Subacute Invasive Pulmonary Aspergillosis in an Immunocompromised Patient: A Case Report
- 1. Department of Pulmonology and Respiratory Disease, University Hospital Center of Casablanca, Morocco
Abstract
A case of semi-invasive pulmonary aspergillosis was diagnosed in a 65-year-old patient with acute myeloid leukemia undergoing chemotherapy. Chest imaging revealed bilateral cavitary associated with reversed halo signs. The diagnosis was confirmed by a high galactomannan level in both bronchial aspirates and blood. Treatment with voriconazole led to a favorable clinical outcome for the patient.
Keywords
• Subacute Aspergillosis; Immunosuppression; Galactomannan; Voriconazole; Man Male
Citation
Ait mouddene N, Arfaoui H, Msika S, Bamha H, Bougteb N, et al. (2025) Subacute Invasive Pulmonary Aspergillosis in an Immunocompromised Patient: A Case Report. JSM Clin Case Rep 13(2): 1261.
INTRODUCTION
Subacute invasive pulmonary aspergillosis (SAIA) is a fungal infection primarily caused by aspergillus fumigatus. Ubiquitous in the environment, its spores are constantly inhaled by humans and Among the species involved in human diseases, aspergillus fumigatus remains the most frequently encountered, involved in 80 to 90% of cases, followed by aspergillus flavus and aspergillus niger. Pulmonary localization is considered the most common infection [1,2]. It mainly affects patients with moderate immunosuppression. Although less aggressive than invasive aspergillosis, SAIA can lead to significant pulmonary damage if not diagnosed and treated in a timely manner [3-5].This article presents a clinical case of SAIA in a patient with acute myeloid leukemia undergoing chemotherapy.
CLINICAL CASE
The patient is a 65-year-old man with a history of chronic smoking (40 pack-years, ceased for 8 months), alcohol consumption, and hashish use (discontinued for over 13 years). He underwent a prostatectomy four years ago without signs of malignancy. He has been under treatment for acute myeloid leukemia for the past year, receiving five cycles of chemotherapy with regular follow ups by his primary physician. The patient was referred to the pulmonology department following the incidental discovery of radiological abnormalities on a routine thoracic CT scan ordered by his hematologist. However, he exhibited no significant respiratory symptoms, including chest pain, cough, hemoptysis, or dyspnea. Clinical examination revealed mild cutaneous and mucosal pallor without other extra-thoracic abnormalities, evolving in an afebrile context with moderate, unquantified weight loss noted by the patient.
Physical examination on admission
The patient had a low body mass index (BMI = 15.7 kg/ m²), was afebrile (37.1°C), normotensive (BP = 110/79 mmHg), normocardic (HR = 91 bpm), eupneic (RR = 21 breaths/min), and had normal oxygen saturation in ambient air (SaO? = 97%). Pulmonary, pleural, cardiovascular, and other systemic examinations were unremarkable.
Radiological Assessment
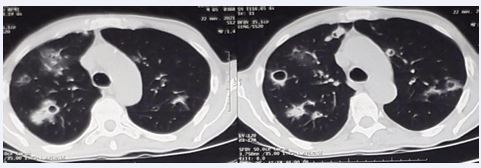
Upon hospital admission, thoracic CT imaging revealed multiple diffuse nodules across both lung fields, distributed in a craniocaudal and peripheral pattern. These included solid nodules, cavitary nodules, and nodules displaying the characteristic reversed halo sign (Figure 1).
Figure 1 CT images showing solid-like, excavated nodules and others with a characteristic inverted halo appearance.
Biological Assessment
The complete blood count revealed normochromic normocytic anemia with a hemoglobin level of 11.5 g/dL,associated with leukocytosis predominantly neutrophilic and lymphopenia (leukocytes: 14,530/mm³; neutrophils: 11,390/mm³; lymphocytes: 1,070/mm³). C-reactive protein (CRP) levels were elevated at 45 mg/L. Renal and hepatic function tests were within normal limits. Specific testing for Aspergillus antigens (galactomannan) in the blood was positive (0.89 ng/mL), while Aspergillus serology was negative (Table 1).
Table 1: Abnormal values measured in the blood.
|
Blood count |
Values before treatment |
Values after treatment |
Reference values |
|
Hémoglobine |
11.5 g/dl |
12 g/dl |
12 – 16 g/dl |
|
Leucocytes |
14530/ mm³ |
10760/mm³ |
4000- 10000/ mm³ |
|
Neutrophils |
11.390/mm³ |
7930/mm³ |
4000- 7000/ mm³ |
|
Lymphocytes |
1070/mm³ |
1492/mm³ |
> 1500/ mm³ |
|
C-reactive protein |
45 mg/L |
15.3 mg/L |
< 6 mg/L |
|
Aspergillus antigens (galactomannan) |
0.89 ng/mL |
|
0,5 ng/ml |
Endoscopic Assessment
Flexible bronchoscopy revealed, at the entrance of the left nasal cavity, a blackish, irregular, cauliflower- like formation covered with a whitish coating. In both the right and left bronchi, diffuse bronchial inflammation of mild intensity (grade 1) was observed, along with the presence of mucopurulent secretions. Bronchial aspirates were obtained to screen for Mycobacterium tuberculosis (MBT) through direct examination and culture, as well as Xpert MTB-Rif testing. Additionally, Pneumocystis jirovecii and other common pathogens were tested, all yielding negative results. The search for Aspergillus antigen in bronchial aspirates, through galactomannan assay, returned a positive result of 4.66 ng/mL (threshold= 0.5 ng/mL) (Table 2).
