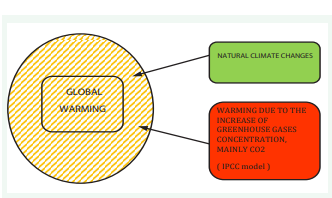
Growing Energy Consumption, Entropy Generation and Global Warming
- 1. Department of Fundamental Research in Energy Engineering, AGH University of Krakow, Poland
- 2. Department of Mechanical Engineering and Science Shibaura Institute of Technology, Japan
Abstract
This paper analyses the potential impact of anthropogenic heat on the global rise in tropospheric air temperature. A significant part of primary energy consumption is converted into heat and, in addition, to solar energy absorbed by the Earth’s atmosphere. We can expect that the increase in energy consumption will significantly impact future climate. The role of energy loss due to irreversibility in any actual process is proposed to analyze global warming through the concept of entropy generation. An increase in entropy always leads to higher energy consumption.
Keywords
• Primary Energy Consumption
• Second Law of Thermodynamics
• Entropy Generation
• Energy Dissipation
CITATION
Kolenda Z, Donizak J, Takasaki A, Szmyd J (2024) Growing Energy Consumption, Entropy Generation and Global Warming. JSM Environ Sci Ecol 12(2): 1093.
INTRODUCTION
“All the talk of global warming and all the conferences, summits and protocols have so far failed to curb global greenhouse gas emissions (…) With current technology, the only real way to stop global warming is to curb economic growth, which no government is prepared to do” [1].
“Even if civilization on Earth stops polluting the biosphere with greenhouse gases, humanity could eventually be awash in too much heat, namely dissipated heat” [2].
Modern civilization runs on energy because all organized, complex systems need energy to survive and prosper. Resources come in and waste - mainly heat - goes out, [2]. An increase in economic activity means an increase in energy consumption, not a decrease. Increasing demand for energy leads to increasing threats to the natural environment. Observed climate change, manifested as increased atmospheric temperature over time, is one of the leading global threats. This phenomenon is called anthropogenic global warming. Proponents of this hypothesis argue that the time to avoid a climate catastrophe is increasingly limited. The future is apocalyptic; gradual melting of ice sheets leading to flooding of densely populated areas, hot summer seasons causing heat shocks dangerous to health, prolonged droughts, increased frequency and destructive power of hurricanes and storms, increased forest fires, destruction of entire ecosystems, slowing world economic growth leading to a significant increase in poverty and hunger and an uncontrolled increase in climate migrants. The global warming hypothesis is the subject of lively debate in the scientific community (understandably so), among politicians and in the mass media. The most common questions in the discussion are;
- whether climate change is the result of human activity?
- what is the scale and rate of changes?
- can the natural environment be adapted to the new living conditions?
Unfortunately, based on the present state of climate science, the answer is unclear.
ENERGY DEMAND FORECAST
All the projections in the literature indicate that primary and final energy consumption will increase over time. There is only one way to meet this demand - by increasing power. More economic activity means more energy consumption, not less. One of the projections of primary energy consumption based on the British Petroleum Statistical Review is shown in (Figure 1) (Table 1) [3,4].
Figure 1: Primary energy consumption [3,4].
Table 1: Primary energy consumption [3,4] (EJ/year)
|
|
History [3] |
Prediction [4] |
||||||
|
Region \ Year |
1980 |
1990 |
2000 |
2010 |
2020 |
2030 |
2040 |
2050 |
|
WORLD |
280.56 |
343.90 |
396.88 |
506.68 |
564.01 |
648.00 |
691.00 |
725.00 |
|
OECD |
176.47 |
198.54 |
231.94 |
236.32 |
220.20 |
240.00 |
218.00 |
215.00 |
|
Non-OECD |
104.09 |
145.36 |
164.94 |
273.36 |
343.89 |
427.00 |
507.00 |
520.00 |
|
EU |
58.55 |
62.86 |
64.79 |
65.82 |
57.74 |
71.00 |
71.00 |
70.00 |
The tendency of the world increasing energy consumption will continue for two reasons-world population growth and requirements for higher living conditions. Increasing global temperature with all negative consequences will be the result.
Influence of Increasing Energy Consumption on Global Warming
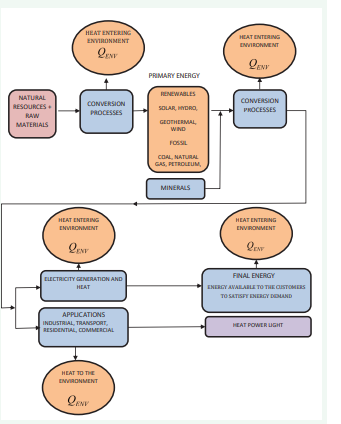
One possible source of atmospheric warming is thermal emissions from direct heat gain from global energy consumption. The constant accumulation of anthropogenic heat in the atmosphere, in addition to solar energy, increases the atmosphere’s temperature. The energy conversion chain and its dissipation into the environment in the form of heat are shown in (Figure 2).
