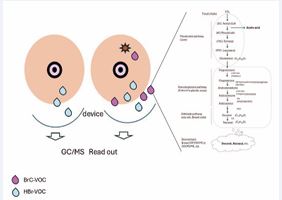
A Novel Non-Invasive Tool for Breast Cancer Detection
- 1. Genomic Oncology Biomedical Research Unit, Gynecology and Pediatrics Hospital 3A, Mexico
- 2. Medical Research Unit in Endocrine Diseases, Specialty Hospital, National Medical Center of the 21st Century-IMSS, Mexican Social Security Institute, Mexico City, Mexico
- 3. Department of Physiology and Biophysics, University of Illinois at Chicago, IL, USA
- 4. Breast Oncology Service, Oncology Hospital, National Medical Center of the 21st Century IMSS, Mexican Social Security Institute, Mexico City, Mexico
- 5. Clínica Londres, Col. Roma, CDMX, Mexico
- 6. Department of Neurophysiology, Center for Research and Advanced Studies of the IPN, CDMX, Mexico
- 7. Medical Research Unit in Oncological Diseases, Oncology Hospital, National Medical Center of the 21st Century-IMSS, Mexican Social Security Institute, Mexico City, Mexico
- 8. Faculty of Chemistry, La Salle University, Mexico
Abstract
Background: Breast Cancer (BrC) is a public health concern, with the Breast Imaging Reporting and Data System (BI-RADS) serving as the primary screening tool. Volatile Organic Compounds (VOC) are being explored as a potential alternative for cancer detection, aiming to overcome challenges in early screening. This study introduces an innovative, non-invasive method employing a bra as prototype BrC detection, with the goal of simplifying and improving the screening procedure.
Materials and methods: A total of 50 women were enrolled, categorized as BI-RADS ≤3 (n =16) and BI-RADS ≥4 (n =34). A bra prototype (device) was designed as a strictly non-invasive auto-collecting system for breast biofluids. Each participant wore the device under a snug fit for 8-10 hours. The collected samples were then analyzed by Gas Chromatography-Mass Spectrometry. The VOC data underwent statistical analysis to assess discriminative ability, with Partial Least Squares Discriminant Analysis (PLS-DA) applied to compare the BI-RADS ≥4 (BrC) and Normal (H) groups.
Results. The device was well received and high compliance among participants. Despite VOC heterogeneity being observed, the chemical classes aldehydes, and alkenes were mostly frequent. A specific BrC-associated VOC profile as common matched the BI-RADS ≥4 patients (BrC-VOC profile) harboring nonanal, 1-Hexene, Ethylbenzene, Benzenesulfonanilide, acetic acid and decanal; while others for BI-RADS ≤3 patients (healthy VOC profile) harboring Benzene, 1,4-dichloro; Methane; ethyl ester, Cyanic acid, 2,4-Octadiyne and Octane compounds.
Conclusion. The auto-collecting non-invasive device demonstrated significative correlation with the clinical outcomes identifying a BrC-associated VOC profile more accurately than BI-RADS ≥4 imaging. This volatolomic method presents a promising, accurate, and emerging adjuvant technique to enhance conventional screening procedures.
Keywords
• Breast Cancer
• Volatile Compounds
• Aldehyde
• Detection
• Gas Chromatography
Citation
Castro-Alba JP, Del Villar AM, Andonegui S, Rodríguez M, Delgado R, et al. (2025) A Novel Non-Invasive Tool for Breast Cancer Detection. JSM Clin Oncol Res 13(1): 1077.
ABBREVIATIONS
BrC: Breast Cancer; BI-RADS: Breast Imaging Reporting and Data System; TNM: Tumor-Node-Metastasis classification; VOC: Volatile Organic Compounds; RT: retention time; IDC: Invasive Ductal Carcinoma; GC/MS: Gas Chromatography-Mass Spectrometry
INTRODUCTION
Breast Cancer (BrC) is the most common cancer type among women worldwide [1]. This neoplasia is a major public health concern, yet it is considered a preventable disease. It has significant physical, psychological, and economic impacts. In 2022, The Global Cancer statistics reported over 2,000,000 new cases of BrC and nearly 700,000 related deaths worldwide [1,2]. In Mexico, approximately 100,000 new cases have been estimated in the last 5 years, with nearly 50% of deaths occurring [3], the majority in women diagnoses at advanced clinical stage (II-IV) during the fifth decade of life. This situation is worse for younger women affected by this cancer type, presenting a more aggressive clinical outcome [4,5]. Multiple barriers such as geographic location, financial constraints, inequities, and cultural factors limit access to diagnosis, with only 30% of women receiving timely diagnostic evaluations [6,7]. BrC diagnosis primarily relies on screening procedures such as mammography, evaluated through the BI-RADS system. BI-RADS <3 imaging is considered as probably benign, whereas BI-RADS 4 subcategories (4A, 4B, 4C) have increasing malignancy probabilities, with 4A presenting a 2–8%, 4B 10-50%, and 4C more than 60% risk of malignancy. BI-RADS 5-6 are classified as highly suspicious for malignant lesions [8]. To confirm a suspicious malignancy tissue, a biopsy is needed. Tumor staging is subsequently determined by using the TNM Classification System [9].Furthermore, to refine BrC prognosis and guide treatment, the Prediction Analysis of Microarray 50, gene-based molecular classification has identified five main subtypes: Luminal A (LumA), Luminal B (LumB), HER2-enriched, Basal, and Normal-like breast cancer. In Mexican BrC patients, hormone-dependent subtypes (LumA/LumB) represent the majority (70%), followed by Her2+ (20%) and triple-negative cases accounting for the remaining percentage [10,11].BrC could be detected early if multiple barriers, whether personal (pain, embarrassment), social (perceptions, stigma, cultural or language barriers), and/ or health-related (limited accessibility, lack of coverage, or negative attitude among healthcare workers) were addressed [12,13]. Overcoming these obstacles requires a multifaceted approach, including education, improved access to affordable healthcare, and strategies to reduce health inequities.Despite the availability of effective clinical procedures for BrC detection such as medical imaging tests, which remain the most commonly used and accepted screening method, the disease continues to be a public health challenge. The role of radiologists, who are trained and licensed to operate imaging technologies and interpret results, remains crucial [14,15]. Additional screening strategies have also been documented [16], including the breast self-exam performed either at home or in clinical setting [15]. Given the persistence challenge in BrC detection, it is essential to explore and develop novel approaches for early diagnosis. This pressing need drives the search for alternatives tools to detect malignant breast lesions. In this context, the metabolomic field, particularly volatolomic, is rapidly advancing, focusing on the identification of Volatile Organic Compounds (VOCs) as potential biomarkers for cancer detection. Several studies suggest that VOC profiling could serve as a noninvasive diagnostic strategy, with reported sensitivity values of 82 94% and specificity of 75-89% [17,18] Specifically, BrC related has identified alkanes, aldehydes, and aromatic compounds as potential markers, detectable in various biofluids such as urine, blood, and breath [19,20]. In this context, the metabolomic field, particularly volatolomic, is rapidly advancing, focusing on the identification of Volatile Organic Compounds (VOCs) as potential biomarkers for cancer detection. Several studies suggest that VOC profiling could serve as a noninvasive diagnostic strategy, with reported sensitivity values of 82 94% and specificity of 75-89% [17,18] Specifically, BrC related has identified alkanes, aldehydes, and aromatic compounds as potential markers, detectable in various biofluids such as urine, blood, and breath [19,20]. Building on this strategy, the present study implements a wearable breast device to define the VOC profile released by the breast. Despite the availability of effective clinical procedures for BrC detection, this disease continues to be a public health challenge. This pressing need drives the search for alternatives tools to detect malignant breast lesions. Avoiding painful, invasive procedure and more cost effective, the present work is highlighting that auto collecting VOC by using a bra as device in simple noninvasive manner, will permit reduce discomfort and acceptance by the patient. We aim to demonstrate the feasibility of detecting a BrC-specific VOC profile using this device which could serve as an adjuvant, non-invasive tool for BrC detection.
MATERIALS AND METHODS
The present study, InnoBRA, was a pilot, cross-sectional, observational, and analytical study conducted in adherence to the Strengthening and the Reporting of Observational Studies in Epidemiology (STROBE) statement [23]. The study was approved by the Scientific and Ethics Committee of the IMSS (Comisión Nacional de Investigación Científica del Instituto Mexicano del Seguro Social, Mexico City R-2018-785-006). All participants provided written informed consent before sample collection. Initially, a total of 90 women were recruited; however, only 50 participants wore and returned the device for analysis. The study included sixteen women with healthy breasts without any evidence of breast lesion (BI-RADS ≤3) and thirty-three diagnosed with BrC (twelve BI-RADS 3, and twenty-one BI-RADS ≥4). To confirm the mammography findings, all patients underwent ultrasound evaluation.The analyzed samples were obtained from a cohort of BrC patients aged 27 to 81 years (mean age: 55.15 years) recruited through the Onco-Breast Service of the Hospital de Oncología, IMSS (Mexico City) between 2020 and 2022. Breast lesions were histologically confirmed and classified according to TNM Classification staging system (stages II-IV) [9,24]. In addition, hormone receptor status (progesterone and estrogen receptors), and Her2 protein expression were determined. Most cases were Invasive Ductal Carcinoma (IDC); seven clinical stage I, fifteen clinical stage II, eight clinical stage III, two bilateral IDC, most were luminal A subtype, two Her2 enriched, and four triple-negative cases. There is a lack of information available for two BI-RADS cases, two clinical stage cases, and seven phenotype cases. Clinical data were also recorded during the visit and some data of BrC tissues are shown in Table 1. The healthy control group (media age: 42 years), included relatives of BrC patients and unrelated women who voluntarily agreed to participate. Notably, among all women approached, none declined participation.
Device Usage
Following previous work [22], a cotton-based textile was selected for the device. To determine the optimal usage duration, various timeframes were tested. After 8-10 hours of wear, non-significant changes in VOC composition were observed. Based on these findings, participants were instructed to wear the “bra prototype medical device” 8-10 hours, preferably overnight, to minimize exposure to external factors. After use, each device was sealed in an individual sterile bag and returned to the designated for storage. Samples were stored at -70°C until analysis. To prevent contamination, each device was gas-sterilized prior to use.