|
Bronchial aspirations and analysis |
Results |
|
Mycobacterium tuberculosis |
Négative |
|
Pneumocystis jirovecii |
Négative |
|
other common pathogens |
Négative |
|
Aspergillus antigen, galactomannan |
Positive : 4.66 ng/mL (0.5 ng/mL) |
Table 2: Analysis carried out on bronchial aspirations
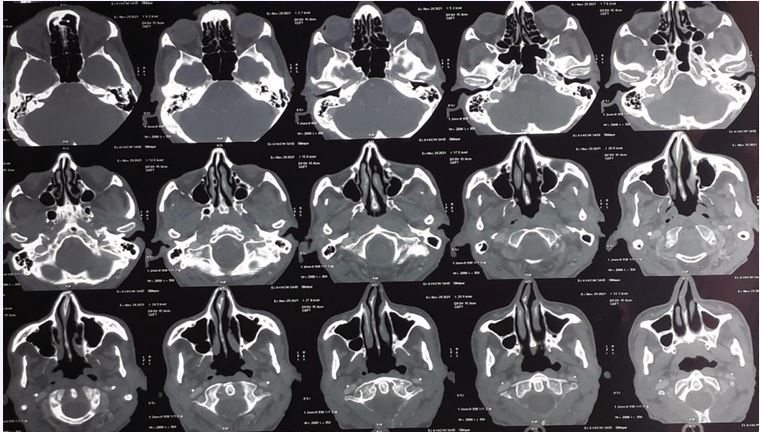
Due to the abnormal appearance of the nasal cavity, a sinus CT scan and a naso-rhino- cavoscopy with biopsy were performed (Figure 2).
Figure 2 CT images of the sinuses showing left septal thickening without signs of maxillary sinusitis
Imaging showed left septal thickening without signs of maxillary sinusitis, while endoscopy revealed a budding tumor in the left middle turbinate. Mycological (direct examination and culture) and bacteriological tests were negative. Histopathological analysis demonstrated chronic inflammatory and hemorrhagic changes with no specific features and no evidence of malignancy.
Final Diagnosis
Considering the presence of moderate immunosuppression due to acute myeloid leukemia (AML) and chemotherapy, the radiological findings of reversed halo sign, diffuse bilateral cavitary and solid nodules, along with elevated galactomannan levels in both bronchial aspirates and blood, a diagnosis of subacute invasive pulmonary aspergillosis was confirmed.
Treatment
The patient received intravenous voriconazole for seven days, with an initial loading dose of 400 mg per day for 48 hours, followed by a maintenance dose of 200 mg per day for five days. Oral maintenance therapy was continued for six months at a dose of 6 mg/kg. Additionally, a seven-day course of antibiotic therapy with amoxicillin clavulanic acid (3g/day) was administered.
Outcome
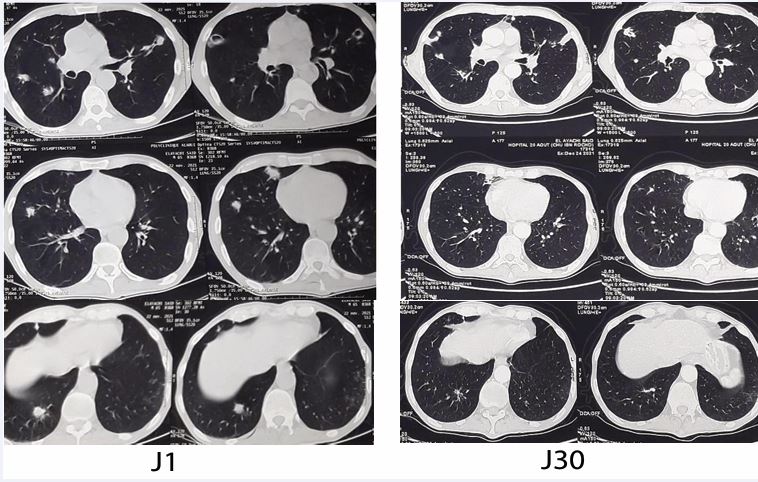
The patient showed favorable clinical and biological improvement, with a decrease in white blood cell count (from 14,530 to 10,760/mm³), neutrophils (from 11,390 to 7,930/mm³), and C-reactive protein (from 45 to 15.3 mg/L), along with an increase in lymphocytes (from 1,070 to 1,492/mm³). Radiological follow-up after one month demonstrated significant regression of pulmonary lesions on CT scan (Figure 3).