Figure 2: Energy conversion processes and energy dissipation (heat wastes).
The rise of the average temperature of the tropospheric air layer can be determined by a simplified equation
1
where by definition β is a coefficient determining the proportion of primary energy absorbed by atmospheric air, t is time, T is average temperature of the tropospheric air layer (K), mat.air is air mass in the troposphere (kg), cp,at.air is average specific heat (kJ/kg. K) and EPE(t) is primary energy consumption (EJ/ year). After integration, temperature rise ?Tβ of the tropospheric air layer over time is:
2
Assuming, in agreement with statistical data, a linear relationship energy consumption-time (see Figure 1), Eq. (2) becomes
3
The estimation of β is important in calculation of ?Tβ . From data in (Figure 3) [6],
Figure 3: Temperature anomaly (K) vs time (year).
the global temperature rise is the sum of the increase of ?TGE as a greenhouse effect and heat emission, ?Tβ , from the conversion processes from primary energy to final energy i.e,
4
This follows from linear increase of temperature anomaly with time (see Figure 3) (according to the analysis in Part 1-temperature changes ?TGE vs time should increase asymptotically). To estimate ?Tβ the data of IPCC and Nikolenko will be used.
According to IPCC [6] (see Figure 3), the estimated average value by 2050 will be ?TGlobal =1.50 K. Nikolenko [5] estimated anthropogenic heating as a burning effect of different kinds of primary fuels as high as ?Tβ =1.10 K/century. Similar values are suggested by Nordel and Gavrat [7] and can be calculated from Flanner [8] and Forman [9].
For the tropospheric air layer with an average thickness 10 000 m and mt = 3,85. 1018 kg atmospheric air, for the years 1985-2015 of [3,4] (Table 1)
EPE(1980) = 280.56 (EJ/year)
EPE (2020)= 564.01 (EJ/year)
Thus from Eq (3) and ?Tβ =0.11 (K/decade) [5].
It means that about 10% of primary energy consumption is transported as heat to the tropospheric air layer and temperature increase will be 0.44 K, from Eq. (3).
The β value can be used for 2020-2050 of warming effect prediction. From data (Table 1)
EPE (2020) = 564.01 (EJ/year)
EPE (2050) = 725.00 (EJ/year)
And from Eq. (3) for β = 0.10 average temperature increase in the tropospheric air layer will be T (2020-2050) = 0.50 K
As global temperature increase ?TGlobal between 1980-2050 (after extrapolation) is [6] ?TGlobal=1.50 K
Thus from Eq (4).
DTGE (1980 - 2050) = DTGlobal - DTb = 1.50 - 0.50 - 0.44 = 0.56
This is average value for tropospheric air layer. Values at Earth surface will be greater and will depend on geographical coordinates. The value ?TGE will not increase significantly over time in comparison to ?T , which is the result of continuous increase in primary energy consumption. „Global Warming is likely to reach 1,5 K between 2030-2050 if it continues to increase at the current rate” [6].
Entropy Generation and Global Warming
Two crucial questions arise in any energy model [2,7]:
- What minimum energy is required, and what is the energy used for?
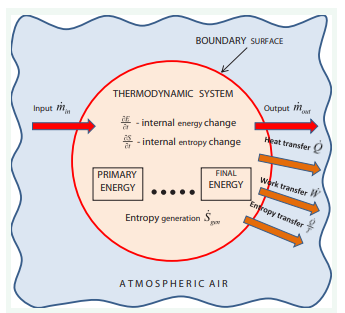
These questions lead us to consider where is wasted and how less energy can be used. Answers to these questions come from the laws of thermodynamics and the concepts of reversibility and entropy generation. The first law of thermodynamics, or energy conservation, states that energy cannot be destroyed or created. It guarantees the exact equivalence of various energy forms but does not ensure interconvertibility. Except for thermal energy (heat), all other forms of energy can be converted entirely into heat; the reverse process is impossible. The second law of thermodynamics defines quality differences between different forms of energy. The net effect of every transformation is the degradation of higher- quality energy to lower-quality energy. The degree of energy degradation, or dissipation, is described by a function proposed by Rudolf Clausius, who introduced the concept of entropy as a new physical quantity as fundamental and universal as energy [11]. The second law of thermodynamics states that every thermodynamic process is accompanied by entropy generation. The general scheme is presented in (Figure 4).
Figure 4: Example of the open thermodynamic system in thermal contact with atmospheric reservoir.
From the First Law of Thermodynamics
Where h is the chemical and physical specific enthalpy.
The Second Law of Thermodynamics defines the rate of entropy generation S gen , a quantity that is always positive
as a measure of a system’s departure from reversibility (s - is specific entropy).
Entropy generation is directly associated with a decrease in the quality of energy . The losses of the available energy (primary energy) δE? , are described by the Gouy [13] and Stodola [14] law, that
7
Where Tenv is the absolute average temperature of the environment.
