The Prognostic Factors and Treatment Outcome of Squamous Cell Carcinoma of the Vulva: A Mono Institutional Study
- 1. Department of Radiation Oncology, DrLütfiK?rdarKartal Training and Research Hospital, Istanbul, Turkey
- 2. Department Of Gynecologic Oncology, Sakarya University Medical Faculty, Sakarya, Turkey
Abstract
Background: Vulvar carcinoma (VC) is rare malignancy with a poor prognosis and rapid progression. The aim of this study was to evaluate the effect of radiotherapy (RT) and/or chemoradiotherapy(CHRT) treatment for survival.
Material Method: Fourty five patients admitted to our clinic with a diagnosis of VC and were evaluated retrospectively between 2003- 2011.Of these patients, 2 of them located in vulvar malignant melanoma, 2 of them for recurrent vulvar cancer, and 15 of them were not complete follow-up were excluded from the study and 30 patients were evaluated. Comparison of iterations based on prognostic factors, survival values were investigated.
Results:Median age was 68, 23 patients had radical vulvectomy and inguinal lymph adenonectomy, 1 patient had vulvectomy and 6 patients were biopsied for diagnostic purposes. According to FzIGO staging 14 patients were III and 12 patients were IV. All of the patients were squamous cell carcinoma and 24 patients received postoperative radiotherapy (PORT).The median RT dose was 5970cGy and median follow-up period was 32 months. The 5 year disease free and overall survival rates were 31% and 30%. FIGO stage, pathological node positivity and lymph node dissection were found to be significant prognostic factors.
Conclusion: The results shown that FIGO stage and pathological node positivity were significant prognostic factors for survival. The use of modern RT technique and concurrent chemotherapy might be the best treatment schedule for locally- advanced VC. Our study was retrospective study and multicenter prospective randomized studies are needed in order to determine the standard treatment of VC.
Keywords
• Gynecologic cancer
• Radiotherapy
• Squamous cell cancer
• Vulvar cancer
Citation
Yetmen Ö, Eren MD, Ozdemir Z, Unal O, Mayadagl? A (2015) The Prognostic Factors and Treatment Outcome of Squamous Cell Carcinoma of the Vulva: A Mono Institutional Study. JSM Clin Oncol Res 3(1): 1040.
INTRODUCTION
Squamous cell carcinoma (SCC) of the vulva is a rare gynecologic malignancy, which is accounting for 3-5% of all gynecological malignancies [1,2]. This disease often occurs in the elderly population with the median age at diagnosis of 65- 70 years [1,2]. Immunosupression, HPV infection and older age are the strongest risk factors identified for vulvar cancer [3]. Lymphatic spread of vulvar cancer cells are usually first affecting the superficial nodes then the deep groin and pelvic nodes [4]. The inguinal lymph node is common metastasis in regional lymph nodes with a frequency of 6-50 % [5]. The most common important prognostic factor is the presence and number of inguinal lymph node metastasis. The other factors are tumor diameter, depth of invasion, margin status, tumor grade, lymphovascular space invasion and age [5-7].
The most common treatment for early stage vulvar cancer is surgery alone, adjuvant radiotherapy (RT) is administered for positive or close margins and positive inguinal lymph node metastasis. The optimal management of locally advanced disease remains controversial. The use of post-operative RT decreases the risk of loco regional tumor recurrence and increases overall survival in patients with locally advanced vulvar cancer [8,9]. RT decreases local recurrence and increases overall survival in patients with fixed ulcerated inguinal nodes and 2 or more lymph nodes [8,9]. However, RT may not be sufficient to distant microscopic metastases which may occur in 12% of patients at 5 years [8,9]. Chemotherapy has also shown limited efficacy in vulvar cancer but the combination of chemotherapy and radiotherapy more efficacious treatment [10]. Although, vulvar cancer occurs in older age and have associated co-morbid conditions because of that reason patients may not be suitable for aggressive treatments like radical surgery or chemo-radiation. In such patients, definitive RT is an option for controlling the disease and can also provide effective palliations of symptoms. In this retrospective study, we reviewed the outcomes of 30 patients with vulvar cancer treated with RT and/or chemo-radiotherapy.
