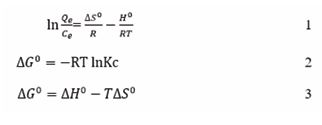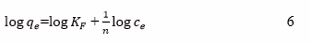Review on Adsorption Techniques for the Removal of Toxic Organic Dyes: Water Treatment Technology
- 1. Department of Chemistry, Borana University, Ethiopia
Abstract
The discharge of toxic organic dyes from industrial effluents presents a significant environmental challenge due to their persistence, toxicity, and potential carcinogenicity. While various physical, chemical, and biological methods exist for wastewater treatment, adsorption is widely regarded as a superior technique due to its high efficiency, operational simplicity, and cost-effectiveness. This review provides a comprehensive overview of adsorption techniques for the removal of toxic organic dyes from aqueous solutions. It critically examines different types of adsorbents, with a particular focus on activated carbon (AC) and the development of low-cost alternatives from agricultural waste materials. The fundamental principles of adsorption, including physisorption and chemisorption, are discussed, along with key factors influencing the process, such as pH, temperature, initial dye concentration, and adsorbent porosity. Furthermore, the application of essential isotherm models like Langmuir and Freundlich for analyzing adsorption equilibrium is detailed. The review highlights the potential of sustainable, bio-derived adsorbents as a promising pathway for efficient and economical dye removal, addressing the limitations of conventional treatment methods.
Keywords
• Adsorption Techniques; Organic Dyes; Activated Carbon; Wastewater Treatment; Low-Cost Adsorbents.
Citation
Shuka Y (2025) Review on Adsorption Techniques for the Removal of Toxic Organic Dyes: Water Treatment Technology. JSM Chem 11(1): 1066.
INTRODUCTION
The discharge of toxic organic dyes from industrial effluents, particularly from textile, paper, printing, and cosmetic industries, poses a significant threat to aquatic ecosystems and human health [1]. These dyes are often non biodegradable, highly stable, and potentially carcinogenic, leading to severe environmental pollution and long-term ecological damage [2]. Conventional wastewater treatment methods, such as coagulation, flocculation, and biological degradation, have shown limited effectiveness in removing these persistent pollutants due to their complex aromatic structures and resistance to degradation [3]. Adsorption has emerged as a highly efficient, cost effective, and versatile technique for the removal of organic dyes from aqueous solutions [4]. The process involves the accumulation of dye molecules onto the surface of an adsorbent material through physical or chemical interactions, offering advantages such as high removal efficiency, ease of operation, and the potential for adsorbent regeneration [5]. A wide range of adsorbents, including activated carbon, clay minerals, biochar, metal-organic frameworks (MOFs), and polymer-based composites, have been explored for dye removal, each with distinct advantages and limitations [6].Recent advancements in adsorption technology have focused on enhancing the adsorption capacity, selectivity, and sustainability of adsorbent materials [7]. Modified and nanocomposite adsorbents, for instance, have demonstrated superior performance due to their high surface area, tunable porosity, and enhanced surface reactivity [8]. Additionally, the development of low-cost and eco-friendly adsorbents derived from agricultural waste and biomass has gained significant attention, aligning with the principles of green chemistry and sustainable wastewater treatment [9]. Currently, activated carbon (AC) is the most widely utilized adsorbent for the removal of dyes due to its microporous structure, significant adsorption capacity, large surface area, and high surface reactivity. In comparison to other adsorbents, AC offers several benefits, including effective removal of odors and tastes, straightforward process design, high molecular selectivity, low energy requirements, reusability, strong adsorption potential, and durability in harsh and toxic conditions [10]. One of the major drawbacks of AC is its high cost of production, which limits its application extensively. In generally, any abundantly available, low-cost and safe organic matter such as agricultural (Plant leaves) can be considered as raw materials for AC production [11]. This is due to plant leaves are easily available and loaded with various functional groups such as alcohols, carboxylic acids, ethers, phenols, etc., which are useful in the adsorption of dye molecules [12]. Some plant-based adsorbents that have been employed to remove MB from solutions include waste grape leave [13], Neem (Azadirachta indica) leaves [14], Typha leaves [15], Rubus idaeus Leaves [16] and Lippia adoensis (LA) plant leaves adsorbent [17]. This review critically examines the latest developments in adsorption techniques for the removal of toxic organic dyes, with a focus on the mechanisms, influencing factors (pH, temperature, contact time, and initial dye concentration), and performance of various adsorbents. Furthermore, the challenges and future prospects of adsorption technology are discussed to guide further research in this field.
WATER POLLUTION
Water pollution is the contamination of water bodies such as lakes, rivers, oceans, aquifers, and groundwater by harmful substances or conditions that degrade water quality, rendering it toxic to humans or the environment. This contamination occurs when pollutants are discharged directly or indirectly into water without adequate treatment to remove them. While natural events like volcanic eruptions or algal blooms can cause pollution, the vast majority is a result of human activities [18]. The impact of a pollutant is relative to the intended use of the water. For example, a river might be considered too polluted for drinking (potable use) but still be suitable for irrigation or industrial cooling. Therefore, managing water pollution involves assessing the specific water body, its designated uses, and the type and concentration of contaminants present [5].
Categories of Water Pollutants
Water pollutants are broadly classified into three main categories: chemical, biological, and physical as shown in Figure 1.
Figure 1 Source of water pollution.
