The Distinctive Properties of Parp and Parg as a Cancer Therapeutic Target
- 1. Division of Genome Stability Research, National Cancer Center Research Institute, Japan
- 2. Department of Radiation Oncology, Juntendo University Faculty of Medicine, Japan
- 3. Shien-Lab, National Cancer Center Research Institute, Japan
Abstract
Poly (ADP-ribose) polymerase (PARP) catalyzes poly ADP-ribosylation reaction using NAD as a substrate. PARG is the main enzyme for poly (ADP-ribose) degradation. Strong PARP inhibitors have been developed and are in clinical trials as single agents or in combination with other chemotherapeutic drugs or radiation therapy. Synthetic lethality of BRCA1/2 mutation in cancer cells with PARP inhibitors led to a new strategy for individualized cancer therapy. Functional studies of PARG revealed that its inhibition leads to poly (ADP-ribose) accumulation, block of DNA repair, epigenetic changes and cell death, suggesting that it could serve as another potential cancer target. The differences and similarities of PARP and PARG as a cancer target will be discussed.
Keywords
• PARP
• PARG
• Cancer therapy
• NAD
• PAR
Citation
Fujimori H, Hirai T, Inoue K, Koizumi F, Masutani M (2014) The Distinctive Properties of Parp and Parg as a Cancer Therapeutic Target. JSM Clin Oncol Res 2(5): 1033.
ABBREVIATIONS
PARP: Poly(ADP-ribose) Polymerase; PARG: Poly(ADP-ribose) Glycohydrrolase; NAD: Nicotinamide Adenosine Dinucleotide; PAR: Poly(ADP-ribose); HR: Homologous Recombination
INTRODUCTION
PolyADP-ribosylation reactions, one of the post-translational modification of proteins, are catalyzed by poly(ADP-ribose) polymerase (PARP) using NAD as a substrate [1]. Among these PARP family proteins, PARP-1, PARP-2 and PARP-3 are activated by DNA strand breaks and are involved in DNA repair [2]. Poly(ADP-ribose) (PAR) was catabolized mainly by poly(ADP-ribose) glycohydrolase (PARG). PARP and PARG inhibitors have been focused for cancer therapy. These two enzymmes have apparently opposite roles in poly (ADP-ribose) metabolism and the consequences of inhibition of their function are distinctive, although partly overlap. Here we discuss how PARP and PARG inhibition leads to cell death through different action mechanisms.
PAR Signaling in Cancer
PARP inhibitors suppress DNA repair induced by spontaneous and exogenously introduced DNA damage [2]. Certain PARP inhibitors also produce bulky DNA lesions by stalling PARP-1 molecules at the sites of DNA breakages [3]. Poly(ADP-ribose) (PAR) signaling by three PAR binding proteins; ALC1, a PAR-dependent helicase, APLF [4], which has PAR-binding zinc finger and a ubiquitin-ligase, CTFR [5], facilitates DNA damage response and are involved in induction of cell cycle arrest.
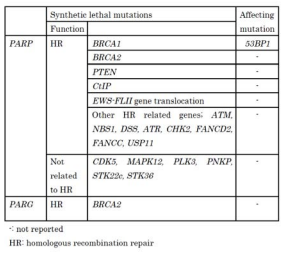
Synthetic lethality of BRCA1/2 mutation in cancer cells with PARP inhibitors [6,7] or PARP-1 knockdown led to a new strategy for cancer therapy (Table 1); a single treatment with a PARP inhibitor is able to target BRCA1/2 mutated cancers [8]. In this strategy, normal cells, which retain at least one BRCA1/2 functional allele will not be affected by PARP inhibitors.
PARP inhibitors for cancer therapy
Several PARP inhibitors were demonstrated to be effective in clinical trials including BRCA-mutated breast cancers, and prostate cancers, triple-negative breast cancers [8,9] and ovarian cancers [10]. However, there are cases in which PARP inhibitors are not effective (Table1). The loss of 53BP1 is frequently observed in breast cancer and such cancers showed resistance to PARP inhibitors even when the BRCA1 gene is mutated [11]. Secondary mutations in the BRCA2 gene, which enable to recover partial activities of the mutated the BRCA2 gene, are reported to cause resistance to treatment with PARP inhibitors [12]. Some cancers may also become resistant to PARP inhibitors through MDR gene overexpression through high levels of drug excretion.
Action mechanism of PARG inhibitors
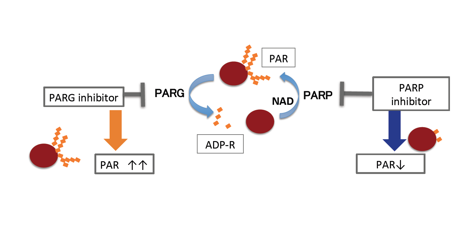
PAR degradation involves PARG [13], ARH3 [14], and TARG [15]. Among them, PARG is the main PAR degradation enzyme and its disruption in the cells leads to accumulation of PAR (Figure 1) [16,17]. PARG functions through interaction with PARP in base excision repair [18]. PARG inhibition thus leads to inhibition of DNA repair directly by blocking the base-excision repair process and indirectly by stalling PAR signaling.