MATERIALS AND METHODS
We retrieved the 45 case records of all SCC vulvar cancer patients who were treated at our center between 2003 and 2011. We gave the information about clinical details, diagnosis, treatment given, survival and complications from the each case record. Of these patients, 2 of them located in vulvar malignant melanoma, 2 of them for recurrent vulvar cancer, and 11 of them are not complete follow-up were excluded from the study. Pre treatment patient’s characteristics, including detailed clinical examination in the gynecologic clinic, age and ECOG performance status were retrospectively collected. Baseline evaluation consisted of a medical history and physical examination, complete blood count, serum chemistries, urinalysis, computed tomography (CT) of the chest, and magnetic resonance imaging (MRI) of the abdominopelvic region and cystosigmoidoscopy were done if necessary. Recent years, we usually use PET-CT scan for locally advanced vulvar cancer for re-staging and metastatic evaluation. Patients underwent repeat physical examination before each cycle of chemotherapy. The complete blood count, serum chemistries were checked weekly during the chemotherapy. Staging was done according to FIGO system [10]. Radiotherapy treatment options were discussed with clinical and surgico-pathological details. PORT indications for vulvar cancer to pelvic and inguinal area were patients with more than one involved inguinal node, extracapsularextansion or gross residual nodal disease. PORT for local area waspositive or close margins, depth of tm invasion > 5mm and lymphovascular invasion. The postoperative dose of RT for microscopic disease was 45-50Gy to the whole pelvic region with AP-PA fields and for residual disease was 60Gy with conventional fractionation (1.8-2 Gy per fraction, 5 days a week). All patients were treated with linear accelerator with 15-18 MV energy and electrons were used for boosting the inguinal node region. Recent years some patients who had involved lymph nodes or gross disease were treated with chemoradiation.All patients were followed up at a multidisciplinary clinic until the end of follow-up or death. Treatment details and outcomes were recorded prospectively. All patients were followed regularly with a physical examination every 3 months for 2 years, then every 6 months up to 5 years and yearly thereafter. Further investigative studies were done according to the patients’ complaints. Loco-regional recurrences were confirmed by a biopsy sample.Prognostic factors that might influence local control, disease-free survival and overall survival were subjected to univariate and multivariate analysis. These factors included age, gender, grade, tumor size, tumor location, type of surgery and surgical margin status. Local control, disease-free survival and overall survival rates were calculated using the Kaplan-Meier method. All time-to-failure end points were calculated from the date of diagnosis. Overall survival was measured from the biopsy date to the time of last follow-up or date of death from any cause. Univariate and multivariate analysis of prognostic factors were performed using log-rank and Cox regression models, respectively. A p value < 0.05 was accepted as statistically significant. Toxicities were recorded and graded from 1-4 according to the NCI Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [11]. Acute and late toxic effects of radiotherapy were scored according to the Radiation Therapy Oncology Group acute and late morbidity scoring criteria [12].
RESULTS
A total of 45 case records were retrieved for this retrospective study. Of these patients, 2 of them located in vulvar malignant melanoma, 2 of them for recurrent vulvar cancer, and 11 of them are not complete follow-up were excluded from the study. As a result, 30 patients were evaluated. Patient characteristics are given in Table 1. Age ranged 35 to 97 years with a median of 67 years. Most of the patients were (73%) post-menopausal at diagnosis and first symptom was vulvar itching (43%). According to FIGO staging, 3 patients were IB, 1 patient was II, 14 patients were III and 12 patients were IV. Twenty-three patients underwent radical vulvectomy and inguinal lymphadenectomy, 1 patient had vulvectomy and 6 patients were biopsied for diagnostic purposes. Lymphadenectomy consisted of removal of both superficial and deep nodes. The median number of removal lymph node was 9. Twenty-four patients underwent PORT, 3 of them had concurrent chemotherapy with cisplatin50mg/m2 on a weekly. Five patients had definitive RT and one of them had concurrent chemotherapy, 1 patient received palliative RT because of advanced disease at diagnosis and elderly age. The median radiation dose was 5970cGy (4500-6600cGy) and the median duration of radiotherapy time was 57 days (32-95 days), respectively. Two of four patients who received weekly chemotherapy had severe hematological and skin complications because of that reason RT treatment was delayed.