Chemical Pollutants: Dissolved or suspended chemical substances pose significant threats to aquatic ecosystems and human health, categorized primarily into inorganic pollutants, organic pollutants, and radionuclides. Inorganic pollutants include heavy metals such as lead (Pb), mercury (Hg), and arsenic (As), which are highly toxic even at low concentrations and can accumulate in living organisms. Additionally, nutrients like nitrates (NO3 −) and phosphates (PO4 3−) from fertilizers and sewage contribute to eutrophication, further degrading water quality. Organic pollutants encompass carbon-based compounds, including pesticides, herbicides, industrial solvents, and petroleum products such as oil and gasoline. Notably, many of these compounds, particularly Polychlorinated Biphenyls (PCBs), are classified as persistent organic pollutants (POPs) due to their resistance to environmental degradation, allowing them to persist in the environment for extended periods. Lastly, radionuclides, which originate from radioactive waste produced by nuclear power plants, mining operations, and military activities, can contaminate water sources with radioactive isotopes, posing severe risks of cancer and genetic damage to exposed populations [19].
Biological Pollutants: This category refers to microorganisms that cause waterborne diseases. They are typically introduced into water from sewage, livestock operations, and septic systems. Common biological pollutants include: Bacteria: Such as Escherichia coli (E. coli) and Vibrio cholerae (the agent of cholera). Viruses: Including Hepatitis A and Norovirus. Protozoa: Such as Giardia lamblia and Cryptosporidium, which cause severe gastrointestinal illness [11-20].
Physical Pollutants: These include materials and conditions that affect the physical properties of water. Suspended Solids: Silt, sediment, and other insoluble particles from soil erosion and industrial discharge increase water turbidity (cloudiness). This blocks sunlight from reaching aquatic plants and can clog the gills of fish.Thermal Pollution: The release of heated water from power plants and industrial facilities raises the temperature of water bodies. Warmer water holds less dissolved oxygen, stressing or killing fish and other aquatic organisms that have specific temperature requirements. Solid Waste: Trash such as plastic bags, bottles, and other debris can choke, entangle, and poison wildlife. Over time, plastics break down into microplastics, which can be ingested by aquatic life and enter the human food chain [21].
SOURCES OF WATER POLLUTION
Generally, the pollutants come from three prominent sources- (i) sewage discharged into the river, into the (ii) river industrial without any pretreatment and (iii) surface run off from agricultural land, where chemical fertilizers, pesticides, insecticides and manures are used [19], as shown in Figure 1. The sources of pollutants are categorized as either point source or non-point source, which dictates the strategy for control and regulation.
Point Source Pollution
Point source pollution refers to contamination that originates from a single, identifiable source, as illustrated in Figure 1. Because the specific point of origin is known, these sources are relatively easy to manage, monitor, and regulate. Common examples include wastewater flowing from a factory’s discharge pipe, effluent from a municipal sewage treatment plant’s outfall, or a direct oil spill from a tanker [18].
Non-point Source Pollution
This type of pollution comes from diffuse, widespread sources rather than a single point of origin. It is carried into water bodies by rainfall or snowmelt moving over and through the ground. Because of its diffuse nature, non point source pollution is much more difficult to control. Some Examples Agricultural Runoff: Fertilizers, pesticides, and animal waste from farms and fields; Urban Runoff: Oil, grease, heavy metals, and trash washed from streets, parking lots, and rooftops and Atmospheric Deposition: Pollutants from the air (like acid rain) that settle into water bodies [17-22].
CONSEQUENCES OF WATER POLLUTION
The effects of water pollution are far-reaching, with devastating consequences for human health, entire ecosystems, and the economy. From a human health perspective, contaminated drinking water is a leading cause of acute illnesses worldwide, including cholera, typhoid, and dysentery. Beyond immediate sickness, long-term exposure to chemical pollutants in water can lead to chronic conditions such as cancer, neurological disorders, and severe organ damage [13-24]. Environmentally, the disruption to ecosystems is profound. One of the most significant impacts is eutrophication, a process where nutrient pollution from fertilizers and sewage triggers massive algal blooms. When effluents discharged these algae die, their decomposition by bacteria consumes vast amounts of dissolved oxygen, creating hypoxic “dead zones” where fish and other aquatic life cannot survive. Furthermore, many industrial chemicals and heavy metals like mercury are persistent toxins that bioaccumulate, building up in the tissues of individual organisms. As these organisms are consumed by others, the toxins become increasingly concentrated up the food chain in a process called biomagnification, ultimately endangering top predators like birds, marine mammals, and humans [14 26]. These health and environmental crises translate directly into significant economic costs. The financial burden includes the high expense of treating contaminated water to make it safe for consumption, major losses in tourism and recreational revenue due to phenomena like beach closures, the collapse of commercial fisheries, and a decrease in property values near polluted water bodies.
ORGANIC DYES
A dye is an organic pigment that selectively absorbs light at specific wavelengths and can adhere to fibers due to its inherent physical and chemical properties. Dyes are employed to impart color to various substrates. The blue dye indigo was the first organic dye utilized approximately 4,000 years ago, notably in the wrappings of mummies in ancient Egypt. Currently, there are around 100,000 different dyes available for commercial purchase worldwide [20]. Dyes can be categorized in various ways according to different research groups, but classifications based on chemical properties and applications have gained significant relevance in contemporary studies. Traditional classifications, primarily used by chemists, focus on the chromophoric groups present in dye molecules. Chromophores are typically electron-withdrawing groups, while auxochromes serve as electron donors. Key chromophores include -C=C-, -C=N-, -C=O-, -N=N-, -NO2 , and -NO, whereas notable auxochromes include -NHR, -NH2 , -OH, -NR2 , -COOH, -SOH, and -OCH3 . Based on the structural characteristics of chromophores, dyes can be divided into 20 to 30 distinct groups, with some of the most significant being azo, anthraquinonoid, phthalocyanine, and triarylmethane. The classification system based on usage or application is recognized by the Color Index (C.I.) and is widely employed by professionals in the dye industry and dye technology [21].Generally, the dyes can be classified into three categories: (i) cationic, (ii) anionic and (iii) non-ionic dyes. Based on the solubility, dyes are classified in to two water-soluble dyes and water-insoluble dyes. Water-soluble anionic dyes are direct, acid, and reactive dyes; a water-soluble cationic dye is basic whereas disperse, Sulphur, and azoic indigo and vat dyes are some other examples of the insoluble dyes [22].