Device Analysis
The area of maximal biofluid concentration was identified, and a 40 cm2 section of the device, covering the axillary region and nipple area, was excised. All procedures were conducted under sterile conditions using surgical grade materials. The excised device sections were placed into the chromatographic vials, ensuring a 30% headspace, then sealed and analyzed using a robotic arm for automated processing. VOCs were extracted by heating the sample at 900C 15 minutes before the injection. VOC analysis was conducted under previously established Gas Chromatography-Mass Spectrometry (GC/ MS) [22]. Briefly, Helium gas was used as a carrier with a 0.7 ml/min continuous flux. For Mass spectrometry the EI 17-350 (4 – 47 min) at 70eVolt the mass ionization mode and a limit of detection of 10-5-10-6 g of solute was used. Raw data were converted to netCDF format using MASSTransit program and subsequently processed with XCMS on-line software. VOCs were identified by cross-referencing the National Institute of Standards and Technology (NIST) 05 MS database, followed by manual visual inspection. To validate the detected VOCs, a C6-C22 alkanes/mix spike standard (Fisher Scientific, Loughborough, UK) was used. Only VOCs with a match score ≥ (including compound name and Retention Time, RT) were selected. Each chromatographic session included both technical and biological samples. Finally, the most prevalent qualitative VOCs across all BrC cancer samples were considered potential biomarkers.
Data Analysis
All data collected were entered into Microsoft Excel database and analyzed using descriptive statistics to define sociodemographic and clinicopathological characteristics. Diagnostic performance analysis was conducted with a 95% Confidence Interval (95% CI) using OpenEpi. To ensure rigor in data processing only samples with ≥ 85% area coverage were included. Compounds with nonzero values were analyzed and logarithmic transformation and scale normalization were applied. Exploratory analysis included density and box plots to facilitate visual comparison. Principal Components Analysis (PCA) was performed to assess potential clustering. To discriminate BrC from Healthy samples based on VOCs, a Partial Least Squares-Discriminant Analysis (PLS-DA) was applied. The perf() function determined the optimal number components, and the final PLS-DA model was built using two components in the plsda() function. Differential VOC analysis was conducted using a linear model (limma library). All statistical analysis were performed in R (version 4.4.0), using mixOmics and factoextra libraries on Ubuntu 22.04.4 LTS.
RESULTS
Acceptance of the device
The women recruited for the study were invited to wear the device for 8-10 hours. In response to the participants’ questions regarding the study’s methodology, they were provided with detailed information about the device and its purpose. Overall, the device was well accepted as a novel tool for detecting the breasts VOCs. Participants were instructed to use the device any day of their menstrual cycle, regardless of whether they had showered, smoked, or taken medicament (drug) or food. However, they were advised to avoid applying any odorous products before using the device.
Clinical characterization of participants
All participants underwent a clinical examination performed by an oncologist. Those categorized as BI-RADS ≤3 was further evaluated by a radiologist confirming the absence of breast lesions. In contrast, BI-RADS ≥4 cases exhibited a radiologically suspicious breast mass.
Table 1: Imaging, staging and molecular phenotypes findings of Breast cancer patients using the device.
|
Age |
BI-RADS |
TNM Stage |
Receptors |
Histopathology |
Phenotype |
|
57 |
4ª |
II |
PR-/ER-/Her2- |
IIDC |
Triple negative |
|
69 |
4ª |
II |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
66 |
3 |
II |
PR+/ER+/Her2- |
ISD |
Luminal A |
|
65 |
4ª |
II |
PR+/ER+/Her2- |
Lobular |
nd |
|
48 |
6 |
II |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
65 |
6 |
I |
PR+/ER+/Her2+ |
IDC |
Her2 enriched |
|
49 |
5 |
I |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
69 |
3 |
I |
PR+/ER+/Her2- |
IIDC |
Luminal A |
|
60 |
3 |
I |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
27 |
5 |
III |
-- |
IDC |
nd |
|
34 |
4ª |
II |
PR+/ER+/Her2- |
Squamous |
Luminal A |
|
38 |
3 |
II |
-- |
Sarcoma |
nd |
|
50 |
3 |
II |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
49 |
3 |
II |
-- |
IDC |
nd |
|
52 |
5 |
II |
PR+/ER+/Her2- |
IIDC |
Luminal A |
|
60 |
3 |
III |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
32 |
3 |
III |
PR+/ER+/Her2+ |
IDC |
Her2 enriched |
|
49 |
4 |
III |
PR+/ER+/Her2- |
bilateral IDC |
Luminal A |
|
57 |
3 |
I |
- |
Phyllodes |
nd |
|
62 |
3 |
I |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
68 |
3 |
I |
PR+/ER+/Her2- |
Canalicular |
Luminal A |
|
81 |
4ª |
II |
PR+/ER+/Her2- |
bilateral IDC |
Luminal A |
|
59 |
6 |
III |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
35 |
3 |
no |
- |
Adenoid |
nd |
|
46 |
4ª |
III |
PR+/ER+/Her2- |
IIDC |
Luminal A |
|
61 |
4 |
no |
- |
Adenoma |
nd |
|
71 |
5 |
II |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
65 |
6 |
II |
PR-/ER-/Her2- |
IDC |
Triple Negative |
|
57 |
5 |
III |
PR-/ER-/Her2- |
IDC |
Triple Negative |
|
69 |
4 |
III |
PR+/ER+/Her2- |
IDC |
Luminal A |
|
57 |
6 |
II |
PR-/ER-/Her2- |
IDC |
Triple Negative |
|
38 |
4 |
II |
PR+/ER+/Her2- |
IDC |
Luminal A |
BI-RADS: mammography image. TNM: Tumor/lymph Node/Metastasis; no: no data available. IDC: Invasive Ductal Carcinoma; IIDC: Invasive Intraductal Carcinoma; BI RADS: mammography image. no: no data
According to clinical findings (Table 1) in the present study: BI-RADS 3, classified as a as probably benign lesion, was associated with TNM I-III. This finding suggests the possibility of false negative imaging results or clinically aggressive tumors. BI-RADS 4-5 cases were observed in TNM II-III, indicating that the detected suspicious lesions correspond to locally advanced tumors. Notably, in one young patient with BI-RADS 5 and TNM III the findings suggested the presence of an aggressive tumor. Finally, BI RADS 6, identified in TNM stages I-III, suggests that imaging findings may not always reflect actual tumor extension. These results underscore the limitations of imaging assessments, highlighting the need for clinical context to ensure accurate diagnostic and staging evaluations. Most of the samples were LumA characterized by PR+/ER+/ Her2- phenotype (Table 1).
