Successful management by Combination of Alternaria alternata Subcutaneous Allergen Immunotherapy with Omalizumab in five cases of Chronic Rhinosinusitis with or without Nasal Polyps Co-morbid Asthma
- 1. Department of Pulmonology, National Allergy Centre, India
Abstract
Allergen Immunotherapy is a unique and effective therapeutic option in patients allergic to molds. Alternaria alternata is known to be a significant source of fungi aeroallergens. Sensitization to A. alternata is a risk factor for development and persistence of Chronic Rhinosinusitis with (CRSwNP) or without (CRSsNP) nasal polyps Co-morbid Asthma. The role of Allergen Immunotherapy (AIT) by Alternaria alternata allergen extract combined with Omalizumab has been evaluated in very few studies. In our five cases, the combined effect of Alternaria alternata Subcutaneous Immunotherapy (SCIT) with Omalizumab was safe, effective, and synergistic, sustained and achieved the maintenance dose faster. Our conclusion was based on significant reduction in symptom and medication scoring by Asthma Control Test (ACT), Rhinitis Control Assessment Test (RCAT), Sino-nasal Outcome Test (SNOT-22), Improvement in Peak Expiratory Flow Rate (PEFR) variability, Immunological (Ratio of Specific IgE to Total IgE for Alternaria alternata), Endoscopic and Radiographic parameters after 1 year of treatment. We suggest a causative role of Alternaria alternata allergen in the pathogenesis of Chronic Rhinosinusitis with or without nasal polyps Co-morbid Asthma.
Keywords
Subcutaneous Immunotherapy (SCIT); Allergen specific Immunotherapy (ASIT); Peak Expiratory Flow Rate (PEFR); Severe Asthma with Fungal sensitization (SAFS); Functional Endoscopic Sinus Surgery (FESS); Acute Exacerbation of respiratory diseases (AERD); Rhinitis Control assessment Test (RCAT); Asthma Control Assessment (ACT); Sino-nasal Outcome Test22 (SNOT-22); Maximum tolerated Dose (MTD).
Citation
Kathuria PC, Rai M, Rai D (2021) Successful management by Combination of Alternaria alternata Subcutaneous Allergen Immunotherapy with Omalizumab in five cases of Chronic Rhinosinusitis with or without Nasal Polyps Co-morbid Asthma. JSM Clin Case Rep 9(1): 1182.
ABBREVIATIONS
CRSwNP: Chronic Rhinosinusitis with Nasal Polyp; CRSsNP: chronic Rhinosinusitis without Nasal Polyp; SCIT: Subcutaneous Immunotherapy; ASIT: Allergen specific Immunotherapy; PEFR: Peak Expiratory Flow Rate; SAFS: Severe Asthma with Fungal Sensitization; FESS: Functional Endoscopic Sinus Surgery; AERD: Acute Exacerbation of respiratory diseases; RCAT: Rhinitis Control assessment Test; ACT: Asthma Control Assessment; SNOT: Sino-nasal Outcome Test; MTD: Maximum tolerated Dose.
INTRODUCTION
Chronic Rhinosinusitis is defined as an inflammatory disorder of the mucosa of nasal passages and the paranasal sinuses that lasts for at least 12 weeks with relevant symptomatology or signs with at least 2 of the following:
a) Nasal Obstruction, b) Facial Pain/ Pressure/ Fullness, c) Hyposmia/ Anosmia, d) Mucopurulent drainage and objective evidence of mucous inflammation of at least 1 of the following: a) Polyps in the nasal cavity in middle meatus, b) Edema in the middle meatus or anterior ethmoid region and c) purulent mucus [1]. Two subtypes of chronic rhinosinusitis comprise distinct clinical syndrome a) Chronic Rhinosinusitis without nasal Polyp (CRSsNP) b) Chronic Rhinosinusitis with nasal polyp (CRSwNP). Approximately 20% of Chronic Rhinosinusitis patients have Asthma and up to 70% of patients with Asthma have chronic rhinosinusitis (CRS) [2,3]. Among severe asthma co-morbidities, one of the most frequent and relevant is chronic rhinosinusitis with nasal polyps (CRSwNP) which affects at least 30% of patients [4].