Reactive dyes
Reactive dyes have become very popular due to their high wet fastness, brilliance and range of hues. These dyes are widely used because of the ability of their reactive groups to bind to the fibers, their stability and their processing conditions, etc., and are the second largest classes of dyes. These dyes are capable of forming a covalent bond with the amine or sulfhydryl groups of proteins in textile fibers [23].
Acid dyes
Acid dyes are predominantly composed of carboxylic or sulfuric acid salts, exhibiting high solubility in water and possessing anionic characteristics. These dyes primarily establish ionic bonds during the dyeing process, although van der Waals and hydrogen bonds may also contribute to the overall interaction. Acid dyes are utilized in an acidic dye bath, making them particularly effective for dyeing protein fibers and polyamide materials. Their application extends to thermoplastic hydrophobic fabrics, as these dyes are water-soluble and demonstrate significant efficacy on the aforementioned substrates [24].
Direct dyes
Direct dyes are applied on rayon, linen, cellulosic fibers and can also be used to dye wool and silk. These dyes are loosely bound to the molecules of the fiber. Thus, after being applied to fabrics, they do not rapidly dry up. Such dyes are used at temperatures between 79.4 ?–93.3 ?. It can only be cold washed because they lack the fixative property. One of the benefits of direct dye is the cheapest price compared to all other dyes [25].
Basic dyes
Basic dyes are characterized by their solubility in water and their ability to generate positively charged cations in aqueous environments. These dyes find application across various domains, including the dyeing of paper, modified nylon, and polyester, as well as cationic polyethylene. Additionally, they are utilized in certain medical applications and were historically employed within industries focused on tannin-etched fabrics [26].
Sulfur Dyes
Sulfur dyes are characterized by their insolubility in water and, with few exceptions, are primarily utilized for dyeing cellulosic fibers. For application to the substrate, these dyes are first reduced to their water-soluble Leuco form using a sodium sulfide solution. Subsequently, the sulfur dye is generated within the pores of the fiber through either atmospheric or chemical oxidation. This class of dyes is significant for producing economically viable tertiary shades, particularly black, on cellulosic materials. A notable example is C.I. Sulphur Black 1, which is synthesized by heating 2,4-dinitrophenol in the presence of sodium polysulfide [27].
Disperse dyes
These dyes exhibit significantly low water solubility. Their molecular structure is characterized by a small, planar configuration that is non-ionic, featuring polar functional groups such as –NO2 and –CN. Primarily, they are employed for dyeing polyester fibers due to their ability to engage with the polyester polymer chains, leading to the formation of dispersed particles [28].
Indigo dyes
Indigo, classified as a vat dye, is characterized by its initial insolubility in water, which is altered through an alkaline reduction process that renders it soluble. During the textile dyeing procedure, the water-soluble leuco form of indigo is utilized. Upon exposure to atmospheric oxygen, this form undergoes oxidation, reverting to its original insoluble keto state, thereby facilitating optimal adhesion of the dye to the fabric. Indigo dyes are predominantly employed in the dyeing of blue denim, resulting in their extensive production on a global scale [29]. In their composition, these dyes typically consist of a keto group and require the vatting process to become water-soluble. This process involves alkaline conditions and is similar to the application techniques used for sulfur dyes. Vat dyes are primarily employed for dyeing cotton-based textiles, particularly denim and jeans [30].
DYE-REMOVAL TECHNOLOGY
Current color removal treatment approaches involve chemical, physical and biological processes. These technologies, however, have both advantages and disadvantages. Most of these traditional procedures are inapplicable on a broad scale because of the high expense and disposal issues associated with the significant the quantity of sludge produced in the final treatment process [28], as highlighted in Figure 2.
Figure 2 Category of Pollutant removal techniques1.
Physical treatment
Table 1: Various physical dye removal methods along with its advantages and disadvantages [29-34].
|
Method |
Description |
Advantages |
Disadvantages |
|
Adsorption |
Adsorbents fashioned from high adsorption capacity materials to adsorb dye molecules. |
Excellent removal method for a wide variety of dyes. Re-generable adsorbent. |
Adsorbents can be costly |
|
Coagulation and flocculation |
Agents are added to dye wastewater where dye particles clump together. Clumps can then be removed through filtration |
Cheap. Robust method. Suitable only for disperse, Sulphur and vat dye effluents. |
Generation of huge amounts of concentrated sludge. Not suitable for water-soluble dyes |
|
Ion exchange |
A reversible chemical process whereby ions from the dye wastewater swaps with similar ions attached to a stationary solid surface. |
Can be regenerated. Good dye removal method. Produces high quality water. |
Effective to a limited number of dyes |
|
Irradiation |
Radiation is used to remove dye molecules from dye wastewater. |
Effective at laboratory scale |
A huge amount of dissolved oxygen is required. Expansive. |
|
Membrane filtration |
Dye wastewater is passed through a membrane which separates dye particles from clean water. |
Effective for water recovery and reusing. |
Costly initial investment. Unsuitable for dye removal. |
|
Reverse osmosis |
Pressure driven system where water is passed through an extremely thin membrane leaving contaminants on one side and water on the other |
Common water recycling method. Effective for decolouring and desalting a variety of dyes. Produces clean and pure water. |
costly. Requires high pressure. |
Physical methods as shown in Table 1 are pre dominantly employed to segregate substantial dissolved substances and to reclaim valuable materials utilized in primary processes. Various physical technologies have been implemented for dye removal, including ion exchange, adsorption, and membrane filtration techniques. Filtration approaches such as reverse osmosis, microfiltration, and nanofiltration are utilized to extract dyes from aqueous solutions for potential reuse; however, these methods are often economically unfeasible due to their high maintenance requirements. Conversely, adsorption has demonstrated greater efficacy in the decolorization of dyes compared to coagulation methods. This technique leverages low-cost adsorbents, such as polymeric resins and bentonite clay; nevertheless, it faces economic limitations as these adsorbents are typically employed in a single-use capacity without opportunities for regeneration [31].