A Large VOC Family from Breasts
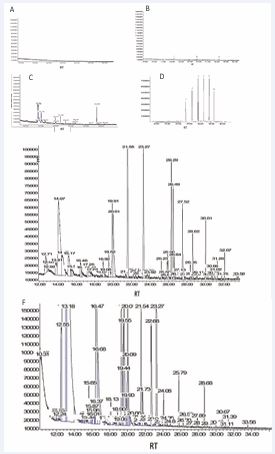
To ensure the accuracy of the chromatographic analysis, technical controls were performed (Figure 1A-D), followed by the sample sessions.
Figure 1: Headspace chromatograms obtained for controls and normal breast or BrC devices. Representative result of (A) chromatogram corresponding to environment lab; (B) empty blank (only vial) small peaks are observed corresponding to 1) 2,4-diamino N,N,5-trimethyl-6-quinolinesulfonamide; 2) cyclopentasiloxane; 3) trimethylsilyl ester; (C) for clean device, increased peaks are observed related to the device; (D) control spike or C6-C12 Normal Hydrocarbon Mix alkanes mix corresponding to 1-dodecane; 2-tridecane; 3-tetradecane; 4-pentadecane; 5-hexadecane; 6-heptadecane. (D) for normal breasts, or (F) BrC devices. The ordered axis shows the relative VOC abundance represented by parts of billion (ppb), while abscissas axis is the Retention Time (RT).
In total, 592 metabolites were identified, including alkanes, aldehydes, ketones, carboxylic acids, and benzene-related chemical compounds, which were the most prevalent VOC classes. The analysis revealed heterogeneity in the number and chemical classes of VOCs detected in breast samples. Interestingly, normal breast samples (n = 379) contained nearly twice as many VOCs as BrC samples (n = 200). Representative chromatograms for normal and BrC samples are shown in Figures 1E and 1F, demonstrating that the device effectively collects VOCs from the breasts.
Breasts with Cancer Emit a Specific VOC profile
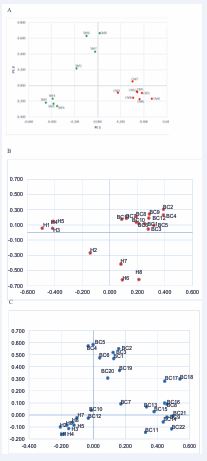
nitial small group of devices was examined, consisting of eight women with healthy breasts (SM, green dots) and eight with BrC (CM, red dots) in a 1:1 ratio. The PCA plot exhibited significant qualitative discrimination between the two groups (Figure 2A).
Figure 2: Qualitative Principal Component Analysis from retention times of VOCs. (A) The retention times data of each sample analyzed, show a good discrimination between eight BrC-affected (CM, red dots) patients and eight healthy (SM, green dots) women groups. The samples were analyzed by Bartlett Kaiser-Meyer-Olkin test (KMO 0.331; x2=0,000). (B) As (A), the retention times data of each sample analyzed, show a good discrimination between twelve BrC-affected (BC) patients and eight Healthy (H) women groups. The samples were analyzed by Bartlett Kaiser-Meyer-Olkin test (KMO 0.651; x2=0,000). (C) Again, a good discrimination between eight H cases and twenty-two BrC (BC) samples is shown (x2=0,000).
Next, to further validate the findings, twelve additional devices from BrC patients were analyzed (8 vs 12; 50% more of BrC devices). Again, clear discrimination between healthy and BrC groups was observed (Figure 2B). Motivated by these results, the sample size was further expanded to include eight additional healthy and twenty two BrC (8 vs 22; 100% more BrC samples). This large data set continued to show clear discrimination between healthy participants and BrC patients (Figure 2C). These findings suggest that VOC profiles can qualitatively discriminate between healthy and BrC samples.
Specific VOCs Are Associated with Breast Cancer
To identify disease-specific VOCs, all data was analyzed.A group of VOCs were consistently detected for the healthy women: Benzene, 1,4-dichloro-; Methane; Cyanic acid; ethyl ester; 1,5-Hexadiyne, and Octane. This was called Healthy Breast Volatolomic (HBrV). Conversely, BrC associated VOCs included Decanal; 1-Hexene; Benzene sulfonanilide; Nonanal; acetic acid, Cyclohexene, 1-butyl-, Ethylbenzene referred to as the Breast Cancer Volatolomic (BrCV) (Table 2).
