Aplastic Anemia and Eltrombopag
- 1. Department of Hematology, All India Institute of Medical Science, India
ABSTRACT
Aplastic anemia which was once considered as rare and invariably fatal disease. Over the years the understanding of its pathophysiology, its relationship with constitutional bone marrow failure syndrome and evolution to myelodysplastic syndrome and leukemia has improved. Evolution of standard immunotherapy and bone marrow transplantation has dramatically improved the survival of patients over the years. The optimum immunotherapy with antithymocyte globulin and cyclosporine has been developed and patients who failed even to second course of immunotherapy were also subjected to bone marrow transplantation. These strategies have achieved and improved the event free but significant numbers of cases were refractory to these treatments.
Initial studies of thrombopoietin-MPL system revealed their activity to increase the number of committed progenitor cells along with their proliferation in all three lineages. Peptide agonists such as eltrombopag can bind to MPL region to initiate lineage responses. Initially it was shown to induce significant stimulus to increase platelets in patients of chronic idiopathic thrombocytopenic purpura. Subsequent clinical trials have revealed that eltrombopag induced erythropoietic stimulus even in patients of refractory aplastic anemia and resulted in improved event free and overall survivals. In the present manuscript the role of eltrombopag along with standard immunotherapy has been briefly reviewed in aplastic anemia.
KEYWORDS
Severe aplastic anemia ; Immunosuppressive therapy ; Stem cell therapy ; Eltrombopag.
CITATION
Choudhry VP (2017) Aplastic Anemia and Eltrombopag. JSM Bone Marrow Res 1(2): 1007
ABBREVIATIONS
SAA: Severe Aplastic Anemia; HLA: Human Leukocyte Antigen; ATG: Antithymocyte Globulin; HSC: Hematopoietic Stem Cells; TPO: Thrombopoietin; FDA: Food and Drug Administration
INTRODUCTION
Aplastic anemia is characterized by severe deficiency of red cells, white cells & platelets. This pancytopenia is the result of hypo cellular bone marrow without any evidence of abnormal cellular infiltration or presence of marrow fibrosis [1]. Aplastic anemia can be mild, moderate or severe based upon the severity of aplastic anemia and natural course of the disease. Severe aplastic anemia (SAA) has been defined with bone marrow cellularity of less 25% or cellularity between 25-50% with residual hematopoietic cells of less than 30% along with two of three parameters (a) absolute neutrophil count <0.5 x 109 /L (b) reticulocyte count <20 x 109 /L and (c) platelet count <20 x 109 /L. These changes are secondary to marked reduction in hematopoietic stem cells and lineage committed progenitor cells in the bone marrow. Aplastic anemia is generally an acquired disorder and prevalence of familial disease is rare. In majority of acquired aplastic anemia the etiology of aplastic anemia can’t be identified. It is believed that cytotoxic T-lymphocytes by immune mechanism interact with hematopoietic stem & progenitor cells resulting in their destruction leading to low number of CD34 cells & specific subpopulation of various progenitor cells. Recently it has been observed that short telomeres of white cells at presentation is associated with higher risk of relapse and chromosomal instability can be detected in bone marrow cells of patients much earlier before the evolution of aplastic anemia to myelodysplastic syndrome or leukemia [2,3]. It is essential to exclude inherited disorders of bone marrow failure syndromes such as Fanconi’s anemia, Schwachman-Diamond syndrome, Dyskeratosis congenita by appropriate tests before establishing the diagnosis of acquired aplastic anemia.
BONE MARROW TRANSPLANTATION
Younger patients (<45 years) with SAA, who havehuman leukocyte antigen(HLA) matched sibling donor have the best option of treatment by allogeneic bone marrow transplantation which offers complete cure in 90% of individuals [1,4,5]. It is unfortunate that matched healthy siblings donors are available in nearly 30% of cases. While results of allogeneic bone marrow transplantation from matched unrelated donors are poor. Improvement in conditioning regimens in future may result in improving the cure rates.
