 2.4 81.5 ± 12.2 |
27.5 ± 2.1 95.8 ± 20.6 |
|
| 65th day postnatal (36 days after pharmacological stress interruption) Final Body weight Final Body weight gain (%) |
262.6 ± 50.3 1909 ± 256 |
276.7 ± 39.3 1850 ± 379 |
> 0.05 |
| Prostatic weight (g) | 0.92 ± 0.09 | 0.99 ± 0.20 | >0.05 |
| Prostate/body weight ratio (%) | 0.35 ± 0.03 | 0.36 ± 0.09 |
>0.05 |
| CORT – corticosterone. Statistical significance for p < 0.05. | |||
A significant difference (p < 0.05) was found between CORT and Control groups in the ratio of Small (area ≤ 0.01 mm2 ) and Medium (0.01< area ≤ 0.1 mm2 ) acini, but not to Large acini (area > 0.1 mm2 ). For group CORT, the mean relative ratios of Small, Medium and Large acini were 21.2%, 51.5% and 27.3%, respectively. For group Control, the relative ratios were 1.7%, 76.8% and 21.2%. It means that 98.0% of prostatic acini of the Control group could reached areas ≥ 0.01 mm2 , and for CORT group, only 78.8%.The number of Small acini in the group CORT was 19.5% (p < 0.05) higher than for the group control, while the number of Medium acini was 25.3% (p < 0.05) lower. For Large acini, the number found in the CORT was 6.1% (p > 0.05) higher than in the Control. In this way, the ratio Medium/Small acini for the group CORT (= 2.4) was very lower than that found for the Control (= 45.2), as well as the ratio Medium/Lager acini for CORT (= 1.9) was higher than for Control (= 3.6).
The height of the epithelium also suffered important CORTinduced change Table (2), since it was significantly lower (p < 0.05) for CORT (10.45 ± 0.43 μm)compared tothe Control (19.31 ± 0.66 μm). Thelevel of the decrease in epithelium height observed by us is close to that found by Mukerjee and Rajan [26] for physiological stress.
On the other hand, Ribeiro et al [19] described prostatic damage in adult Wistar rats after short-term treatment (five consecutive days) with dexametas one more important than observed with CORT. They reported atrophy and decreased proliferative activity of prostatic epithelial cells, as well as changes in the epithelium-stroma interface, with some interruptions in the basement membrane. However, these results refer to acute alterations caused by a synthetic GC recognized as 25 to 80-fold more potent than cortisol, with 5-fold longer time of action. Rats received diary 1 mg of dexamethasone/kg body, which is a high GC dose even to cortisol, and histological analysis was performed immediately after the end of treatment.
Concerning the collagen distribution (Table 2), we observed discretely lower in the group CORT (2.47 ± 2.22%) than Control (3.53 ± 1.99%), but the difference was not significant (p > 0.05). Collagen is an important component of the fibro muscular stroma, taking a relevant role in the extracellular matrix modulation [27]. This is a complex structural network constituted by glycosaminoglycan, collagens and adhesion molecules, which has important functions related to adhesion, migration and recognizing micro environment. Alteration in collagen content or distribution in prostatic tissue represents an important change in the fibro muscular stroma function and in the modulation capacity of extracellular matrix [29].
It was already demonstrated that long-term systemic GC therapy interferes in the type I and III pro-collagen synthesis in skin and liver [30], and may cause atrophy in certain tissues [31]. Tenius et al [32] showed that first occurs a decrease in contend of type III collagen and then the reduction of type I collagen.
Using high dexamethasone, Ribeiro et al [19] noted a significant decrease in prostatic collagen contend for short-term treatment, in spite of GC play a stimulating role on fibroblasts. The authors suggested that together increased collagen synthesis, higher levels of degradation might be present, and this might be an explanation for the slight difference in collagen distribution between the two groups in our study. There are evidences that a decrease of the CG-induced collagen synthesis accompanies the reduction in DNA contend and RNAm synthesis in fibroblasts [33].
The large spectrum of GC actions can explain the variety of morphological tissue alterations resulting from pharmacological and physiological stress described in literature. Considering the physiological dependence of the conjoint action of GC and other hormones, as well as others factors that interfere in prostatic homeostasis, it is possible understand why different experimental protocols have sometimes yielded conflicting results. One important point to note is that alterations promoted by stress-induced and exogenous GC excess may be different [34], and another is the age of experimental animals can be a critical variable.