Table 2: The most representative Volatile Organic Compounds for Health or Breast cancer devices.
|
Breast Cancer Volatile Organic Compounds |
|
Decanal |
|
1-Hexene |
|
Benzenesulfonanilide |
|
Nonanal |
|
Acetic acid |
|
Cyclohexene, 1-butyl- |
|
Ethylbenzene Health Breast Volatile Organic Compounds |
|
Benzene, 1,4-dichloro- |
|
Methane |
|
Cyanic acid, ethyl ester |
|
1,5-Hexadiyne |
|
Octane |
|
Cyclohexene, l-methyl-5-(1-meth |
|
2,4-Octadiyne |
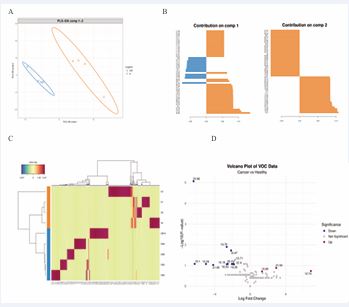
A strong correlation (p=0,000) was observed between VOC profiles and the BI-RADS classification, indicating their agreement and potential comparability. To determine whether VOCs can reliably differentiate health and disease, PLS-DA and volcano plot analyses were conducted. This analysis identified nonanal, decanal, and acetic acid as significant discriminators for BI-RADS ≥4 patients, while benzene 1,4-dichloro- is associated to healthy women. Table 3 presents the top thirty-four discriminatory VOCs based on Retention Time (RT). Using high-area percent samples and non-zero compounds’ data, the PLS-DA model applied with two components clearly separated the groups (Figure 3A), clustering four BrC samples and four healthy samples separately. The top 50 variables associated with each component were identified (Figure 3B), highlighting RT 20.98 for healthy samples and RT 21.54 for BrC in component 1, while RT 22.4 and RT 12.42 were relevant for healthy samples in Component 2. Figure 3C expands the list of VOCs associated with each group, revealing a significant number of group-specific compounds. Finally, a linear model was applied to calculate fold changes between groups. The top compounds and their log-fold changes are shown in Figure 3D, demonstrating that many VOCs were significantly reduced by BrC samples.
Figure 3: Defining specific VOC BrC-related. The samples were analyzed to define in stringent conditions probable VOC biomarkers. After normalizing the samples, for analyzing the high-dimensional data set the multivariate analysis method PLS-DA (A) was performed. Again, a very good discrimination between BrC (orange triangles) and healthy samples (blue circles) was observed. B) Contribution of component 1 and component 2 by using the PLS-DA model. Orange bars for BrC samples and blue bars for healthy samples. C) Heat map for health (blue bar) or BrC (orange bar) samples. Clear differences of compounds are shown. D) Volcano plot of the log 10-fold-change difference in VOC abundance of devices with or without BrC. Metabolites in blue (healthy) or in red (BrC) devices (p<0,1). The fold-changes and p-values are presented in Supplementary in Table 3. Compounds most up-represented were nonanal p=1.437889 e-06; hexanal 1.776822041604 e-06; and acetic acid p=1.711915 e-05.
Table 3 Partial discriminative retention times for VOC from Breast cancer and healthy devices. It shows the retention time and the x value according to PLS-DA analysis. Retention time,; x value.
Regarding VOC origin, classic biochemical pathways such as β-oxidation, fatty-acid metabolism, steroidogenesis and mevalonate-cholesterol pathway are likely contributors to the production of nonanal and decanal (Figure 4). We did not discard the contribution from microbiome molecules as exogenous source.
Figure 4: Biochemical pathways model for breast odoromic. A probable biochemical route for emitting specific BrC-related aldehydes is 1) mevalonate-cholesterol pathway (in liver); 2) steroidogenesis pathway in endocrine glands, and 3) Aldehyde pathway (in any cells) by REDOX reactions. At least, nonanal, decanal aldehydes (purple drops), etc. are secreted to generating part of the breast exposome. These and some more VOC are collected by the device as shown in the picture and then analyzed by GC/MS (electronic nose). The blue drops represent normal VOCs; the star represents a tumoral mass.
DISCUSSION
This study demonstrates that wearing a bra-like device for 8-10 hours enables the non-invasive qualitative collection of VOCs, significantly distinguishing BrC patients from healthy individuals. This novel approach may serve as an alternative BI-RADS imaging, complementing mammography as the gold standard for BrC screening. Epidemiological data highlight the high prevalence and mortality of BrC in low- and middle-income countries, partially due to limited participation in screening programs, and numerous barriers to early detection [1,12,13]. Our findings suggest that alternative BrC detection strategies represent a valuable opportunity.
Scaling Up: Acceptability of a new device
We initially considered whether patients might hesitate to accept an unfamiliar prototype. Research indicates that even effective interventions may face challenges with scalability and sustainability if not properly implemented [25]. To address this, we prioritize implementation strategies and patient education. During the educational phase, patients were introduced to the study and fully informed about the procedure. Remarkably, all patients accepted the device, no refusal, which we consider a promising first step. Future adoption strategies should emphasize education and awareness.