There is a debate as to what exactly triggers the inflammatory pathways of Chronic Rhinosinusitis (CRS) where infections, superantigens, allergens, biofilms, asthma and aspirin hypersensitivity have all been implicated. The co-existence of CRS and Asthma is explained by the involvement of similar inflammatory pathophysiological pathways in these two conditions, particularly sensitization to allergens and increased serum specific IgE. There is a higher prevalence of positive allergy skin tests and nasal polyposis with most of the evidence pointing to nasal polyps as a manifestation of a systemic disease [5] A possible mechanism for the relationship between severe Chronic Rhino-sinusitis and Asthma has been proposed by Bachert et al, where the production of inflammatory cytokines induces bone marrow to upregulate Eosinophils, mast cells and basophils, which ultimately migrate to the airway mucosa and cause a reactive inflammatory response [6] Currently the success rate of medical management of CRS is approximately 50% [7]The risk of failure in CRS is higher in patients with certain conditions such as nasal polyposis, co-morbid Asthma, Acute Exacerbation of Respiratory Disease (AERD) and allergic fungal rhinosinusitis [8]
Since surgery of CRSwNP, referred to as Functional Endoscopic Sinus Surgery (FESS), is a standard treatment with good functional results in patients resistant to medical treatment and need long term follow up, but most nasal polyps do re-occur with varied post-operative recurrence rate ranging from 13-75% [9] Two of our patients (case 1& 2) have had history multiple surgical interventions (FESS) done due to recurrent nasal polyps.
Molds are considered the third most frequent cause of allergic respiratory disease after dust mites and pollens. Most studies concluded that fungal extracts were not tolerated [10] Alternaria is a risk factor for asthma which activates the innate immune system and enhances lung inflammation, has been linked to airway hyper-responsiveness [11] Alternaria alternata is considered to be a marker of primary sensitization and a ubiquitous saprophytic fungus found in soil and plants, has been described both indoors and outdoors as an allergen associated with Allergic Rhinitis and severe Asthma with fungal sensitization (SAFS) [12]. It is mainly present in the spore wall and respond molecularly to a 30 kDa dimer specific to fungi kingdom. Sensitization to Alt a 1 is identified in more than 90% of patients allergic to A. alternate [13]Alt a 1 is a predominant allergen of the 17 reactive IgE proteins identified in A. alternata, 12 of which are described in the international union of Immunological societies (www.allergen.org). This allergen is released in considerable amount by A. alternata and related species which access the respiratory tract and elicit allergic reaction in these patients.
Omalizumab is a humanized, recombinant antihuman immunoglobulin E (IgE) antibody derived from murine monoclonal antibodies with proven efficacy and effectiveness for adult and paediatric patients with severe allergic asthma [14]. Omalizumab reduces allergen induced responses by decreasing level of free serum IgE and expression of high affinity IgE receptors [15].
The combination of Specific Immunotherapy (SIT) and antiIgE resulted in prolonged inhibition of allergen IgE binding compared with either treatment alone, which might contribute to enhanced clinical efficacy and safety, has a synergistic mode of action and is superior [16,17]. The combined AIT with Omalizumab (a humanized monoclonal antibody) is proven in numerous clinical trials comprising patients with either allergic rhino-conjunctivitis or allergic Asthma. In one study treatment with omalizumab before an intensified rush immunotherapy compared with specific immunotherapy (SIT) alone significantly decreased allergic side effects including anaphylaxis reaction in patients with ragweed induced Seasonal Allergic Rhinitis (SAR) [18] Allergen specific Immunotherapy (SIT) alone induces anti-inflammatory effect, T-cell tolerance by decreasing allergen induced proliferation, alteration of secreted cytokines, stimulation of apoptosis and the production of T-regulatory cells.
In our five cases, two of our cases of Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) and three cases of Chronic Rhinosinusitis without Nasal Polyps (CRSsNP), were given combined A. alternata Allergen Immunotherapy with Omalizumab resulted in faster and sustained build-up dose, better safety profile, improvement in symptom & medication scoring, PEFR and Immunological scoring.
CASE PRESENTATION
Case 1
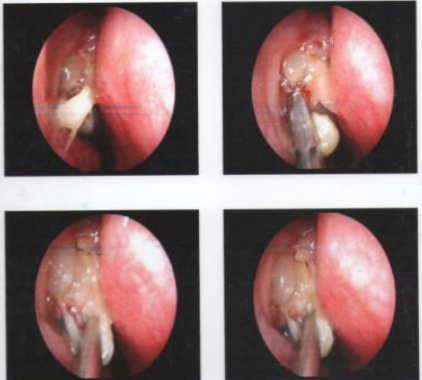
13year old male with history of Chronic Rhinosinusitis with Nasal Polyp (CRSwNP) and Seasonal Bronchial Asthma since five years was prescribed symptomatic treatment (nasal and inhaled corticosteroids, cetirizine, antibiotic and on & off oral corticosteroids) but had poor relief. He has had history of recurrent episodes of nose block and nasal congestion, progressive loss of smell (hyposmia) and seasonal exacerbations (March-April), underwent surgical intervention thrice (FESS with right ethmoidectomy-year 2011, 2014, 2015) (Figure 1 (a) & (b)) for recurrent nasal polyps. Comprehensive allergy testing by Skin Prick Test (SPT) & Specific IgE was done in 2016.