GC receptors are highly expressed in the prostatic stroma, while androgen receptors are more frequent in the epithelial cells [15]. This means that GC may play an important role in alteration of the total prostatic volume during chronic stress, since it acts directly on thestroma, and influence direct and indirectlyon the proliferation of the epithelium [35]. On the other hand, by indirect via, GC excess binds to GC-receptors in Leydig cells and decreases the testicular response to gonadotropins, reducing the testosterone secretion [36]. Furthermore, increased GC levels can cause peripheral insulin resistance, and subsequent hyperglycaemia and hyperinsulinaemia. In turn, hyperinsulinaemia is frequently related to low levels of androgens [19].
Martikainen [35], reported that insulin acting independently of androgen is a potent mitogen and exerts its effect mainly on prostatic epithelium. In physiological conditions, he verified that the combination of CORT, insulin and testosterone is able to preserve the structure and the amounts of components in prostate tissue cultures for at least 2 weeks, showing an important GC role in the physiology of the gland. Thus, four main mechanisms acting together or alone might explain the prostate alterations by GC excess: (1) direct action of GC, (2) reduction of testosterone; (3) the high concentration of glucose in the tissue, and (3) the action of insulin [19,34].
Our propose was to verify whether prostatic alterations caused in rats by chronic pharmacological stress during the infantile period and beginning of the juvenile period [22] could still be present in post-pubertal stage, after stress-free weeks. In this way, treatment with CORT in the rats was interrupted (at 29th day of life) with the testicular descent, proceeding to the first significant increase of plasma testosterone, which normally happens between 40 and 50 days of age. The prostatic increase after 8 weeks (56 days) is especially due to the action of testicular androgens [37]. According to Vilamaior et al [20], the prostate growth begins with the lumen formation in first three weeks. After a resting period (4th to 6th postnatal week), an increase absolute volume of epithelium normally occurs from 8th to 10th week. A further gland increase coincident with plasmatic increase of testosterone occurs at the 12th week (84 days).
Table 2: Ratio of acini by size, mean epithelial height and collagen distribution by area at 65th postnatal day (36 days after pharmacological stress interruption).
| Parameter | Control | CORT | p |
|---|---|---|---|
| Small acini (area ≤ 0.01 mm2 ) % | 21.2 ± 0.1 | 1.7 ± 0.1 | < 0.05 |
| Medium acini (0.01 < area ≤ 0.1 mm2 ) % | 51.5 ± 0.3 | 76.8 ± 0.2 | < 0.05 |
| Large acini (area > 0.1 mm2 ) % | 27.3 ± 0.2 | 21.2 ± 0.1 | < 0.05 |
| Mean epithelial height (mm) | 19.31 ± 0.66 | 10.45 ± 0.43 | < 0.05 |
| Mean collagen distribution by area (%) | 3.53 ± 1.99 | 2.47 ± 2.22 | < 0.05 |
| CORT – corticosterone. Statistical significance for p < 0.05 | |||
CONCLUSION
In present study, significant retard in the body weight gain of pre pubertal Wistar rats was observed during the two first weeks of exposure to pharmacologic stress with CORT compared to the control group. But, the difference between weight gains of two groups gradually became insignificant, showing that early stressinduced alterations can be offset by natural adaptive processes.
Prepubertal exposure of rats to CORT was extended by 22 days, including infantile period and part of the juvenile period, and was able to induce structural prostatic alterations that could still be evaluated in post-pubertal stage, after stress-free 36 days. Results evidence the CORT excess capacity in early age to retard the prostatic acini growth and the epithelial development, independent of the action of androgen, since the treatment in the rats was interrupted (at 29th day of life) with the testicular descent, before the first significant increase of plasma testosterone.
Thus, the prostatic changes observed in our study are the result of the direct CORT action on prostatic tissue and its indirect effects through varied mechanisms, especially involving insulin and hyperglycemia. The slight difference found in the collagen contend between two groups, for example, can have origin in simultaneity of the GC actions on estroma, which is a stimulating action on fibroblasts and highly degrading on the protein matrix. It is also possible that the natural tendency of prostatic tissue regeneration is responsible by the insignificant values found for some parameters measured after free-stress period.
In previous study [9], we observed that the number of nephrons in adult rats decreased in response to early stress (restrict to pre pubertal period), but the levels of blood creatinine was normal. Certainly, this recovering of secretory and filtering functions do not guarantee the absence of later disorders. Similarly, alterations from early stress might effectively harm the prostate morphophysiolgy and program disorders to the adult life.
ACKNOWLEDGEMENTS
This work was supported by FAPERJ (Foundation for Research Support of the Rio de Janeiro State) and CNPq (National Council for Scientific and Technological Development).