CURRENT IMMUNOSUPPRESSIVE THERAPY
Several retrospective studies along with randomized studies have demonstrated that horse antithymocyte globulin (ATG) along with cyclosporine forms the standard therapy for patients with SAA [2,6,7]. Hematologic responses with blood transfusion independence can be achieved nearly in 70 % of cases. These responses are not prolonged as 30-40% cases disease relapses or patients require prolonged cyclosporine maintenance therapy. Prolonged cyclosporine administration usually delays the relapse. Now it is believed that low dose cyclosporine is adequate for maintenance. Patients who respond to standards therapy do well while patients who fail to respond or relapse on standard therapy can be treated with second course of immunotherapy by rabbit ATG. In earlier studies it was observed that rabbit ATG was more effective in treating patients who had failed to horse ATG therapy [8,9]. Rabbit ATG therapy resulted in profound lymphocyte depletion of CD-4 T cells and T regulatory cells which indicate that rabbit ATG is more potent immunosuppressive than horse ATG. However rabbit ATG therapy in standard dose does not improve the response rates in SAA when it was administered as first line therapy. Intensification of immunotherapy by high dose cyclophosphamide, mycophenolate mofetil either alone or in combination with ATG have failed to improve the response rates [10-12].
STEM CELL STIMULATION THERAPY
The principle of combining the immunosuppressive therapy with growth factors was that the immunotherapy (ATG & cyclosporine) will suppress the cytotoxic T lymphocyte while growth factors will increase the stem cell proliferation and self-renewal synergistically to improve the event free survival. Erythropoietin and G-CSF were used in various regimens to correct the anemia and to improve the neutropenia though the levels of these growth factors were significantly higher initially because of increased endogenous production. Professor Marsh in his review has summarized that these growth factors (erythropoietin and G-CSF) have limited or little utility in treatment of aplastic anemia [13].
The observation that presence of MPL receptor on hematopoietic stem cells (HSC), megakaryocyte colony forming units (CFU-MK3), along with myeloid and erythroid precursor cells play a significant role in hematopoiesis [14]. MPL receptor was so named as it was initially identified as proto-oncogene homolog of murine myeloproferative leukemia virus oncogene. MPL per-se lacks intrinsic kinase activity but its ligation with thrombopoietin (TPO) leads to phosphorylation and activation of associated proteins such as JAK2, TYK2 which in turn stimulates various kinase pathways (STAT-3, STAT-5, RAF-1/MAP) [15-17]. Stimulation of MPL by TPO also activates CYN, CNK, SOCS which are negative regulators [18]. TPO-MPL complexes are rapidly internalized leading to its degradation. Kuter in his review article summarized that TPO along with various cytokines (IL-3, IL-11) and stem cell factors increase the development and proliferative rate of stem cells [19]. TPO also stimulates the megakaryocyte maturation and expression of various glycoproteins such as GPIb & GPII b / IIIa.
TPO mimetic as small chemical molecules have been developed which have high affinity to bind with MPL. Eltrombopag is only TPO non-peptide molecule has been approved by FDA. It comprises of 14-amino acid peptide which has high affinity for MPL and has no sequence homology with TPO. Romiplastin is also approved TPO peptide mimetic. These are genetically engineered to bind with high affinity to stimulate MPL. Eltrombopag binds with MPL to activate JAK / STAT and MAPK pathways [19]. Its binding site on MPL is different from that of TPO. Therefore TPO & eltrombopag have additive effects. It is rapidly absorbed after oral administration & its peak levels are achieved within 2-6 hours [20]. It should be taken on empty stomach for its better absorption. It is metabolized in the liver by cytochrome 450 system. Clearance of eltrombopag is 33-52% lower in Asian people & therefore its dose recommended for Asians is half as compared with other ethinic people [21]. Based upon various phase III clinical trials it has been approved for use in patients with chronic immune thrombocytopenia purpura [21- 24], and for management of thrombocytopenia in patients with chronic liver disease secondary to hepatitis C infection [25-27]?.