Figure 3 CT images showing regression of excavated nodules after one month of Voriconazole treatment.
DISCUSSION
Aspergillus is a filamentous fungus (or mold) that develops in the form of filaments called hyphae and produces spores that are dispersed by air or water. Ubiquitous in the environment, its spores are constantly inhaled by humans. Their suspension in the air can be amplified by certain activities, such as construction work or handling compost and moldy hay. Among the species involved in human diseases, aspergillus fumigatus remains the most frequently encountered, involved in 80 to 90% of cases, followed by aspergillus flavus and aspergillus niger. Pulmonary localization is considered the most common infection [1,2]. This germ is responsible for several forms of pulmonary aspergillosis, including subacute pulmonary aspergillosis or semi-invasive aspergillosis (formerly called chronic necrotizing pulmonary aspergillosis), which has a rapid progression (<3 months). This form of aspergillosis is generally observed in moderately immunocompromised patients, such as the case of our patient who has acute myeloid leukemia under chemotherapy [3], but not sufficiently immunocompromised to develop an invasive angioinvasive aspergillosis [4,5]. The clinical expression is non-specific and depends on aspergillus exposure, including the quantity and virulence of the inhaled particles, local factors such as the integrity of the anti infectious defenses of the tracheobronchial tree, and the host’s immune response, which plays a key role in pathogenesis [2]. Thus, the clinical and radiological characteristics of semi-invasive aspergillosis are similar to those of chronic cavitary pulmonary aspergillosis, but its evolution is faster [3]. Among the clinical presentations encountered, respiratory symptoms such as cough, dyspnea, expectoration, and hemoptysis are common, as well as general symptoms like fever, fatigue, weight loss, or loss of appetite [4,5]. Chest CT scans show often excavated and bilateral opacities, usually in the upper lobes and sometimes in the Fowler segments [2-6]. The positive diagnosis of semi-invasive aspergillosis is not always straightforward, as the diagnostic yield of bronchial washings and biopsy is low [7]. In fact, the diagnosis requires a combination of characteristics: one or more cavities with or without a fungal mass present, or visible nodules on thoracic imaging, such as excavated nodules or reversed halos, with direct evidence of Aspergillus infection (microscopy or culture from a biopsy) or an immunological response to Aspergillus. It has been shown that the serum galactomannan antigen from aspergillus is correlated with the diagnosis of invasive pulmonary aspergillosis [8]. Indeed, the galactomannan assay is a key diagnostic tool, with increased sensitivity in bronchial aspirations and the exclusion of alternative diagnoses, persisting for at least 3 months [2,3]. The prolonged antifungal treatment lasts from three to six months, probably until the aspergillus serology becomes negative or as part of a long-term suppressive treatment. Itraconazole seems to be the preferred drug because of its long history of use. Studies report good results with voriconazole, which is available in our national context [2].
CONCLUSION
Semi-invasive a pulmonary aspergillosis remains serious condition in patients with moderate immunosuppression. The diagnosis relies on a combination of clinical, radiological, and biological signs, with a central role for galactomannan testing. Antifungal treatment, primarily with itraconazole or voriconazole, allows for favorable outcomes when initiated early.
REFERENCES
- Ledoux MP, Guffroy B, Nivoix Y, Simand C, Herbrecht R. Invasive Pulmonary Aspergillosis. Semin Respir Crit Care Med. 2020; 41: 80- 98.
- Blandin S, David G. L’aspergillose en pratique pour le pneumologue. Rev Pneumol Clin. 2008; 64: 202-210.
- Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, et al. European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016; 47: 45-68.
- Müller NL, Franquet T, Lee KS, Silva CIS. Imaging of Pulmonary Infections. Lippincott Williams & Wilkins. 2007; 198.
- Marshall H, Jones S, Williams A. Chronic pulmonary aspergillosis - longterm follow-up over 20 years, a case report. J Radiol Case Rep. 2010; 4: 23-30.
- Young Kim SU, Kyung Soo Lee, Joungho Han, Jhingook Kim, Tae Sung Kim, Sung Wook Choo, et al. Semiinvasive Pulmonary Aspergillosis. Am J Roentgenol. 2012; 174.
- Saraceno JL, Phelps DT, Ferro TJ, Futerfas R, Schwartz DB. Chronic necrotizing pulmonary aspergillosis: approach to management. Chest. 1997; 112: 541-548.
- Melancon CC, Lindsey J, Russell GB, Clinger JD. The role of galactomannan Aspergillus antigen in diagnosing acute invasive fungal sinusitis. Int Forum Allergy Rhinol. 2019; 9: 60-66.