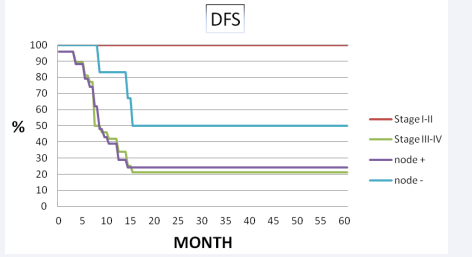
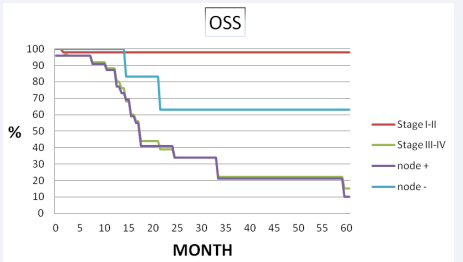
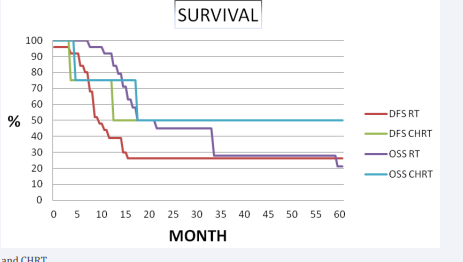
Median follow-up period was 32 months (range 4-98 months). The 5 years disease free survival and overall survival rates were 31% and 30% (Figure 1, 2 3).
Figure 1
Figure 1 Disease free survival and overall survival for stage and pathological node status.
Figure 2
Figure 2 Disease free survival and overall survival for stage and pathological node status.
Figure 3
Figure 3 Survival for RT and CHRT.
The 5- years distance metastasis free survival rate was 65%, respectively. Out of 30 patients 9 had no evidence of disease and 21 had failure. Of the 21 failures, 10 (33.3%) had distant metastasis, 10 (33.3%) were confined to loco-regional recurrence and 1 (3.4%) had both distant metastasis and loco-regional recurrence. Eleven of 30 patients showed recurrent disease after radiotherapy. Four of them had vulvar, 3 of them had inguinal lymph node and 4 of them had both vulvar and regional lymph node recurrence was developed. Eight of them underwent radical vulvectomy and inguinal lymphadenectomy, 1 patient had vulvectomy and 2 patient was treated definitive RT. Among the patients with loco-regional recurrence, none of them had chemotherapy. Eleven patients had developed distant metastases. The most common site of first distant metastases was the lung in 5 patients and the bone 3 patients. The median time to metastasis was 12 months. At the completion of the study, nine patients were alive and disease free, 1 patient was alive with metastases, and twenty patients had died of disease. In univariate analysis, lymph node dissection was significant difference in loco-regional recurrence (83% vs 50%, p=0.02) and disease free survival (54% vs 20%, p=0.03, respectively). Pathological node positive patients had significantly inferior overall survival (34% vs 63%, p= 0.06) and loco-regional recurrence (50% vs 18%, p=0.05) than node negative patients. FIGO stage was the most important prognostic factor on loco-regional recurrence (100% vs 75%, p= 0.08), disease free survival (100 % vs 42%, p= 0.03), overall survival (100% vs 88%, p=0.04) (Table 2, Figure 3).
