Etanercept-Induced Transverse Myelitis: A Clinical Image
- 1. Rheumatology Department, Cork University Hospital, Ireland
- 2. Radiology Department, Cork University Hospital, Ireland
Citation
Mohamed Ashraf FA, Sum L, Harney S (2013) Etanercept-Induced Transverse Myelitis: A Clinical Image. JSM Clin Case Rep 1(1): 1004
CLINICAL IMAGE
A 29-year-old healthy gentleman was diagnosed with ankylosing spondylitis (AS) and was treated with etanercept 50 mg subcutaneously weekly, after failing conservative treatment with non-steroidal anti-inflammatory drugs (NSAIDs) and exercise. Twenty months after initiation of etanercept, over thecourse of two weeks, he developed bilateral lower limb pain,numbness and weakness with bilateral upper limb paraesthesia.In the ensuing week, he developed night sweats, back pain andurinary retention. Magnetic resonance imaging (MRI) of thewhole spine showed diffuse intramedullary central cervical and thoracic cord T2 hyperintense lesions with relative sparing ofthe periphery. These areas also showed patchy enhancement post gadolinium administration (Figure 1, 2 and 3).
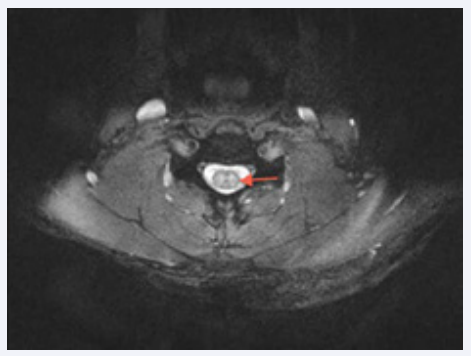
Figure 2 Axial T2-weighted MRI cervical spine showing intramedullary predominantly central hyperintense signal within the cervical cord.
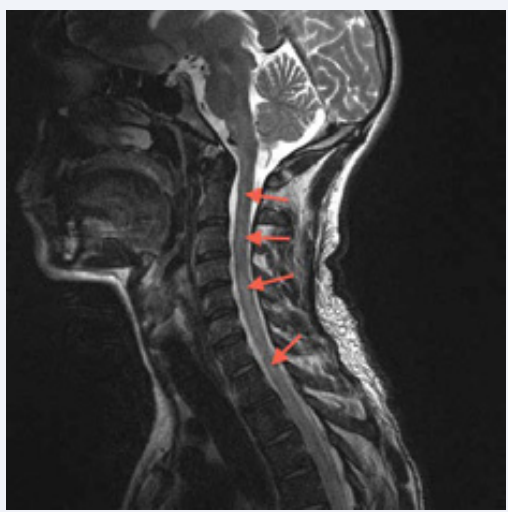
Figure 1 Sagittal T2-weighted MRI of the cervical spine showing extensive intramedullary hyperintense lesions within the cervical and upper thoracic cord (arrowheads).
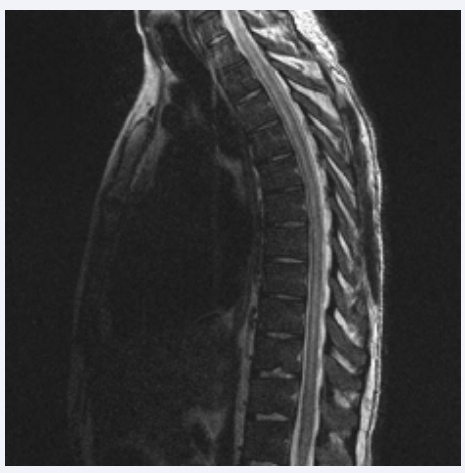
Figure 3 Sagittal T2-weighted MRI thoracic spine showing extensive intramedullary hyperintense lesions centrally within the thoracic cord.
There was no significant expansion of the spinal cord and MRI of the brain did not demonstrate any abnormality. Lumbar puncture and blood serology ruled out infection, malignancy and other metabolic causes. His blood cultures were sterile, and his skin testing for tuberculosis (TB) with a subsequent interferon-gamma release assay (IGRA) test were both negative. Overall clinical presentation and imaging features were compatible with transverse myelitis (TM).
His etanercept was held and he was initially treated with three daily pulses of intravenous methylprednisolone (total of three grams) for its anti-inflammatory activity and continued on high-dose oral steroid therapy 60 mg daily with gradual tapering to zero over the course of two months with complete clinical recovery. Repeat MRI of the spine three months later showed marked improvement with faint residual T2 hyperintense areas within the spinal cord and complete resolution of these findings were seen a year later.
Etanercept is a human tumor necrosis factor receptor: Fc fusion protein designed to antagonize tumor necrosis factor alpha (TNF-alpha) function. Since its development more than a decade ago, biologic disease modifying anti rheumatic drugs (DMARDs) such as etanercept have been shown to be associated with neurological manifestations including demyelination and transverse myelitis [1-3]. The presence of night sweats in our patient despite a negative tuberculosis skin testing (TST), in the setting of an immunocompromised patient on a TNF-alpha blocker, prompted us to the possibility of tuberculosis (TB) with a false-negative TST result, as there is a high incidence of skin anergy associated with the immunosuppressed as well as in inflammatory arthritis patients such as ankylosing spondylitis. We followed up the TST with an IGRA blood test that has superior sensitivity in immunocompromised patients [4], which was negative. We remained vigilant for TB, and the patient was carefully monitored throughout the course of his treatment and was reviewed by a TB specialist, despite his negative initial screening for TB prior to the commencement of etanercept (including no history of prior TB infection, exposure or treatment, a negative chest X-ray, TST and clinical examination). Unreliable if negative in patients on biologic DMARDs, TST has however been proven effective as a positive test should be taken as evidence of TB infection [5].
We acknowledge that even though the clinical and diagnostic findings in our patient were temporally related to the biologic DMARD with occurrence after its initiation and complete resolution of symptoms and MRI findings after discontinuation of therapy, we could not completely rule out the possibility that the transverse myelitis may have been due to his ankylosing spondylitis, which had been previously described [6,7].
There is very little medical literature to guide treatment of acute TM and there are no controlled studies for the treatment with corticosteroids. Despite some studies that have shown steroids (especially intravenous methylprednisolone) hasten neurologic recovery [8] and shorten the duration of the attack [9,10], there are no available studies discussing when treatment of steroid is no longer beneficial in acute TM. While we know that acute TM is usually a transient process, we also acknowledge the possibility of a more subacute course in patients with underlying systemic autoimmune or inflammatory diseases [11-13], the reason for the longer duration of steroid therapy given to our patient.
Even though demyelination and transverse myelitis are rare side effects of biologic DMARDs, its potentially disabling and lifethreatening effects merits attention, and extra caution should be taken in patients with existing neurological symptoms or disorders.