Metabolomic and Volatolomic Appraches for BrC Detection
Volatolomics and metabolomic offer novel diagnostic avenues. Various biofluids including blood, urine, and exhaled breath, have been explored for BrC detection using animals’ models or analytical methods such as e-noses and GC/MS [26-30]. Access to the target site, the dynamic range, molecular abundance, and chemical complexity are critical factors for identifying reliable disease markers [21]. Therefore, non-invasive biofluid collection methods located near the target site could provide an optimal approach for biomarker discovery. As expected, different VOC profiles were obtained from the breasts samples compared to other biofluids analyzed in previous studies [26-30]. The variability and heterogeneity of VOCs detected may be due to differences in fluid composition and technical conditions. While these factors pose certain limitations in the clinical application of VOCs for cancer detection [19,22,29,30], it is noteworthy that several VOCs identified in our study, such as 1-hexene and Cyclohexene, 1-butyl- have been previously reported in cancer research [26-32], reinforcing the validity of our findings.
VOC discrimination and Influencing Factors
A semiquantitative analysis of VOCs revealed a tendency toward discrimination between normal or BrC groups, although some heterogeneity was observed. This variability may be influenced by environmental factors, diet, lifestyle or genetics, all of which impact personal odor.
Auto-collection system, an alternative
The auto-collection of biofluids using a device placed near the genital area has been successfully used to predict cervical cancer based on specific VOC profiles [22]. Similarly, our study demonstrates that a bra-like device, used in non-invasive fashion can effectively predict and discriminate against the presence or absence of BrC with high sensitivity and specificity. Our results reinforce that GC/MS technical conditions combined with a device positioned near the target site, can facilitate the collection of cancer-specific VOCs, offering a novel detection strategy.
VOC Profiles in Cancer Research
Most VOC in cancer research report alkane, aldehyde, and alkenes as predominant chemical classes [19]. Our findings are aligned with these reports, showing that aldehydes were the most prevalent VOCs detected in BrC samples. Recent studies suggest that breast cancer cell lines produce unsaturated fatty acids, which serve as precursors of saturated octanal, nonanal and decanal, aldehydes found in high concentrations in the breath of cancer patients compared to healthy individuals [31]. Our study confirms the presence of nonanal and decanal aldehydes in BrC samples supporting the hypothesis these compounds are produced and emitted by transformed cells and may serve as strong cancer biomarkers [27-33]. These aldehydes are known to form DNA adducts in esophageal cancer cells, highlighting their genotoxicity potential and role in inadequate detoxification processes [34]. Acetic acid has been previously reported in fluids from BrC patients and other cancer types [35, 36]. Additionally, 1-hexene and Cyclohexene, 1-butyl- compound consistently detected in BrC samples, have been poorly explored in cancer research [37]. Our results suggest that BrC tissues and cells predominantly emit aldehydes and alkenes, which can permeate through biological membranes and be collected as VOCs. These findings align with previous reports [35 39] and reinforce the potential of VOCs as biomarkers for BrC detection.
Potential VOC origins in breast cancer cells
The biochemical origin of VOCs in BrC remains complex and may involve multiple intrinsic and extrinsic factors. However, the biochemical pathways already reported in the transformed cells [37-44], are supporting the present results, highlighting that the present strategy could be feasible and promising for BrC detection. We hypothesize that breast tissue cells release a mixture of VOCs, creating a distinctive volatolomic profile that can be collected non-invasively. This collection system is entirely pain-free, radiation-free and represents a significant opportunity for early cancer detection. Moreover, this methodologically could be adapted for other diseases beyond BrC.
Future Directions and Limitations: This study is pilot phase, yet a statistical correlation between VOC profiles and the BI-RADS classification was observed. Imaging limitations highlight the need for additional parameters in BrC detection. If further studies confirm the alignment between VOC profile and BI-RADS classifications, this biochemical non-invasive strategy could become a viable diagnostic tool, warranting phase-2/3 clinical trial for validation. Given the small sample size analyzed, further research is needed to confirm these findings. However, our device shows promise as a novel tool for BrC screening, offering advantages such as acceptability, overcoming many barriers associated with traditional screening methods.
The current protocol groups all BrC subtypes under a general, BrC-associated VOC profile. However, genetic background diversity may contribute to specific VOC variations among different molecular subtypes. Further studies should investigate these metabolic differences through extensive VOC profiling. Additionally, standardization of this strategy is necessary to strengthen the reliability and reproducibility of our findings. This approach has the potential to become an accurate, feasible, and promising tool for early detection of transformed cells.
Gender Considerations: Gender inclusivity is essential for standardization efforts. The inclusion of men, particularly those with hereditary breast cancer risk, should be mandatory for future studies. Ongoing research is evaluating patients under follow-up and surveillance including male cases.
Key findings
Normal breasts show a specific VOC profile associated with BI-RADS 4.
Strengths and limitations
This is a novel noninvasive alternative method for detecting BI-RADS >4 breast cancer using a bra-type device that auto collects VOCs emitted by transformed cells. This is a multiple exhibition collecting system working in a range of part per billion of sensitivity. The accepted procedures are X-ray-based or ultrasound representing only one exhibition (one picture). The number of samples analyzed could be a limitation. According to statistical analysis, we hypothesize that increasing the number of samples will strength the system. A phase 2/3 clinical trial must be conducted and to know false positive/negatives. Moreover, this study should show the role for follow up and surveillance for oncological patients, in “benign results” and mainly for suspicious masses. These results will strengthen our present proposal.
Comparison with similar research
At present, there is no other similar method to detect breast cancer. The methods closest to the present study are those noninvasive methods analyzing urine and other molecular markers (microRNAs, proteomic) or using trained dogs.