By functional inhibition of PARG, PAR catabolism could be severely disturbed and causes extensive accumulation of PAR, which leads to cell death. In cancer cells and neuronal cells, apoptosis-inducing factor (AIF) dependent parthanatos is induced by PAR accumulation [19,20] (Figure 1).
Figure 1 PARP and PARG inhibition both lead to cell death through different action mechanisms. PARP inhibition causes decrease of PAR levels and PARP stalling at DNA ends that severely block DNA repair. On the other hand, PARG inhibition will induce PAR accumulation and may block DNA repair. Epigenetic and transcriptional dysregulation by PARG inhibitors may also contribute to inhibiting cancer cell growth.
Other types of cell death such as apoptosis and necrotic cell death are also enhanced when PARG deficient cells are treated with DNA damaging agents [21] or ionizing radiation [22-24]. PARG inhibition also disturbs growth signaling pathway and micro RNA regulation [25].
PARP-1 levels in cancer cells are frequently upregulated, and PARG levels in cancer cells are also shown to be divergent [26,27] and these PARP and PARG levels may affect the endogenous PAR levels. However, the relationship between the levels of PARP or PAR and the efficiency of cell death induction by PARG inhibition has not been studied yet. Because PARG inhibition also leads to indirect inhibition of PARP-1 by enhancing auto-PARylation of PARP-1, PARG inhibition is also expected to cause PARP-1 inhibition.
Factors affecting therapeutic effects of PARG inhibitors
A strong synthetic lethal combination has not been reported with PARG functional inhibition, however, a mild synthetic lethal action has been reported (Table 1)
with BRCA2 mutation [28]. There is a lack of clinically useful strong inhibitor of PARG, and finding of synthetic lethal target molecules or a pathway for PARG has not been progressed. Because of the recent elucidation of the structure of PARG molecules, development of effective PARG inhibitors are expected to progress in the near future. The approach with synthetic lethality could be effective and useful, therefore further studies are expected to be carried out.
Combination of PARG inhibitors with chemotherapy and radiation therapy
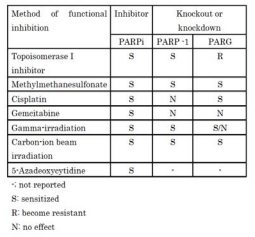
Combinations of PARG inhibition with chemotherapy or radiation therapy have been also suggested to be useful. As shown (Table 2),
PARG inhibitors show some overlapping spectra of sensitization in combination with chemotherapeutic agents. Of note, PARG inhibition attenuates the cytotoxicity of a topoisomerase inhibitor, camptothecin [29]. This is a contrasting phenomenon from the PARP inhibitor combination [30]. Another difference between PARP and PARG inhibition is the combined effect with cisplatin. PARP1 functional inhibition did not enhance cytotoxicity of cisplatin but PARG functional inhibition augmented cisplatin cytotoxicity [29] indicating the action mechanism of PARG inhibition should be different from that of PARP inhibitor. PARG inhibition also showed radiosensitization to particular cancer cells [23]. PARG inhibition was also shown to be effective for radiosensitization of particle-ion radiotherapy by accompanying augmented PAR accumulation [22].
PAR pathway addiction
PARylation signaling is upregulated in many cancer cells [31]. The dependence levels of cancer cell growth on PAR signaling, namely the addiction level of cancer cells to PAR signaling, may determine the effectiveness of PAR pathway blocking drugs. The more tumor cells acquire the high levels of genomic instability, the more the addiction level to PAR signaling may increase.
PERSPECTIVES
As described here, action mechanisms of PARP and PARG inhibitors show differences. This suggests that different cancers should be targeted by PARP and PARG inhibitors. PARG inhibitors also indirectly inhibit PARP. The NAD level of cancer cells should be divergent and certain cancers show dysfunction of nicotinate phosphoribosyltransferase, NAPRT [32]. PARG inhibitors may be more effective if higher levels of NAD are present because augmented levels of PAR accumulation could be expected to be induced. In contrast, lower NAD levels may be effective for treatment with PARP inhibitors. Further studies of PARP and PARG function and development of their inhibitors will enable the clinical application of the cancer drugs targeting the PAR pathway.
ACKNOWLEDGMENT
This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan (11104962), the Third Term Comprehensive 10-Year Strategy for Cancer Control (10103833) from the Ministry of Health, Labor and Welfare of Japan, and from the MEXT of Japan (22300343).