Figure 1a Case 1, (2011) before surgery- Endoscopy revealed fungal sinusitis with nasal polyp.
Figure 1b Case 1, (2018) Repeat Endoscopy with biopsy was suggestive of mucosal edema.
In-vivo and In-vitro test: (Table 1 (a)) Skin Prick Test (SPT) were positive for Alternaria alternata-10mm, Aspergillus fumigatus-6mm, Candida albicans-4mm, Yeast-3mm.
|
Table 1 (a): Clinical characteristics of Case 1. |
||
|
|
Case 1 |
|
|
|
2016 |
2018 |
|
RCAT/ACT scoring |
11/12 Baseline |
23/21 |
|
SNOT-22 |
73 |
17 |
|
PEFR (Variability) |
450-550 L/mts (20%) |
500-550 L/mts (9%) |
|
Total IgE |
2248 IU/ML |
3024 IU/ml |
|
Specific IgE/ Total IgE % (Alternaria alternata) |
4.1% |
2.24% |
|
Eosinophil % / AEC |
7.2 % / 710 cells/ul |
3.9 % / 363 cells/ul |
|
Serum cortisol |
3.6 mcg/ml |
6.0 mcg/ml |
|
SPT
Histamine Alternaria alternata Aspergillus fumigatus Candida albicans Yeast |
wheal size Specific IgE
|
Wheal size Specific IgE
|
Specific IgE were positive for Alternaria alternata-93.0 kUA/l, Aspergillus fumigatus-27.7 kUA/l, Candida albicans-25.6 kUA/l, yeast-8.01 kUA/l. Total IgE- 2248 IU/ml, Absolute Eosinophil Count-710 cells/Ul.
Pulmonary Function Tests (2016) were normal FEV1/FVC86.5% with LABA and ICS
Recommendation: Combined Alternaria alternata Subcutaneous Allergen Immunotherapy (SCIT) along with Inj Omalizumab 150mg once a month was started by gradual updosing protocol of build-up phase to achieve MTD (2.5 mg per month maintenance dose) every 4 weeks with supportive therapy for 2 years (Table 1 (b)). His nasal and respiratory symptoms were better controlled with normal Lung function and no variability by PEFR measurement. RCAT (11 Baseline /23), ACT 12 Baseline /21) and SNOT 22 (73 Baseline /17) improved after 1 year. The patient is asymptomatic and under follow-up.
| Table 1 (b): Duration of Combined Alternaria alternata extract conc. (5mg/ml) for Cluster AIT as per schedule along with Inj Omalizumab (150mg) X 15 days before AIT followed by once in a month for 2 years*. | ||||||
|---|---|---|---|---|---|---|
| No. of Visits | 1st Visit | 2nd Visit | 3rd Visit | 4th Visit | 5th Visit | 6th Visit |
| Cluster Dose AIT (5mg/ ml) Per visit Combined with Inj Omalizumab (150mg)* | 0.05/0.05ml @ 60 mts interval | 0.1/0.1 ml @ 60 mts interval | 0.2/0.2 ml @ 60 mts interval | 0.3/0.2 ml @ 60 mts interval | 0.5 ml | l 0.5 ml (2.5 mg) M.D |
| Cluster Dose frequency | First day | 10-12 days | s 10-12 days | 15-20 days | 20-30 days | Every 4 weeks |
Case 2
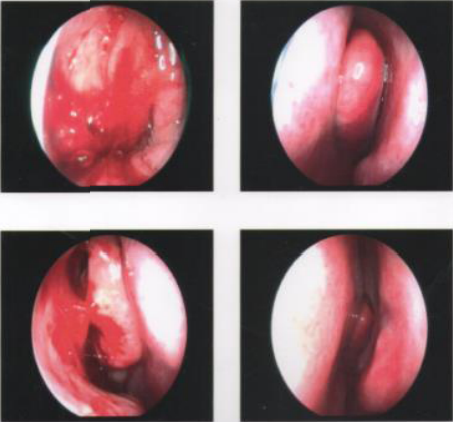
24 years old male, known case of Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) and Co-morbid Bronchial Asthma for the last 11 years. He has had symptoms of nasal obstruction and thick nasal mucus since early childhood. He has been on regular intake of montelukast, cetirizine and indigenous Ayurvedic treatment with no relief. There is history of exacerbation of symptoms of symptoms during change of season (March/April). He consulted an Ear, Nose & Throat specialist and was found to have polypoidal lesion occupying both nasal cavities with yellow and highly viscous mucus. He has had four nasal surgeries during this period and underwent polypectomy and Radiofrequency Sinus Surgery (Figure 2).