Explanations of findings
In the present global time, several drugs, equipment’s, novel molecular genetics tests (mammaprint, oncotype, prosigna, etc.), new antibodies, AI algorithms, etc., are reshaping oncology by expanding access, improving patient outcomes, and driving cost efficiencies. These data are generating a hallmark of disruptive change, why not perform it for cancer detection methods.
Looking for a novel noninvasive breast cancer detection system, avoiding accessing to target breast tissue, we thought of a collecting device supporting our previous data. Further, the breasts are emitting several VOC generating the breast odor. The present method of detecting VOC profile by using a simple device dressed for 8-10 hours, in part would simplify complex processes and we would have greater accessibility. It would reduce costs, making the system more democratic. In the global time, new disruptive innovations must be comfortable, accurate, accessible to the entire population, sensitive, specific, and reliable. The present method rather than replace the present invasive procedures, could optimize the early cancer process and the diagnostic efficacy.
Implications and actions needed
The present study could represent a disruptive innovation (low risk and cost) that lowers barriers to care, shifts focus to prevention contributing to novel innovations, increasing frequent monitoring. Optimizing this method will expand access to rural and urban populations, reducing costs, increasing the screening programs, making it more democratizing process. This should avoid regulatory hurdles and get official approval, reducing reliance on traditional approaches being a harmonious blend of safety.
CONCLUSION
In summary, this study (as a disruptive innovation) presents a novel, non-invasive, self-collecting metabolomic strategy for BrC detection. This emergent proof-of concept approach demonstrates the feasibility of VOC based diagnostics, with promising potential for global BrC screening programs
ACKNOWLEDGEMENTS
This work was partially fulfilled by JPC for his PhD degree at Programa de Doctorado en Ciencias de la Salud, ESM, IPN, CDMX México. During of the resent work, ADV, ES, were recipients from a CONACY-Mexico fellowship. We would like to thank Dr. Rodrigo Aldair, MSc. Reynaldo Hernandez and the nurse’s oncological group for technical assistance.
Funding
This work was partially supported by IMSS MX grant.
Ethical Statement
The study was approved by the Scientific and Ethics Committee of the IMSS (Comisión Nacional de Investigación Científica del Instituto Mexicano del Seguro Social, Mexico City R-2018-785-006). All participants provided written informed consent before sample collection.
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024; 74: 229-263.
- Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022; 66: 15-23.
- Gómez-Dantés H, Lamadrid-Figueroa H, Cahuana-Hurtado L, Silverman-Retana O, Montero P, González-Robledo MC, et al. The burden of cancer in Mexico, 1990-2013. Salud Publica Mex. 2016; 58: 118-131.
- Ángeles-Llerenas A, Torres-Mejía G, Lazcano-Ponce E, Uscanga- Sánchez S, Mainero-Ratchelous F, Hernández-Ávila JE, et al. Effect of care-delivery delay on the survival of Mexican women with breast cancer. Salud Publica Mex. 2016; 58: 237-250.
- Justo N, Wilking N, Jönsson B, Luciani S, Cazap E. A review of breast cancer care and outcomes in Latin America. Oncologist. 2013; 18: 248-256.
- https://ensanut.insp.mx/encuestas/ensanutcontinua2021/index. php
- https://www.paho.org/es, access February 2025
- Carriero A, Groenhoff L, Vologina E, Basile P, Albera M. Deep Learning in Breast Cancer Imaging: State of the art and recent advancements in early 2024. Diagnostics (Basel). 2024; 14: 848.
- Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA, Bianchi-Micheli G, et al. ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann Oncol. 2022; 33: 1097-1118.
- Wallden B, Storhoff J, Nielsen T, Dowidar N, Schaper C, Ferree S, et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genomics. 2015; 8: 54.
- Turova P, Kushnarev V, Baranov O, Butusova A, Menshikova S, Yong ST, et al. The Breast Cancer classifier refines molecular breast cancer classification to delineate the HER2-low subtype. NPJ Breast Cancer. 2025; 11: 19.
- Tsapatsaris A, Babagbemi K, Reichman MB. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: A review. Clin Imaging. 2022; 82: 224-227.
- Özkan ?, Taylan S. Barriers to women’s breast cancer screening behaviors in several countries: A meta-synthesis study. Health Care Women Int. 2021; 42: 1013-1043.
- European Society of Radiology 2009. The future role of radiology inhealthcare. Insights Imaging. 2010; 1: 2-11.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009; 151: 716-26, W-236.
- Zuluaga-Gomez J, Zerhouni N, Al Masry Z, Devalland C, Varnier C. A survey of breast cancer screening techniques: Thermography and electrical impedance tomography. J Med Eng Technol. 2019; 43: 305-322.
- Shahbazi Khamas S, Alizadeh Bahmani AH, Vijverberg SJH, Brinkman P, Maitland-van der Zee AH. Exhaled volatile organic compounds associated with risk factors for obstructive pulmonary diseases: a systematic review. ERJ Open Res. 2023; 9: 00143-2023.
- Kaloumenou M, Skotadis E, Lagopati N, Efstathopoulos E, TsoukalasD. Breath Analysis: A Promising tool for disease diagnosis-The Role of Sensors. Sensors (Basel). 2022; 22: 1238.
- Phillips M, Cataneo RN, Cruz-Ramos JA, Huston J, Ornelas O, Pappas N, et al. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res Treat. 2018; 170: 343-350.