Chemical treatment
Physical methods alone are inadequate for the complete removal of dyes from textile effluents, as they necessitate additional treatment to eliminate solid waste, thereby incurring extra costs in the overall treatment process. (Table 3)
Table 3: Various biological dye removal methods along with its advantages and disadvantages [34-41].
|
Method |
Description |
Advantages |
Disadvantages |
|
Adsorption by microbial biomass |
Mixture of organic living organisms fashioned to adsorb dye molecules. |
Selected dyes have an exceptional affinity towards microbial biomass. |
Not an effective method for all dyes. |
|
Algae degradation |
Algae consumes dye particle for self- growth. |
Able to consume dyes. Cheap. Easily assessable. Environmental friendly process. |
Unstable system. |
|
Enzyme degradation |
Extracted enzyme used to degrade dye molecules. |
Cheap. High efficiency. Non-toxic. Possesses the ability to degrade dyes using enzymes. Reusable. |
Unreliable amount of enzyme production. |
|
Fungal cultures |
Fungus breaks down dye molecules and consumes them for self-growth |
Can eliminate various types of dyes at once. Flexible method. |
Lengthy growth phase. Requires large reactors for complete dye removal. |
|
Microbial cultures such as mixed bacterial |
Bacteria mixed with chemicals or other bacteria to remove dye particles. |
Takes a maximum of 30 h in decolourization of dye wastewater which is considered fast |
Effective to a limited number of dyes |
also present certain disadvantages, they are frequently favored due to their relative ease of implementation and cost-effectiveness. Techniques such as flocculation and coagulation are commonly employed to remove organic pollutants. Although coagulation is particularly effective in degrading insoluble dyes, its efficacy diminishes when addressing soluble dyes present in textile effluents. A significant limitation of these chemical methods is the generation of sludge, which requires further management and disposal, ultimately contributing to an increase in the total operational costs associated with the treatment process [32].
Biological Treatment
Biodegradation and bioremediation as shown in Table 3 naturally through a diverse array of specialized microorganisms, including bacteria, fungi, algae, and yeast, that inhabit wastewater and contaminated environments. This process can also be artificially stimulated in a laboratory setting by isolating and characterizing suitable microorganisms, followed by scaling up the process to facilitate the treatment and decolorization of textile effluents.
ADSORPTION PROCESS
Adsorption is a surface phenomenon in multi component fluid (gas or liquid) where a molecule (solute) is attached to the surface of a solid substance by chemical and physical bonds. Such substances that provide the surface (space) are called adsorbents, while the molecule which is removed from the liquid phase is known as the adsorbate [42].
Type of adsorption
Depending upon the energy of the interaction of adsorbed species with the adsorbent, one can differentiate physisorption and chemisorption.
Physisorption: It involved intermolecular force of attraction. It is based on the fact that there is a concentration gradient ofadsorbate in solution and adsorbent so that adsorbate migrates from solution into the pores of adsorbent to reach the point of maximum force of attraction and thus get adsorbed. Physisorption is generally reversible, nonselective, and associated with smaller enthalpy change, typically 5–40 kJ/mol [43].
Chemisorption: Chemisorption involves chemical bonding between the adsorbent and adsorbate molecule. The chemical bonds may be covalent or ionic in nature. In general most of the solids have a property to adsorb the solute from solution but few of them are actually used commercially. It is usually deemed to be irreversible, selective and accompanied with higher heat change usually in the range of 40–125 kJ/mol. The removal of chemicals by chemisorption is more challenging than the removal of substances by physisorption because of the type of bonding and forces between the adsorbent and adsorbate. If the circumstances are right, the two processes may happen one after the other or simultaneously [44].
Activated Carbon
Activated carbon is one of the commonly adopted adsorbent material for the removal of organic pollutants from textile effluents. Dyes which are less soluble in water shows slow rate of adsorption on carbon content while, Physisorption: It involved intermolecular force of attraction. It is based on the fact that there is a concentration gradient ofadsorbate in solution and adsorbent so that adsorbate migrates from solution into the pores of adsorbent to reach the point of maximum force of attraction and thus get adsorbed. Physisorption is generally reversible, nonselective, and associated with smaller enthalpy change, typically 5–40 kJ/mol [43]. Chemisorption: Chemisorption involves chemical bonding between the adsorbent and adsorbate molecule. The chemical bonds may be covalent or ionic in nature. In general most of the solids have a property to adsorb the solute from solution but few of them are actually used commercially. It is usually deemed to be irreversible, selective and accompanied with higher heat change usually in the range of 40–125 kJ/mol. The removal of chemicals by chemisorption is more challenging than the removal of substances by physisorption because of the type of bonding and forces between the adsorbent and adsorbate. If the circumstances are right, the two processes may happen one after the other or simultaneously [44]. water soluble dyes like acidic-basic dyes and reactive dyes do not get readily adsorbed on carbon. The reason behind their poor adsorption is the polar nature of these dyes vs. non polar nature of carbon. Hence, adsorption on carbon would be less efficient when used alone. But it becomes more efficient adsorbent when used along with coagulants. Although, Activated carbon has been found to be quite effective in removal of dyes but due to its high cost and loss of adsorbent during the deactivation, forces the researchers in seek of replacing it with some low cost adsorbents [45]. Production of AC was achieved typically through two routes, physical activation and chemical activation [46].