Figure 2 Case 2, (2014) before surgery Endoscopy was suggestive of fungal sinusitis with nasal polyp
NCCT PNS (2014) done before surgery was suggestive of low density polypoidal mucosal thickening involving bilateral maxillary sinuses (left>right). DNS to right side with mucosal thickening on right side.
Repeat Endoscopy (2017) after 1 year of AIT: revealed mucosal edema.
In-vivo and In-vitro test: (Table 2 (a)) Total IgE-217 IU/ml. Skin Prick Test (SPT) were positive for Alternaria alternata8mm, Aspergillus fumigatus-7mm, Candida albicans-5mm, 5mm, Yeast-4mm. Specific IgE were positive for Alternaria alternata-5.58 kua/l, Aspergillus fumigatus-1.49 kua/l, Candida albicans-1.18 kua/l, Yeast-1.12 kua/l
|
Table 2 (a): Clinical characteristics of Case 2. |
|||||||
|
|
Case 2 |
|
|||||
|
|
2016 |
2017 |
|||||
|
RCAT/ ACT Scoring |
13/12 Baseline |
23/22 |
|||||
|
SNOT-22 |
60 |
16 |
|||||
|
PEFR (variability) |
400-500 L/mts (20%) |
500-550 L/mts (9%) |
|||||
|
Total IgE |
217 IU/ML |
320 IU/ML |
|||||
|
Specific IgE/ Total IgE % (Alternaria alternata) |
2.5% |
0.24% |
|||||
|
Eosinophil % / AEC |
0.7 % / 52 cells/ul |
0.5% / 50 cells/ul |
|||||
|
Serum cortisol |
5.9 mcg/ml |
10.6 mcg/dl |
|||||
|
SPT wheal size Specific IgE
|
SPT wheal size Specific IgE
|
|||||
Pulmonary Function Test (2016): FEV1- 76% and FEV1/ FVC 86%
Recommendation: He was given combined Alternaria alternata Allergen Immunotherapy with effective dose by gradual up-dosing protocol of build-up phase to achieve MTD (2.5 mg per month maintenance dose) along with Inj Omalizumab 150mg once a month with supportive therapy for 1 year (Table 2 (b). His nasal and respiratory symptoms were well controlled. RCAT (13BASELINE/23), ACT(12 BASELINE/22), SNOT 22 (60 BASELINE/16) improved after 1 year. Patient is asymptomatic and on regular follow-up
|
Table 2 (b): Duration of Combined Alternaria alternata extract conc. (5mg/ml) for Cluster AIT as per schedule along with Inj Omalizumab (150mg) X 15 days before AIT followed by once in a month for 2 years*. |
||||||
|
No. of Visits |
1st Visit |
2nd Visit |
3rd Visit |
4th Visit |
5th Visit |
6th Visit |
|
Cluster Dose AIT (5mg/ ml) Per visit Combined with Inj Omalizumab (150mg)* |
0.05/0.05ml @ 60 mts interval |
0.1/0.1 ml @ 60 mts interval |
0.2/0.2 ml @ 60 mts interval |
0.3/0.2 ml @ 60 mts interval |
0.5 ml |
0.5 ml (2.5 mg) M.D |
|
Cluster Dose frequency |
First day |
10-12 days |
10-12 days |
15-20 days |
20-30 days |
Every 4 weeks |
|
maintenance dose (2.5mg); Conc. : concentration; AIT: Allergen Immunotherapy |
||||||
Case 3
12 years old male, known case of Chronic Rhino-sinusitis without nasal polyp (CRSsNP) and Co-morbid Bronchial Asthma with hypothyroidism, presented with history of recurrent rhinorrhea, nose block, shortness of breath, sneezing, watering from eyes and cough on and off, onset at the age of 2 years. Symptoms were initially mild to moderate but since 6-7 years, exacerbated during months of March-April. He gives h/o hospitalization on 2 occasions for pneumonia at 8 years of age. His vitals were otherwise normal.