All of 30 patients showed grad 1-2acute skin complications. Of the 24 patients who had PORT 5 (22%) had acute severe complications the form of grade IV skin toxicity. Skin complications caused treatment break at least 1 week. Of the 24 patients who underwentlymphadenectomy 20 (87%) patients had chronic lymphedema at the mean 15 months after completion of radiotherapy. Among 24 patients treated with postoperative radiotherapy with late complications as 12 (50%) had fibrosis and 5 (21%) wound disruptions developed. Two patients treated with concurrent chemotherapy showed grade II leucopenia and moist desquamation.
DISCUSSION
Surgery is the main treatmentof vulvar carcinoma when diagnosed at an early stage. Radical vulvectomy with dissection of bilateral inguino-femoral and pelvic lymph nodes improves overall survival [13,14]. Radiotherapy may need either as primary treatment for unresectable disease or postoperative therapy for positive lymph node. Twenty-five patients (80%) in our series presented lymph node metastasis of the disease. This is almost the same limitations in the study by Sharma and Bafina et al. [15,16]. The median age of our study was 68 years is also approximately the same as in the literature [5]. In GOG study [7] reported stage III and IV patients had poor outcome as compared to stage I-II and survival analysis of 588 patients, observed that 5 years survival rates was 31% at advanced stage, respectively. Our study demonstrated that 5 years overall survival was also 30% but the survival results of our study may not be strictly comparable in the literature and 86% of our patients were advanced stage at diagnosis and a few number of patients were included in this study.
Maggino et al. [17] also reported stage and presence of positive nodes significant prognostic factors for survival. Our results have also demonstrated that lymph node dissection, pathological node positivity and FIGO stage of disease at presentation to be the significant prognostic factors for survival. Radical vulvectomy and pelvic lymphadenectomy was the commonest surgical procedure in our study due to advanced disease status and weekly chemotherapy was done only in 4 patients. The recent trend is shifting toward surgery with combined use of preoperative chemo-radiotherapy treatment. The optimal treatment for advanced vulvar cancer is controversial. PORT is not routinely used in all vulvar cancer. Homesley et al. [18]were randomized to radical vulvectomy and bilateral inguinal node dissection versus PORT. Dose of radiation was 45-50Gy delivered bilaterally pelvic and inguinal nodes using anterior-posterior fields. This study showed that significant 2 years survival advantages 68% vs 54% (p=0.03) and 5% vs 24% (p=0.02) rates of relapse for patients receiving PORT. PORT shown to improve local control and survival for patients who had close or positive margin, depth of invasion >5 mm, lymphovasculer space invasion and two or more groin nodes. In our study 23 of radical vulvectomy and bilateral pelvic-inguinal node dissection was done due to advanced disease and 18 of them had two or more node groin nodes. Five of them had no more groin lymph nodes but 4 of them had positive or close margin status and one of them had residual tumor after operation. The role of PORT to the vulva was less clear in patients with negative lymph nodes. Larger tumor size, close surgical margin, deep invasion and lymphovascular space invasion were the surgical-pathological factors with a higher risk of local recurrence. There were several retrospective studies suggested that adjuvant radiation therapy of the primary tumor site reduces the risk of local recurrence [19-20].However, this hypothesis was not successfully tested.
PORT is highly effective in preventing inguinal node recurrence but severe complications such as chronic lymphedema, wound disruption, infection, urinary or sexual dysfunction are common who receive both surgery and radiotherapy [21-22]. In GOG 37 study reported long term outcome and toxicities of PORT inguinal and pelvic region compared with pelvic dissection. Pelvic radiotherapy from 45 to 50Gy improved 6 years survival in patients with two or more positive groin nodes (p< 0.001) and clinically fixed and suspected groin nodes (p=0.004)[23]. In our study, 22 patients treated radical vulvectomy with bilateral pelvic-inguinal node dissection followed PORT. Only 5 (21.7%) patients had inguinal recurrence and 2 (8.7%) patients had vulvar recurrence. The 2 years regional recurrence rate was 28%. Despite good local control, PORT had severe late complications such as lymphedema, wound disruption and fibrosis.