Physical activation: Physical activation is a two-step process. It involves carbonization of raw material followed by activation at elevated temperatures in the presence of suitable oxidizing gases such as carbon dioxide, steam, air or their mixtures. Carbonization temperature ranges between 400 ? to 800 ?, and activation temperature ranges between 800 ? to 1100 ? [47].
Chemical Activation: Preparation of activated carbon by chemical activation is a single step process in which carbonization and activation is carried out simultaneously. Initially the precursor is mixed with chemical activating agent, which acts as dehydrating agent and oxidant. Chemical activation offers several advantages over physical activation which mainly include (i) lower activation temperature (< 800 ?) compared to the physical activation temperature (800 – 1100 ?), (ii) single activation step, (iii) higher yields, (iv) better porous characteristics, and (v) shorter activation times. The most commonly used chemical activating agents are H3 PO4 , ZnCl2 , NaOH, H2 SO4 , NaCl and KOH [48].
Factors Affecting Adsorption of Dye
There are many factors affecting dye adsorption such as solution pH, temperature, initial dye concentration, etc. Thus, the effects of these parameters are to be taken into account. Optimization of such conditions will greatly help in the development of industrial-scale dye removal treatment process. In this section, some of the factors affecting adsorption of dyes are discussed below.
Effect of initial dye concentration: The impact of initial concentration on adsorption is contingent upon its ratio to the available binding sites on the adsorbent’s surface. At low concentrations, only a limited number of adsorption sites are occupied. As the concentration of pollutants increases, a greater proportion of these sites becomes occupied until saturation is achieved, at which point no additional binding sites are available. Consequently, for a constant quantity of adsorbent, the percentage of the pollutant removed through adsorption diminishes as the concentration increases [49].
Temperature: The adsorption efficacy of an adsorbent is significantly influenced by the temperature of the adsorbate solution. The process of dye adsorption onto an adsorbent can be classified as either endothermic or exothermic. In endothermic adsorption processes, an increase in temperature enhances adsorption efficiency due to heightened mobility of dye molecules and greater accessibility of active adsorption sites, which is a consequence of thermal energy. Conversely, in exothermic adsorption processes, higher temperatures lead to a decline in uptake; this phenomenon occurs because elevated temperatures weaken the binding interactions between the dye molecules and the active sites on the surface of the adsorbent, resulting in diminished adsorption capacity. The adsorption thermodynamics are measured using the Gibbs equation, as follows:
In Eq. (1), qe is the equilibrium adsorption capacity, Ce is the equilibrium adsorbate concentration ΔS0 is the standard entropy, R is the gas constant, ΔH0 is the standard enthalpy, and T is the absolute temperature. In Eq. (3), ΔG0 indicates the nature of the adsorption reaction. If the randomness (ΔS) of the dye adsorption increases, then the change in enthalpy (ΔH) indicates the nature of the reaction, such as whether it is an endothermic or exothermic reaction [50].
pH: The pH of a solution is a critical parameter influencing the adsorption of dyes, as it affects both the surface characteristics of the adsorbent and the chemical speciation of the adsorbate. The effect of pH is typically assessed by preparing a suspension in an electrolyte solution, followed by the introduction of the adsorbate into the equilibrated mixture. The pH is then adjusted to the desired level using standardized solutions of HNO3 and NaOH. Generally, an increase in solution pH correlates with an enhancement in the adsorption capacity for cationic dyes, whereas the adsorption of anionic dyes tends to decline with rising pH levels. Therefore, optimizing the solution pH is essential for maximizing dye removal efficiency; however, the specific optimal pH value is contingent upon the chemical nature of the dye involved [51].
Porosity of the Adsorbent: The efficacy of adsorption is significantly influenced by the accessibility of the internal surface of the adsorbent. A critical characteristic of adsorbent materials is their pore structure, which encompasses the total number, morphology, and dimensions of pores. These factors collectively dictate both the adsorption capacity and the kinetics of the adsorption process. The role of pores in adsorption mechanisms is predominantly contingent upon their dimensional attributes. Many solid adsorbents exhibit intricate architectures comprising pores of varying sizes and geometries, which contribute to their overall adsorption performance [52]. The International Union of Pure and Applied Chemistry (IUPAC) classifies the size of pores for adsorbents as, i, micropores (r25 nm). Macropores exist at the entrance of activated carbon, and serve as carriers. The size of pore must conform to the particle diameter of pollutants. If the molecular size of the adsorbate is close to the pore size of the adsorbent, the force of gravity will increase. The surface area of the activated carbon is important, because pollutants are adsorbed on the surface of the activated carbon [43].
Application of various isotherm models in dye removal Equilibrium adsorption isotherm models are essential requirements for the design of adsorption systems and the interaction between adsorbent and adsorbent. Models used to analyze equilibrium adsorption data include the Langmuir and Freundlich models [53].
Langmuir isotherm: Langmuir is one of the most prominent isotherm models that describe the nonlinear equilibrium between the amount of adsorbed analyte and its free amount in solution at a constant temperature. This model is simple and provides a good description of the experimental behavior in a wide range of working conditions. This isotherm is based on the assumption of monolayer adsorption on an adsorbent with a homogeneous structure that (i) all adsorption sites are uniform and equal in energy, (ii) only one sorbate, (iii) one sorbate molecule reacts with one active site , (iv) no interaction between adsorbed species The Langmuir relationship is expressed as follows [27].