In-vivo and In-vitro test: (Table 3 (a)) Total IgE-1196 IU/ml, Absolute Eosinophil Count (AEC)-833 cells/Ul, Skin Prick Test (SPT) were positive for Alternaria alternata-8mm, Aspergillus fumigatus-4mm, Candida albicans-4mm, Yeast-4mm,. Specific IgE were positive for Alternaria alternata-66.6 Kua/L (2018), Candida albicans-1.47 kua/l, Aspergillus fumigatus-4.03 Kua/l, yeast-1.03 kua/l.
Pulmonary Function test (2018)-FEV1-91%, FEV/FVC92.2% (on LABA 12ug and ICS 800ug/ day)
Recommendation: He was given combined Subcutaneous Alternaria alternata Allergen Immunotherapy with effective dose by gradual up-dosing protocol of build-up phase to achieve MTD (2.5 mg per month maintenance dose) along with Inj Omalizumab 150mg once a month with supportive therapy for 2 years (Table 3 (b)). RCAT (10BASELINE/22), ACT (11 BASELINE/21), SNOT 22 (61BASELINE/17) improved after 1 year. Patient is asymptomatic and on regular follow-up.
|
Table 3 (b): Duration of Combined Alternaria alternata extract conc. (5mg/ml) for Cluster AIT as per schedule along with Inj Omalizumab (150mg) X 15 days before AIT followed by once in a month for 2 years*. |
||||||
|
No. of Visits |
1st Visit |
2nd Visit |
3rd Visit |
4th Visit |
5th Visit |
6th Visit |
|
Cluster Dose AIT (5mg/ ml) Per visit Combined with Inj Omalizumab (150mg)* |
0.05/0.05ml @ 60 mts interval |
0.1/0.1 ml @ 60 mts interval |
0.2/0.2 ml @ 60 mts interval |
0.3/0.2 ml @ 60 mts interval |
0.5 ml |
0.5 ml (2.5 mg) M.D |
|
Cluster Dose frequency |
First day |
10-12 days |
10-12 days |
15-20 days |
20-30 days |
Every 4 weeks |
|
Maintenance Dose (2.5mg); conc.: concentration; AIT: Allergen Immunotherapy |
||||||
|
Table 3 (a): Clinical characteristics of Case 3. |
||||||||||||||||||||||
|
|
Case 3 |
|
||||||||||||||||||||
|
|
2018 |
2020 |
||||||||||||||||||||
|
RCAT/ACT scoring |
10/11 Baseline |
22/21 |
||||||||||||||||||||
|
SNOT-22 |
61 |
17 |
||||||||||||||||||||
|
PEFR (variability) |
300-400 L/mts (25%) |
450-500 L/mts (10%) |
||||||||||||||||||||
|
Total IgE |
1196 IU/ML |
1206 IU/ml |
||||||||||||||||||||
|
Specific IgE/ Total IgE % (Alternaria alternata) |
5.5% |
1.7% |
||||||||||||||||||||
|
Eosinophil % / AEC |
9.8 % / 833cells/ul |
0.8 % / 60 cells/ul |
||||||||||||||||||||
|
Serum cortisol |
25.2 mcg/ml (with 10mg OCS) |
2.61 mcg/ml (without OCS) |
||||||||||||||||||||
|
TSH |
7.54 mIU/l |
2.2 mIU/L |
||||||||||||||||||||
|
Histamine Alternaria alternata Aspergillus fumigatus Candida albicans Yeast |
|
|
||||||||||||||||||||
case 4
9 years old mal, known case of Chronic Rhinosinusitis without nasal polyp, Co-morbid seasonal Bronchial Asthma. He had onset of symptoms at 6 months of age. He frequently suffered from night time cough and cold, often complicated by wheezing. He had recurrent maxillary sinusitis and each episode was associated with green nasal discharge and exacerbation of asthma. Worse months (March-April). He has been on symptomatic treatment with on and off courses of oral corticosteroids, anti-histamines montelukast with no relief. Comprehensive allergy testing done.