ELTROMBOPAG IN SAA
Eltrombopag being a small molecule & therefore it enters the bone marrow niche more effectively than endogenous TPO [28]. It being oral drug & thus it can be administrated easily for prolonged periods. Initially phase II study was conducted at National Institute of Health (NIH) in USA where 25 cases of SAA who had failed to respond to one or more courses of ATG were included to evaluate its efficacy over 12 weeks study period. Its starting dose was 50mg/day which was escalated every 15 days until a maximum dose of 150mg/day [29]. Eleven of 25 (44%) patients achieved the primary response at least in one lineage at 12 weeks (9 patients had platelet response and 2 patients each had neutrophil and hemoglobin response).Seven of these patients continued to receive eltrombopag (150mg/day) for 8-32 (median 16) months. Bone marrow examination revealed normalization of hematopoiesis in all three cell lines without any increase in myelofibrosis. This was first landmark study which demonstrated that eltrombopag induced multilineage response in SAA. Subsequently 18 more patients were included in the above study which revealed that 17 of 43 (40%) patients had either bilineage or trilineage response at 3-4 months [30]. An important observation in the study was that patients having unilineage or bilineage response at 12 weeks on continuation of eltrombopag therapy had trilineage response. Seventeen patients had normalization of blood counts &five of them discontinued eltrombopag after a median of 28.5 (9-37) months. All these patients were in remission with a median follow up of 13 (1- 15) months [30]. Gill & their colleagues in their review [31], reported retrospective analysis of 10 patients with AA/ SAA who were treated with eltrombopag had overall response rate of 70% and 30% of these patients had trilineage response. They further observed that responses were dose dependent and a dose of 450 mg/day (white patients equivalent) had no significant toxicities on prolonged administration. In another phase II study 88 newly diagnosed cases of SAA who were undergoing ATG and cyclosporine therapy were also given eltrombopag [32]. In this study patients were divided in 3 cohorts, cohort 1 (n=30) patients received eltrombopag from 3rd week to 6 months, in cohort 2 (n=31) eltrombopag was given from 3rd week to 3 months while in cohort 3 (n=27) it was given from day 1 to 6 months with primary endpoint of complete response at 6 months. Overall and complete responses were seen in 80% & 30% in cohort 1, 87% & 36% in cohort 2 and in 92% & 54 % in cohort 3 patients. Patients who responded, the median time to become independent of red cell transfusion was 42 days & 32 days for platelets. In this study 12 patients underwent hematopoietic stem cell transplantation for relapse in 3 cases, evolution to myelodysplastic syndrome in 3 and refractoriness to therapy in 6 cases. These results are much superior to patients receiving standard immunotherapy [31]. Among three cohorts in the study overall complete responses were superior in patients of cohort 3.Transaminitis was frequent adverse effect which was reversible with short interruption of therapy without needing any dose reduction [28,30]. While gastrointestinal adverse effects (dyspepsia, abdominal pain etc) can be controlled without reducing the dose by symptomatic therapy. Eltrombopag therapy in SAA has resulted in cytogenetic abnormalities including partial deletion, loss of chromosome 7, [30], and monosomy 7 [31].
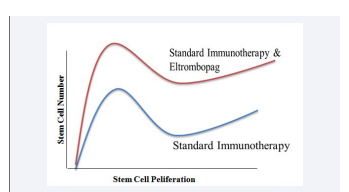
Over all these studies indicate that eltrombopag along with standard immunotherapy is able to increase the HSC number and also increases the proliferation of stem cells in the bone marrow (Figure 1).
Figure 1 Hypothetical diagram showing the effect of standard immunotherapy with and without eltrombopag on stem cell number and stem cell proliferation.
It is quite clear from all these studies that addition of eltrombopag to standard immunotherapy with ATG & cyclosporine combination is very promising. However large multicentric studies over prolonged periods are essential to determine the efficacy, optimal dose and duration of eltrombopag therapy in patients of SAA. Long term follow up studies after stopping eltrombopag will reveal complete cure rates or evolution of SAA to other stem cell disorders. If these studies confirm the present results then standard immunotherapy along with eltrombopag may even replace the bone marrow transplantation in SAA.
CONFLICT OF INTEREST
It is to certify that the author has no financial interest in preparation of this brief review. There is no conflict of interest with the studies cited while reviewing the literature on the subject.
REFERENCES
- Killick S, Bown N, Cavenagh J, Dokal I, Hill Anita, Hillmen Peter, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016; 172: 187-207.
- Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011; 365: 430-438.
- Calado RT, Cooper JN, Padilla-Nash HM, Sloand EM, Wu CO, Scheinberg P, et al. Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia. 2012; 26: 700-707.
- Deeg H, Leisenring W, Storb R, Nims J, Flowers M, Witherspoon R, et al. Longterm outcome after marrow transplantation for severe aplastic anemia. Blood. 1998; 91: 3637-3645.
- Desmond R, Townsley D, Dunbar C,Young N. Eltrombopag in aplastic anemia. Semin Hematol. 2015; 52: 31-37.
- Scheinberg P, Young NS. How I treat aplastic anemia. Blood. 2012; 120: 1185-1196.
- Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Amer Soc Hemotol. 2013; 76-81.
- Di Bona E, Rodeghiero F, Burano B, Gabbas A, Foa P, Locasciulli A, et al. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. GruppoItlaianTrapianto di Midollo Osseo (GITMO). Br J Haematol.1999; 107: 330-334.
- Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and cyclosporine for patients with relapsed or refractory severe aplastic anemia. Br J Haematol. 2006; 133: 622-627.
- Zhang F, Zhang L, Jing L, Zhou K, Wang H, Peng G, et al. High-dose cyclophosphamide compared with antithymocyte globulin for treatment of acquired severe aplastic anemia. Exp Hematol. 2013; 41: 328-334.
- Scheinberg P, Townsley DM, Dumitriu B, Rios O, Weinstein B, Battiwalla M, et al. Even “moderate” dose cyclophosphamide for severe aplastic anemia is associated with significant toxicities and does not prevent relapse and clonal evolution. Blood. 2012; 120: 1259.
- Li X, Shi J, Ge M, Shao Y, Huang J, Huang Z, et al. Outcomes of optimized over standard protocol of rabbit antithymocyte globulin for severe aplastic anemia: a single-center experience. PLoS One. 2013; 8: e56648.
- Marsh JCW, Kulasekararaj AG. Management of the refractory aplastic anemia patient: what are the options? Hematology Am Soc Hematol Educ Program. 2013; 2013: 87-94.
- Kaushansky K. Thrombopoietin: the primary regulator of megakaryocyte and platelet production. Thromb Haemost. 1995; 74: 521-525.
- Bacon C, Tortolani P, Shimosaka A, Rees R, Longo D, O’Shea J. Thrombopoietin (TPO) induces tyrosine phosphorylation and activation of STAT5 and STAT3. FEBS Lett. 1995; 370: 63-68.
- DrachmanJ, Kaushansky K. Dissecting the thrombopoietin receptor: functional elements of the MPL cytoplasmic domain. Proc Natl Acad Sci USA. 1997; 94: 2350-2355.
- Miyakawa Y, Rojnuckarin P, Habib T, Kaushansky K. Thrombopoietininducesphosphoinositol 3-kinase activation through SHP2, Gab, and insulin receptor substrate proteins in BAF3 cells and primary murine megakaryocytes. J Biol Chem. 2001; 276: 2494-2502.
- Kaushansky K. Thrombopoiesis. Semin Hematol. 2015; 52: 4-11.
- Kuter D. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013; 98: 10-23.
- Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011; 51: 842-856.
- Cheng G, Saleh M, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (Raise): a 6-month, randomised, phase 3 study. Lancet. 2011; 377: 393-402.
- Bussel J, De Miguel P, Despotovic J, Grainger J, Sevilla J, Blanchette V, et al. Eltrombopag for the treatment of children with Persistent and Chronic Immune Thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015; 2: e315-e325.
- Bussel J, Saleh M, Khelif A, Meddeb B, Salama AO, Portella M, et al. Final safety and efficacy results from the extend study: treatment with eltrombopag (Epag) in adults with chronic immune thrombocytopenia (CITP). Haematologica. 2016; 101: 193.
- Wong R, Bakshi K, Brainsky A. Thrombophilia in patients with chronic immune thrombocytopenia. Scand J Clin Lab Invest. 2015; 75: 13-17.
- Schmid M, Kreil A, Jessner W, Homoncik M, Datz C, Gangl A, et al. Suppression of haematopoiesis during therapy of chronic hepatitis C with different interferon alpha mono and combination therapy regimens. Gut. 2005; 54: 1014-1020.
- Brouwer W, Xie Q, Sonneveld M, Zhang N, Zhang Q, Tabak F, et al. Adding pegylated interferon to entecavir for hepatitis B E antigen positive chronic hepatitis B: a multicenter randomized trial (ares study). Hepatol. 2015; 61: 1512-1522.
- Afdhal N, Dusheiko G, Giannini E, Chen P, Han K, Mohsin A, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterol. 2014; 146: 442-452.
- Roth M, Will B, Simkin G, Narayanagari S, Barreyro L, Bartholdy B, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012; 120: 386-394.
- Olnes M, Scheinberg P, Calvo K, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012; 367: 11-19.
- Desmond R, Townsley D, Dumitriu B, Olnes M, Scheinberg P, Bevans M, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014; 123: 1818-1825.
- Gill H, SM Raymond and YL Kwong, et al. From chronic immune thrombocytopenia to severe aplastic anemia: recent insights into the evolution of eltrombopag. Ther Adv Hematol. 2017; 8: 159-174.
- Townsley D, Dumitriu B, Scheinberg P, Desmond R, Feng X, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia accelerates count recovery and increases response rates. Blood. 2015; 126: LBA-2.