Where qe : equilibrium adsorption capacity, qmax : maximum adsorption capacity (mg/g), KL : Langmuir constant, Ce : equilibrium adsorbate concentration (mg/L). Further analysis of the Langmuir equation can be made using a dimensionless equilibrium parameter, also known as the separation factor, given by
Where: KL = Langmuir adsorption constant related to the free energy of adsorption (L/mg), Co = The initial solution concentration (mg/L). The value of RL reflects the type of isotherm to be either: (i) irreversible (RL = 0); (ii) favorable (0 < RL < 1); (iii) linear (RL = 1); or (iv) unfavorable (RL < 1) [54,55].
Freundlich isotherm: The Freundlich adsorption model Eq. (6) is an empirical relation which indicates that adsorption takes place in multiple layers. The linear equation of Freundlich isothermal adsorption equation is as follows:
where qe : Equilibrium adsorption capacity (mg/g), KF : Freundlich constant, Ce : equilibrium adsorbate concentration (mg/L), n: a constant, always larger than 1. The Freundlich model shows that a higher concentration of initial analyte would lead to a higher adsorption rate of nanoparticles. The Freundlich model assumes that the number of positions in the adsorption action with free energy can be potentially reduced by increasing free energy [31]. According to this assumption, with an increasing solute concentration in the solution, the surface concentration never reaches saturation due to high free energy surface sites for adsorption. The values of KF and n can be obtained from the slope and intercept of the plot of log qe against log Ce of the Freundlich Plots. The Freundlich isotherm basically indicates whether the adsorption proceeds with ease or difficulty. Where n is dimensionless, represents Freundlich constants characteristics of the system and the measure of the nature and strength of the adsorption process and the distribution of active sites. The relationship between the Freundlich constant characteristics (n) and the strength of the bond energy in the sorption process (N) indicates that as surface density decreases (n > 1), all surface sites become equivalent (n = 0) [47].
SUMMARY
This review addresses the critical issue of water pollution caused by toxic organic dyes released from various industries. It begins by outlining the classification of dyes and the severe environmental and health impacts associated with their discharge into aquatic systems. A comparative analysis of different dye-removal technologies physical, chemical, and biological is presented, highlighting their respective advantages and disadvantages. The review establishes that among these methods, adsorption is a particularly effective and economically viable approach for treating dye-laden wastewater. The core of the review focuses on the principles of the adsorption process, differentiating between physisorption and chemisorption mechanisms. It extensively discusses the role of activated carbon (AC) as a benchmark adsorbent, noting its high efficiency but also its significant drawback of high production cost. To counter this limitation, the review emphasizes the growing trend towards developing low-cost, sustainable adsorbents from widely available agricultural byproducts and plant-based materials, which are rich in functional groups conducive to dye uptake.Key operational parameters that govern the efficiency of the adsorption process, including solution pH, temperature, initial dye concentration, and the physical characteristics of the adsorbent, are thoroughly analyzed. The importance of understanding the equilibrium of the adsorption process through isotherm models is explained, with a detailed look at the Langmuir and Freundlich models. In essence, this review synthesizes the current knowledge on adsorption-based dye removal, underscoring the shift towards sustainable and green adsorbent materials as the future of wastewater treatment.
FUTURE INSIGHT
While significant progress has been made in using adsorption for dye removal, several areas warrant further investigation to enhance its practical applicability and sustainability.
- Development of Advanced Adsorbents: Future research should focus on fabricating novel composite and hybrid adsorbents (e.g., polymer-biochar composites, magnetic nanocomposites) that offer enhanced adsorption capacity, selectivity for specific dyes, and easier separation from water. Tailoring the surface chemistry of adsorbents through advanced modification techniques can lead to materials with superior performance for complex, multi dye industrial effluents.
- Regeneration and Reusability: The economic feasibility of adsorption on an industrial scale heavily depends on the ability to regenerate and reuse the adsorbent. More research is needed to develop efficient, low-cost, and environmentally friendly regeneration methods that do not damage the adsorbent’s structure or create secondary pollutants.
- Bridging Lab-Scale to Industrial Application: Most studies are conducted under controlled laboratory conditions using single-dye solutions. Future work must focus on testing the performance of promising adsorbents with real industrial wastewater, which contains a complex mixture of dyes, salts, and other organic matter. Pilot scale studies are crucial to validate the practicality and scalability of these technologies.
- Mechanistic Understanding: Deeper insights into the adsorption mechanisms at a molecular level are needed. The use of advanced analytical techniques and computational modeling can help elucidate the interactions between dye molecules and adsorbent surfaces, enabling the rational design of next-generation adsorbents..
- Integrated Treatment Systems: Combining adsorption with other treatment processes, such as photocatalysis or biodegradation, could create powerful hybrid systems. Adsorption can concentrate the pollutants, which can then be degraded by a secondary process, leading to complete mineralization of the dyes and overcoming the limitations of any single method. By addressing these areas, the field of adsorption can move towards more robust, sustainable, and commercially viable solutions for mitigating the environmental impact of dye pollution.
REFERENCE
- Denga Ramutshatsha-Makhwedzha, Avhafunani Mavhungu, Mapula Lucey Moropeng, Richard Mbaya. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon. 2022; 8: 1-9.
- Kamal Al-Mokhalelati, Iman Al-Bakri, Nesrin Al Shibeh Al Wattar. Adsorption of methylene blue onto sugarcane bagasse-based adsorbent materials. J Phys Org Chem. 2021; 34: 7.