In-vivo and In-vitro test: (Table 4 (a)) Total IgE-1679.0 IU/ml, Absolute Eosinophil Count-685 cells/Ul, Skin Prick Test (SPT) were positive for Alternaria alternata-8mm, Aspergillus fumigatus-5mm, Candida albicans-4mm, Yeast-4mm. Specific IgE were positive for Alternaria alternata-56.6 Kua/L, Candida albicans-4.25 kua/l, Aspergillus fumigatus-4.77 Kua/l, yeast-1.33 kua/l.
|
Table 4 (a): Clinical characteristics of Case 4. |
|||||||
|
|
Case 4 |
|
|||||
|
|
2018 |
2019 |
|||||
|
RCAT/ACT Scoring |
13/14 Baseline |
22/21 |
|||||
|
SNOT-22 |
54 |
14 |
|||||
|
PEFR (variability) |
300-400 L/mts (25%) |
400-450 L/mts (11%) |
|||||
|
Total IgE |
1679 IU/ml |
1880 IU/ml |
|||||
|
Specific IgE/ Total IgE % (Alternaria alternata) |
3.9% |
1.25% |
|||||
|
Eosinophil % / AEC |
10.7 % / 685cells/ul |
5 % / 350 cells/ul |
|||||
|
Serum cortisol |
2.3 mcg/dl |
5 mcg/dl |
|||||
|
SPT wheal size Specific IgE
|
|
|||||
Pulmonary Function Test- FEV1 78%, FEV1/FVC-80%
Recommendation: He was given combined Subcutaneous Alternaria alternata Allergen Immunotherapy with effective dose by gradual up-dosing protocol of build-up phase to achieve MTD (2mg per month) along with Inj Omalizumab 150mg once a month with supportive therapy for 1 year with no adverse effects (Table 4 (b)). His symptoms are well controlled with regular long acting B2 agonist and inhaled corticosteroid combination (Formeterol 12ug and Budesonide 400 ug daily) along with nasal topical steroids. RCAT (13BASELINE/22), ACT (14 BASELINE/21), SNOT-22 (54 BASELINE/14) improved after 1 year. Patient is asymptomatic and on regular follow-up.
|
Table 4 (b): Duration of Combined Alternaria alternata extract conc. (5mg/ml) for Cluster AIT as per schedule along with Inj Omalizumab (150mg) X 15 days before AIT followed by once in a month for 1 year.* |
||||||
|
No. of Visits |
1st Visit |
2nd Visit |
3rd Visit |
4th Visit |
5th Visit |
6th Visit |
|
Cluster Doses (5mg/ml) Per visit with Inj Omalizumab* |
0.05/0.05ml @ 60 mts interval |
0.1/0.1 ml @ 60 mts interval |
0.15/0.15 ml @ 60 mts interval |
0.2/0.2 ml @ 60 mts interval |
0.4ml |
0.4 ml (2mg) M.T.D |
|
Cluster Dose frequency |
First day |
10-12 days |
10-12 days |
15-20 days |
20-30 days |
Every 4 weeks |
|
M.T.D: Maximum Tolerated Dose; Conc: Concentration; AIT: Allergen Immunotherapy |
||||||
Case 5
16 years old female with history of Chronic Rhinosinusitis without nasal polyp (CRSsNP), Co-morbid asthma, onset at 2 years of age, had symptoms of nasal congestion with on and off migraine like headache and difficulty in breathing. Symptoms got aggravated during change of season (worse months-March-April-May). She had recurrent attack of coughing, wheezing and nasal congestion and each episode was associated with exacerbation. Her symptoms did not respond to conventional and supportive treatment of rhinosinusitis and asthma. Comprehensive allergy testing was done.
5.5.1 In-vivo and In-vitro test: (Table 5 (a)) Total IgE-1101.4 IU/ml, Absolute Eosinophil Count-958 cells/Ul, Skin Prick Test (SPT) were positive for Alternaria alternata-9mm, Aspergillus fumigatus-6mm, Candida albicans-4mm, Yeast-3mm, Specific IgE were positive for Alternaria alternata-18.4 Kua/L, Candida albicans-3.15 kua/l, Aspergillus fumigatus-4.18 Kua/l, yeast-2.03 kua/l.
Pulmonary function test (2016) FEV1-77% and FEV1/FVC- 78%.
Recommendation: She was given combined Subcutaneous Alternaria alternata Allergen Immunotherapy with effective dose by gradual up-dosing protocol of build-up phase to achieve MTD (2 mg per month) along with Inj Omalizumab 150mg once a month with supportive therapy for 1 year (Table 5 (b)). RCAT (11BASELINE/22), ACT(12 BASELINE/21), SNOT 22 (57 BASELINE/17) improved after 1 year. Patient is asymptomatic and on regular follow-up.