- Leemans M, Bauër P, Cuzuel V, Audureau E, Fromantin I. Volatile organic compounds analysis as a potential novel screening tool for Breast Cancer: A Systematic Review. Biomark Insights. 2022; 17: 11772719221100709.
- Schiess R, Wollscheid B, Aebersold R. Targeted proteomic strategyfor clinical biomarker discovery. Mol Oncol. 2009; 3: 33-44.
- Rodríguez-Esquivel M, Rosales J, Castro R, Apresa-García T, Garay Ó, Romero-Morelos P, et al. Volatolome of the Female Genitourinary Area: Toward the Metabolome of Cervical Cancer. Arch Med Res. 2018; 49: 27-35.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007; 370: 1453-1457.
- Tavassoli, F.A. Pathology of the Breast. 2nd Edition, McGraw-HillProfessional Publishing, Appleton & Lange, Stanford, CA, USA. 1999.
- Ben Charif A, Zomahoun HTV, Gogovor A, Abdoulaye Samri M, Massougbodji J, et al. Tools for assessing the scalability of innovations in health: A systematic review. Health Res Policy Syst. 2022; 20: 34.
- Li J, Guan X, Fan Z, Ching LM, Li Y, Wang X, et al. Non-Invasive biomarkers for early detection of Breast Cancer. Cancers (Basel). 2020; 12: 2767.
- Leemans M, Bauër P, Cuzuel V, Audureau E, Fromantin I. Volatile organic compounds analysis as a potential novel screening tool for Breast Cancer: A Systematic Review. Biomark Insights. 2022; 17: 11772719221100709.
- Giró Benet J, Seo M, Khine M, Gumà Padró J, Pardo Martnez A, Kurdahi F. Breast cancer detection by analyzing the Volatile Organic Compound (VOC) signature in human urine. Sci Rep. 2022; 12: 14873.
- Yang HY, Wang YC, Peng HY, Huang CH. Breath biopsy of breast cancer using sensor array signals and machine learning analysis. Sci Rep. 2021; 11: 103.
- Bauër P, Leemans M, Audureau E, Gilbert C, Armal C, Fromantin I. Remote medical scent detection of cancer and infectious diseases with Dogs and Rats: A Systematic Review. Integr Cancer Ther. 2022; 21: 15347354221140516.
- Cavaco C, Pereira JAM, Taunk K, Taware R, Rapole S, Nagarajaram H, et al. Screening of salivary volatiles for putative breast cancer discrimination: An exploratory study involving geographically distant populations. Anal Bioanal Chem. 2018; 410: 4459-4468.
- Amann A, Costello Bde L, Miekisch W, Schubert J, Buszewski B, Pleil J, et al. The human volatilome: Volatile Organic Compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res. 2014; 8: 034001.
- Tanaka M, Hsuan C, Oeki M, Shen W, Goda A, Tahara Y, et al. Identification of characteristic compounds of moderate volatility in breast cancer cell lines. PLoS One. 2020; 15: e0235442.
- Antonowicz S, Bodai Z, Wiggins T, Markar SR, Boshier PR, Goh YM, et al. Endogenous aldehyde accumulation generates genotoxicity and exhaled biomarkers in esophageal adenocarcinoma. Nat Commun. 2021; 12: 1454.
- Silva C, Perestrelo R, Silva P, Capelinha F, Tomás H, Câmara JS.Volatomic pattern of breast cancer and cancer-free tissues as a powerful strategy to identify potential biomarkers. Analyst. 2019; 144: 4153-4161
- Terasaki M, Ito H, Kurokawa H, Tamura M, Okabe S, Matsui H, et al. Acetic acid is an oxidative stressor in gastric cancer cells. J Clin Biochem Nutr. 2018; 63: 36-41.
- Huff J, Chan P, Melnick R. Clarifying carcinogenicity of ethylbenzene. Regul Toxicol Pharmacol. 2010; 58: 167-169; discussion 170-172.
- Floss MA, Fink T, Maurer F, Volk T, Kreuer S, Müller-Wirtz LM. Exhaled aldehydes as biomarkers for Lung Diseases: A Narrative Review. Molecules. 2022; 27: 5258.
- Janfaza S, Khorsand B, Nikkhah M, Zahiri J. Digging deeper into volatile organic compounds associated with cancer. Biol Methods Protoc. 2019; 4: bpz014.
- De Bo I, Van Langenhove H, Pruuost P, De Neve J, Pieters J, Vankelecom I, et al. Investigation of the permeability and selectivity of gases and volatile organic compounds for polydimethylsiloxane membranes. J Membrane Scie. 2003; 215(1–2):303–319
- Davis VW, Bathe OF, Schiller DE, Slupsky CM, Sawyer MB. Metabolomics and surgical oncology: Potential role for small molecule biomarkers. J Surg Oncol. 2011; 103: 451-459.
- Vijayraghavan S, Saini N. Aldehyde-Associated mutagenesis?current state of knowledge. Chem Res Toxicol. 2023; 36: 983-1001.
- Jin Z, Chai YD, Hu S. Fatty Acid Metabolism and Cancer. Adv Exp MedBiol. 2021; 1280: 231-241.
- Rodriguez-Esquivel M, Flores-Valdivia A, Salcedo E, Nambo-LucioM. de Jesús, Salcedo M. In volatile biomarkers for human health. From Nature to Artificial Senses, ed. H. Haick, The Royal Society of Chemistry, 2022; 8: 134-151