- Lalit Goswami, Anamika Kushwaha, Saroj Raj Kafle, Beom -Soo Kim. Surface Modification of Biochar for Dye Removal from Wastewater. Catalysts. 2022; 12: 1-27.
- Dagme Zewde, Belete Geremew. Removal of Congo red using Vernonia amygdalina leaf powder: optimization, isotherms, kinetics, and thermodynamics studies. Environ Pollut Bioavailab. 2022; 34: 88-101.
- Asmaa Elsherbeny Moharm, Gamal A. El Naeem, Hesham MA. Soliman, Ahmed I. Abd-Elhamid, Ali A. El-Bardan, Taher S. Kassem, et al. Fabrication and Characterization of Effective Biochar Biosorbent Derived from Agricultural Waste to Remove Cationic Dyes from Wastewater. Polymers. 2022; 14: 1-16.
- Tra Huong Do, Van Tu Nguyen, Nguyen Quoc Dung, Manh Nhuong Chu, Doan Van Kiet, Tran Thi Kim Ngan, et al. Study on methylene blue adsorption of activated carbon made from Moringa oleifera leaf. Materials Today: Proceedings. 2020; 38: 3405-3413.
- Lorena Robles-Melchor, Maribel Cornejo-Mazón, Diana Maylet Hernández-Martínez, Gustavo F. Gutiérrez-López, Santiago García- Pinilla, Edgar Oliver López-Villegas, et al. Removal of Methylene Blue from Aqueous Solutions by Using Nance (Byrsonima crassifolia) Seeds and Peels as Natural Biosorbents. J Chem. 2021; 1-13.
- Modi S, Yadav VK, Gacem A, Ali IH, Dave D, Khan SH, et al. Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles. Water. 2022; 14: 2-26.
- Massoud Kaykhaii, Mojtaba Sasani, Sahar Marghzari. Removal of Dyes from the Environment by Adsorption Process. 2018; 6: 31-35.
- Sangeeta Sharma, Amandeep Kaur. Various methods for removal of dyes from industrial effluents - a review. Indian J Sci Technol. 2018; 11: 1-21.
- Ikram M, Naeem M, Zahoor M, Hanafiah MM, Oyekanmi AA, Ullah R, et al. Biological Degradation of the Azo Dye Basic Orange 2 by Escherichia coli: A Sustainable and Ecofriendly Approach for the Treatment of Textile Wastewater. Water. 2022; 14: 2063.
- Jiaqi Cheng, Conghua Zhan, Jiahui Wu, Zhixiang Cui, Junhui Si, Qianting Wang, et al. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega. 2020; 5: 5389-5400.
- Nompumelelo Malatji, Edwin Makhado, Kwena D Modibane, Kabelo E Ramohlola, Thabiso C Maponya, Gobeng R Monama, et al. Removal of methylene blue from wastewater using hydrogel nanocomposites : A review. 2021; 11: 1-27.
- Seyyed Alireza Mousavi, Davood Shahbazi, Arezoo Mahmoudi, Parastoo Darvishi. Methylene blue removal using prepared activated carbon from grape wood wastes: adsorption process analysis and modeling. Water Qual Res J. 2022; 57: 1-19.
- Das M, Mishra C. Jackfruit leaf as an adsorbent of malachite green: recovery and reuse of the dye. SN Appl Sci. 2019; 1.
- Seyyed Alireza Mousavi, Arezoo Mahmoudi, Samira Amiri, Parastoo Darvishi, Elham Noori. Methylene blue removal using grape leaves waste: optimization and modeling. Appl Water Sci. 2022; 15: 1-11.
- Krishna G. Bhattacharyya, Arunima Sharma. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dyes and Pigments. 2005; 65: 51-59.
- Sánchez Orozco R, Martínez-Juan M, García-Sánchez J, Ureña-NúñezF. Removal of methylene blue from aqueous solution using Typha stems and leaves. BioResources. 2018; 13: 1696-1710.
- Mosoarca G, Popa S, Vancea C, Dan M, Boran S. Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent-Raspberry (Rubus idaeus) Leaves Powder. Polymers (Basel). 2022; 14: 1966.
- Mengesha M, Shuka Y, Eyoel T, Tesfaye T. Novel Biomaterial-Derived Activated Carbon from Lippia Adoensis (Var. Koseret) Leaf for Efficient Organic Pollutant Dye Removal from Water Solution. Am J Appl Chem. 2024; 12: 29-46.
- Gemechu Ameya Buli, Abdella Gure Duga, Engda Dessalegn. Antimicrobial Activity of Lippia adoensis var. koseret Against Human Pathogenic Bacteria and Fungi. Am J Clin Exp Med. 2015: 3; 118-123.
- Woldewosen Z. Identification of Lippia adoensis for Access and Benefit sharing. 2016.
- Belachew G, Engeda D, Tilku D. In vitro antioxidant activity of Lippia adoensis Var. Koseret, Thymus schimperi Ronniger and Rosmarinus officinalis Leaf extracts and their effects on oxidative stability of ground raw beef meat during refrigeration storage. 2022; 6: 319-327.
- Tadele Assefa Aragaw, Fekadu Mazengiaw Bogale. Biomass-Based Adsorbents for Removal of Dyes From Wastewater: A Review. Front Environ Sci. 2021; 9.
- Lafi R, Montasser I, Hafiane A. Adsorption of congo red dye from aqueous solutions by prepared activated carbon with oxygen- containing functional groups and its regeneration. Adsorpt Sci Technol. 2019; 37: 160-181.