|
Table 5 (a): Clinical characteristics of Case 5. |
|||||||
|
|
Case 5 |
|
|||||
|
|
2016 |
2017 |
|||||
|
RCAT/ ACT Scoring |
11/12 Baseline |
22/21 |
|||||
|
SNOT-22 |
57 |
17 |
|||||
|
PEFR (variability) |
300-400 L/mts (25%) |
500-550 (9%) |
|||||
|
Total IgE |
1101.4 IU/ml |
1300 IU/ml |
|||||
|
Specific IgE/ Total IgE % (Alternaria alternata) |
1.67% |
0.5% |
|||||
|
Eosinophil % / AEC |
36.8 % / 958 cells/ul |
12% / 600 cells/ul |
|||||
|
Serum cortisol |
4.9 mcg/dl |
5.8 mcg/dl |
|||||
|
SPT wheal size Specific IgE
|
SPT wheal size Specific IgE
|
|||||
|
Table 5 (b): Duration of Combined Alternaria alternata extract conc. (5mg/ml) for Cluster AIT as per schedule along with Inj Omalizumab (150mg) X 15 days before AIT followed by once in a month for 1 year.* |
||||||
|
No. of Visits |
1st Visit |
2nd Visit |
3rd Visit |
4th Visit |
5th Visit |
6th Visit |
|
Cluster Doses (5mg/ml) Per visit with Inj Omalizumab 150 mg * |
0.05/0.05ml @ 60 mts interval |
0.1/0.1 ml @ 60 mts interval |
0.15/0.15 ml @ 60 mts interval |
0.2/0.2 ml @ 60 mts interval |
0.4ml |
0.4 ml (2mg) M.T.D |
|
Cluster Dose frequency |
First day |
10-12 days |
10-12 days |
15-20 days |
20-30 days |
Every 4 weeks |
|
M.T.D: Maximum Tolerated Dose; Conc. : concentration; AIT: Allergen Immunotherapy |
||||||
DISCUSSION
Chronic rhinosinusitis is a prevalent and debilitating disease with nasal polyposis (CRSwNP) & without nasal polyps (CRSsNP). Up to 90% of patients with mild to moderate asthma have abnormal CT findings for the paranasal sinuses and up to 100% of patients with severe asthma have sino-nasal involvement [19]. CRSwNP has a 20-60% association with Co-morbid asthma and has a poorer prognosis with recurrence rate of 38-60% at 12 months [20]. Alternaria alternata is mainly an outdoor aeroallergen, its avoidance as a preventive therapeutic measure is difficult to achieve. Regular cleaning to avoid accumulation of Debris, Dust and Dampness in buildings and to prevent moisture related problems and avoiding indoor smoking are the protective measures that may significantly abate indoor fungal growth.
In our 5 patients, the diagnosis of Chronic Rhinosinusitis was defined as per parameters of American Academy of Otolaryngology-Head and Neck Surgery Task Force and diagnosis of Bronchial asthma (mild to moderate persistent) according to the Global Initiative for Asthma 2002 criteria [21]. The combination of Skin Prick Test (SPT) and determination of Specific IgE in the serum is currently recommended for a reliable assessment of sensitization to Alternaria alternata. In children, studies reported 30-38.3% skin test positivity for Alternaria [22]. Our five cases underwent Skin Prick Test (SPT) (Allergo Pharma, Germany/ Greer Lab, USA) to various groups of aeroallergens after giving an informed consent. A positive skin test response was defined as a wheal size >3mm than negative control. Histamine was used a positive control. Specific IgE to Alternaria alternata and other allergens >0.35 IU/ml was based according to ImmunoCAP criteria (Thermo-Fisher, Uppsala, Sweden).
The allergen vaccine used for SCIT in our cases was prepared by (Greer Lab, USA) of aqueous vaccine method (weight/volume 1:20 concentration). The Maximum Tolerated Dose (MTD) for Allergen specific Immunotherapy (ASIT) was determined and defined as one which produced no systemic adverse reaction [23] AIT was given by gradual up-dosing protocol consisting of two phases; build-up dose and maintenance dose. All five patients received increasing doses starting with 1:200 concentration of Alternaria allergen extract to achieve maximum tolerated dose (Maximum Tolerated Dose 2-2.5 mg of 1:200 concentration) given every 4 weeks, along with Inj Omalizumab 150mg, given 15 days before AIT followed by once in a month for 1 year. Two of our cases (Case 1&3) took combined ASIT with Omalizumab for 2 years while 3 cases (Case 2, 4 & 5) took for 1 year. All of our 5 cases responded well by 12 months and needed no Oral corticosteroids or surgical interference. The improvement was interpreted by the Immunological biomarker [reduction in ratio of specific IgE to Total IgE (Case 1- 4.1% to 2.24%, Case 2- 2.5% to 0.24%, Case 3- 5.5% to 1.7%, Case 4- 3.9% to 1.25%, Case 5- 1.67% to 0.5%) along with 50% reduction in Skin Test reactivity. Changes in the level of Total IgE as perceived are probably not an important biomarker, but there are observational studies that might indicate suppressive effect of SIT on the ratio of specific IgE to Total IgE, as also found in our cases but increase in IgG4 and its role has not been elucidated [24].