According to recent studies advanced vulvar cancer is better treated with chemo-radiotherapy. In GOG 101 study [24], locally advanced 71 patients were treated with preoperative chemo radiotherapy. Pre-operative chemo-radiation was consisting of 2 cycles of 5-flourourocil and cisplatin, radiation therapy was delivered a dose of 47.6Gy with split-course regimen. Patients underwent resection 4-8 weeks after chemo-radiation. There were 34 (48%) clinical complete tumor response. Only 2 had residual unresectable disease. Surgical wound complication and acute cuteneous reactions were the most common adverse effects. With a median follow-up of 50 months, the vulva was the most common recurrence in 8 patients, and 3 had vulvar recurrence with lymph nodes in the groin or pelvis. In GOG 205 [25] study, 58 locally advanced vulvar cancer were treated with radiation 57.6Gy plus weekly cisplatin (40mg/m2 ) followed by surgical resection of residual tumor. There were 40 (69%) patients completed study, 37 (64%) of them had with a complete clinical response. Leukopenia, radiation dermatitis and pain was the common adverse effects. With median follow-up time of 24.8 months, 35 women were alive and 23 have died as a result of cancer. In our institution, most of patients were locally advanced stage and referred our department after operation, only 4 patients were treated with chemo-radiotherapy with cisplatin 40 mg/m2 weekly (3 were treated with PORT and 1 was medically inoperable). Number of patients with chemo-radiotherapy treatment group was too small to attain statistical power. Neo adjuvant therapy might be promising therapeutic strategy for locally advanced squamous cell carcinoma of the vulva, but these patients are frequently elderly with significant coexisting medical problems. These problems are associated with increased severity of complications after chemo-radiation and surgery; carefully selected patients with a good baseline performance status were needed for preoperative treatment.The conventional radiation therapy may treat a large amount of normal tissue and exist radiation related toxicity; RT can be interrupted due totoxicity.Intensity- modulated radiation therapy (IMRT) may have benefits by reducing normal tissue dose, these include reduction in treatment volume for bladder, rectum and small intestine while delivering a higher dose to the tumor [26]. IMRT may reduce lower rates of grade 2 gastrointestinal and genitourinary symptoms without interruption of treatment [26]. Beriwal et al. [27] reported patients with locally advanced vulvar cancer treated with preoperative chemotherapy and IMRT. The dose of subcutaneous tissue, small bowel, bladder and rectumwas reduced, despite this acute desquamations in the vulva and perineum were seen in all patients. There was no grade III-IV of skin reactions in the groin region. This study results may promise IMRT combination with chemotherapy for the treatment of advanced vulvar cancer with a low incidence of severe toxicity, future trials are needed with larger patient population. About two-thirds of the patients in our series were treated 3-D conformal therapy in this retrospective study.
CONCLUSION
The current retrospective study, about two-thirds of the patients were referred our clinic after operation and were in stage III or IV of the disease. Because of rarity of vulvar cancer, the number of patients was too small for statistical power. Forty-five patients were admitted to our clinic, however 11 of them were not complete follow-up were excluded from the study.The short median follow-up of 32 month has limitations in nature. The low socio-economic status, elderly age and co-morbidite were the most important reasons for short follow-up period. The results have shown that 5 year OS of %30 and 5 year DFS of % 31 for all patients. FIGO stage, pathological node positivity and lymph node dissection were found to be significant prognostic factors for survival. Neoadjuvantchemoradiotherapy with modern RT technique may represent a promising therapeutic treatment for locally advanced vulvar carcinoma and it may improve operability. Even among carefully selected patients with good performance status were needed and prospective trials are necessary to further evaluate the advantages and disadvantages in a larger group of patients.