- Rodríguez-Arellano G, Barajas-Fernández J, García-Alamilla R, Lagunes-Gálvez LM, Lara-Rivera AH, García-Alamilla P. Evaluation of Cocoa Beans Shell Powder as a Bioadsorbent of Congo Red Dye Aqueous Solutions. Materials. 2021; 14: 2-14.
- Salunkhe B, Schuman TP. Super-Adsorbent Hydrogels for Removal of Methylene Blue from Aqueous Solution: Dye Adsorption Isotherms, Kinetics, and Thermodynamic Properties. Macromol. 2021; 1: 256-275.
- Roy M, Saha R. Dyes and their removal technologies from wastewater: A critical review. Elsevier Inc. 2020.
- Senthil Kumar P, Janet Joshiba G, Carolin C. Femina, Varshini PS Priyadharshini, Arun Karthick MS, et al. A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalin. Water Treat. 2019: 172: 395-416.
- Deyou Yu, Wang Lili, Minghua. Wu. Simultaneous removal of dye andheavy metal by banana peels derived hierarchically porous carbons. J Taiwan Inst Chem Eng. 2018; 93: 543-553.
- Khote B, Sharma S, Singh N, Sharma A. Dye Removal from Low Cost Adsorbent:-A Review. Int Res J Eng Technol. 2019; 6: 1030-1035.
- Velusamy S, Roy A, Sundaram S, Kumar Mallick T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide- Based Adsorption Strategies for Textile Wastewater Treatment. Chem Rec. 2021; 21: 1570-1610.
- Said Benkhaya, Souad M rabet, Ahmed El Harfi. A review on classifications, recent synthesis and applications of textile dyes. Inorg Chem Commun. 2020; 115: 107891.
- Chavan R B. Environmentally friendly dyes. Handbook of Textile and Industrial Dyeing. 2011; 1: 515-561.
- Jyotshana Sharma, Shubhangani Sharma, Vineet Soni. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg Stud Mar Sci. 2021; 45: 101802.
- Uddin MJ, Ampiaw RE, Lee W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere. 2021; 284: 131314.
- Eren E, Afsin B. Investigation of a basic dye adsorption from aqueous solution onto raw and pre-treated sepiolite surfaces. Dye. Pigment. 2007; 73: 162-167.
- Nirav P. Raval, Prapti U. Shah, Nisha K. Shah. Malachite green ‘a cationic dye’ and its removal from aqueous solution by adsorption. Applied Water Science. 2017; 7: 3407-3445.
- Slama HB, Chenari Bouket A, Pourhassan Z, Alenezi FN, Silini A, Cherif-Silini H, et al. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Applied Sci. 2021; 11: 1-21.
- Pranay Dutta, Razaya Rabbi, Mohammad Abu Sufian, Shahnaz Mahjebin. Effects of textile dyeing effluent on the environment and its treatment: A review. Eng Appl Sci Lett. 2022; 5: 1-17.
- Hao Y S, Othman N, Abbas M, Zaini A. Methylene blue and Congo red removal by activated carbons: A current literature. Agric Environ. 2022; 14: 29-44.
- Khan I, Saeed K, Zekker I, Zhang B, Hendi AH, Ahmad A, et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water. 2022; 14.
- Abdullah T. Al-Fawwaz, Mufida Abdullah. Decolorization of Methylene Blue and Malachite Green by Immobilized Desmodesmus sp. Isolated from North Jordan. Int J Environ Sci Dev. 2016; 7: 95-99.
- Durão C, Pedrosa F, Dinis-Oliveira RJ. Greenish-blue discoloration of the brain and heart after treatment with methylene blue. Forensic Sci Med Pathol. 2021; 17: 148-151.
- Asha Singh, Rozi Sharma, Deepak Pant, Piyush Malaviya. Engineered algal biochar for contaminant remediation and electrochemical applications. Sci Total Environ. 2021; 774: 145676.
- Aditya Moorthy, Rohith Gaikwad, Shreya Krishna, Raghuraj Hegde, Tripathi K K, Preeti G. Kale, et al. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids—An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J Maxillofac Oral Surg. 2021; 20: 418-425.
- Hamad HN, Idrus S. Recent Developments in the Application of Bio- Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers. 2022; 14.
- Anisuzzaman SM, Joseph CG, Pang CK, Affandi NA, Maruja SN, VijayanV. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. Chem Engineering. 2022; 6.
- Vikas Dinkar Gosavi, Sandip Sharma. A general review on various treatment methods for textile wastewater. J Environ Sci. 2013; 3: 29- 39.
- Vanitha Katheresan, Jibrail Kansedo, Sie Yon Lau. Efficiency of various recent wastewater dye removal methods: A review. J Environ Chem Eng. 2018; 6: 4676-4697.
- Yagub MT, Sen TK, Afroze S, Ang HM. Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci. 2014; 209: 172-184.
- Asha Srinivasan, Thiruvenkatachari Viraraghavan. Decolorization ofdye wastewaters by biosorbents: A review. J Environ Manage. 2010;91: 1915-1929.
- Nusrat Tara, Sharf I. Siddiqui, Geetanjali Rathi, Saif Ali Chaudhry, Inamuddin, Abdullah M. Asiri. Nano-Engineered Adsorbent for the Removal of Dyes from Water: A Review. 2020; 16: 14-40.
- Mandake M B, Walke S, Naniwadikar M, Patil G, Jadhav S G. Experimental Investigations of the Removal of Methylene Blue from Waste Water using Agricultural Adsorbant. Int J Membr Sci Technol. 2023; 10: 1-7.
- Omowumi D. Agboola, Nsikak U. Benson. Physisorption and Chemisorption Mechanisms Influencing Micro (Nano) Plastics- Organic Chemical Contaminants Interactions: A Review. Front Environmental Sci. 2021; 9: 1-27.