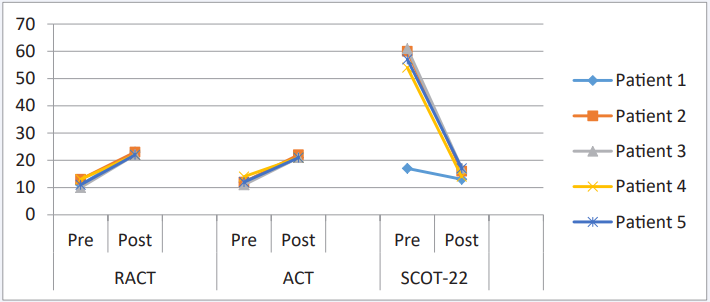
In our patients, the severity of symptoms was assessed by (Figure 3):
Figure 3 Comparative scoring of RCAT, ACT and SNOT-22 RCAT: Rhinitis Control Assessment Test; ACT: Asthma Control Test; SNOT: Sino-nasal Outcome Test
Increase in the RCAT scoring (Case 1- 11/23, Case 2- 13/23, Case 3- 10/22, Case 4-13/22, Case 5- 11/22. [RCAT scoringRhinitis Control Assessment Test- A 5-point Likert scale where names of categories/ domains include Frequency of nasal congestion, sneezing, and watery eyes; sleep disruption; activity limitation caused by symptoms; and self-rating of symptom control. Scores range from 6 to 30, with higher scores indicating better rhinitis symptom control].
Increase in the ACT scoring- (Case 1- 12/21, Case 2- 12/22, Case 3- 11/21, Case 4- 14/21, Case 5- 12/21). ACT scoring- Asthma Control Test [A 5-point scale which assesses the frequency of shortness of breath and general asthma symptoms, use of rescue medications, the effect of asthma on daily functioning, and overall self-assessment of asthma control.]
Reduction in the SNOT 22 scoring- (Case 1- 17/13, Case 2- 60/16, Case 3- 61/17, Case 4- 54/14, Case 5- 57/17) SNOT-22- Sino-nasal Outcome Test [A validated patient-reported measure of outcome established to delineate the presence and severity of Sino-nasal disorders. The questions are based on a 0–5 scale] for Quality of life and the dose of medication were scored as (None0, LABA + ICS-1, LABA + ICS + LAMA-2, LAM + ICS + LAMA + OCS-2) patients with Asthma were instructed to measure PEFR and to record it as the best of three tries, twice a day (Morning/ Evening) to look for variability and reversibility [25,26].
We have described in our five cases that a combined therapy of omalizumab and Alternaria alternata Allergen Immunotherapy has an excellent safety profile and efficacy.
CONCLUSION
In our experience, five cases of Chronic Rhinosinusitis with or without Nasal Polyps Co morbid Asthma had significant improvement after combination of A. alternata Subcutaneous Allergen Immunotherapy with Omalizumab. The combination therapy provides faster symptom relief, better lung function and reducing the risk of systemic adverse reactions. This can be explained by prolonged inhibition of IgE binding to receptors on various inflammatory cells. Double-blind placebo-controlled trials are rare and most of the available studies are case reports. We need further studies to recommend this combined treatment of Omalizumab and Allergen Specific Immunotherapy (ASIT) for use in such patients.
ACKNOWLEDGMENT
The authors were assisted in the proof reading and dataanalysis of the manuscript by Dr. Kripashankar Gupta, HeadMedical Affairs, Care Ability.
REFERENCES
1. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997; 117: S1-S7.
4. Novelli F, Bacci E, Latorre M, Seccia V, Bartoli ML, Cianchetti S, et al. Comorbidities are associated with diferent features of severe asthma. Clin Mol Allergy. 2018; 16: 25.
10.Di Bona D, Frisenda F, Albanesi M, Di Lorenzo G, Caiaffa MF, Macchia L. Efficacy and safety of allergen immunotherapy in patients with allergy to molds: A systematic review. Clin Exp Allergy. 2018; 48:1391-1401.