Extracellular Functions of Galectin-3: An Update
- 1. Department of Biochemistry and Cancer Biology, Meharry Medical College, Nashville, TN, USA
Citation
Ochieng J (2014) Extracellular Functions of Galectin-3: An Update. JSM Clin Oncol Res 2(1): 1006.
EDITORIAL
Galectin-3 is a multifunctional protein which plays pivotal roles in cell physiology, covering diverse mechanisms ranging from immune surveillance [1] to metastatic spread of cancer [2]. Even though it lacks signal peptide, it can be secreted via the non-classical secretory pathways [3]. For example, it was shown to be secreted via vesicular structures that concentrated on the cytoplasmic side of the plasma membrane, that were then externalized upon signaling [4]. Such signaling may involve intracellular calcium ion spikes [5] or mechano-transduction such as detachment from substratum [6]. More recent studies have also indicated that galectin-3 could be externalized via microvesicles or exosomes [7]. These studies bring to focus the question of whether or not galectin-3 plays a role in the biogenesis of either micro-vesicles or exosomes. It has been demonstrated that galectin-3 interacts with Alix, a member of the endocytic trafficking complexes or ESCRT [8]. Could galectin-3 be an organizer of exosomal biogenesis or trafficking?
Examination of galectin-3 shows that it contains one PSAP (T) motif that is a hallmark of ESCRT-1 subunits such as TSG-101 [9]. It would be interesting if mutating this motif in galectin-3 results in the modulation of exosomal biogenesis in a variety of cells including tumor cells. Interestingly, tumor cells with the propensity for rapid adhesion and cell spreading express high levels of galectin-3 [6], and readily secrete exosomes (unpublished information). It therefore appears that galectin-3 modulates adhesion and cell spreading indirectly via exosomes or micro-vesicles. There is an urgent need to investigate these interrelated mechanisms.
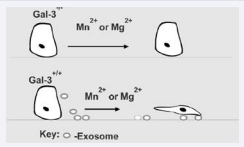
It has been our experience and that of other laboratories over the years that cells (both normal and transformed) with high plating efficiencies in cell cultures express galectin-3. Cells that do not express galectin-3 such as LNCaP prostate cancer cell line [10], attach poorly to culture dishes and do not spread. We therefore propose here that synthesis and secretion of adequate quantities of exosomes is crucial for in vitro cell growth and that galectin-3 drives exosomal biogenesis. Interestingly, exosomes from donor cells easily support the plating and growth of recipient cells. For example the breast carcinoma cell line BT-549 forced to express galectin-3 synthesizes and secretes large quantities of exosomes into the conditioned medium. When these exosomes were isolated from the conditioned medium, they supported the adhesion and growth of recipient cells [11].
Figure 1 Rapid adhesion and Cell spreading driven by exosomes in galectin-3 expressing cells. Cells that lack galectin-3 have poor plating efficiency in culture and take almost 24 h to attach. Galectin-3 expressing cells on the other hand adhere rapidly and also secrete exosomes that speed the adhesion process.