Two equivalent methods of irreversibility analysis are based on availability or exergy concepts, which, in general, can be formulated as follows;
• The availability of a system is the maximum amount of work that can be obtained from it, in combination with an assumed uniform background environment with which it interacts [15].
• Exergy expresses the amount of work required to produce a material in its specified state in a natural environment from constant components in a reversible manner, with heat exchange only with the environment [16].
Both methods can answer the questions of where energy losses occur and where improvements can be achieved to save energy, increasing process effectiveness.
For elementary process “j” in the industrial chain
8
and
9
The energy dissipated Ej δ ? appears mainly in the form of heat and warms the atmospheric air layer. Conversion efficiency based on the first law of thermodynamics is misleading, as it cannot identify energy dissipation, possible improvements of energy systems and more effective use of natural resources. For example, Rose [15], explains the differences between these two approaches (energy flow of the US energy system), (see Figure 5).
Figure 5: Principal energy flow in United States, 1979 (Quads) [15].
From the first law of thermodynamics, global energy balance is:
• Primary energy - 86.83 (EJ)
• Final energy (used) - 36.72 (EJ)
• Energy wasted - 50.11 (EJ)
This means that from the first law efficiency is (36.72/86.83).
100 = 42.29 % From availability analysis:
• Final energy (used) - 12.35 (EJ)
• Wasted energy - 74.48 (EJ)
and thermodynamic efficiency is: (12.35/86.83)100 = 14.22 %
It includes unavoidable irreversibilities and is always significantly less than the usually used first law efficiency. The availability analysis was proposed by Keenan [17] and the exergy method was proposed by Rant and developed by Szargut [16]. Both methods can identify the location of energy dissipation. It is essential to underline that the analysis based on the second law of thermodynamics shows that the substitution of coal, natural gas and oil, usually fossil fuels, by renewable energy sources reduces anthropogenic global warming because energy losses are lower due to less irreversibility in the actual process. In general,“Dissipation of energy causes a decrease of the useful effect or increased consumption of driven energy if the required effect is assumed” [16].
The contemporary climate modelling literature takes little account of the methods discussed above, particularly the constraints imposed by the second law of thermodynamics. Kleidon and Lorenz [18] and Paixoto and Oort [19] have discussed selected problems. However, their analyses were limited to entropy generation within the atmospheric layer (absorption of solar and terrestrial radiation, melting of snow and ice, evaporation, etc.). Entropy is the result of thermodynamic irreversibilities that are constantly present in all real processes involved in the chain of transformation from natural resources to final usable energy. Clearly, energy wastage should be minimized. To this end, Bejan [12] has proposed and developed a method based on the concept of reducing entropy generation.
DISCUSSION
“In the 21st century, the climate may be influenced greatly by the increase in energy consumption. Almost all of this energy turns into heat, additionally to the solar energy absorbed by Earth’s atmosphere” [20]. According to Hafele [21] and Penner [10], the potential impact of anthropogenic heat loads on global mean temperature rise, if the world population is 10 billion people in 2050 and the increase in global mean temperature due to heat gain will be ?Tatm,air = 0.27 K and is too significant to be ignored. Global warming and all the negative consequences for the development of our civilization are a matter of widespread social concern. All forecasts suggest that global warming is inevitable. This is due to natural changes in our environment as a result of human activity and increasing energy consumption. It is evident that we have no control over natural climate change. Different hypotheses suggest possible actions to reduce the adverse effects of global warming. However, the question remains whether the proposed technological solutions are feasible. Unfortunately, the answer is no. The IPCC hypothesis assumes a net reduction of CO2 emissions to zero in 2050 (via the Paris Agreement). Gates [22] also advocates such an approach. Penner [10], on the other hand, proposes a reduction in energy consumption and intensive use of renewable energy sources. It is worth taking into account estimates that the increase in primary and final energy consumption will rise to around 40 % by 2050 compared to current values, leading to an increase in global warming. The negative consequences will mainly affect developing countries and increase areas of poverty and hunger. It is necessary to stress that the primary source of global warming and manufactured environmental degradation is the overuse of fossil fuels, mainly coal.
CONCLUSIONS
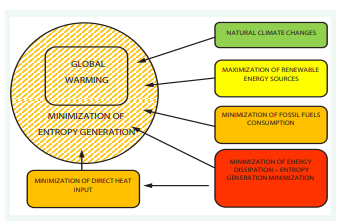
Possible activities to avoid global warming negative consequences are suggested below. The scenario assumes the maximum use of renewable energy sources, greater energy efficiency, and minimization of direct heat addition occurring as the effect of unavoidable energy dissipation resulting from the second law of thermodynamics.
Global Warming Phenomena Scenarios
Two possible effects of global warming are presented below.
Scenario 1: As a result, the temperature of the natural environment will rise (see Figure 6).
Figure 6; Continuation of present trend (known as business as usual).
Scenario 2: Assumes that renewable energy sources are used as much as possible, energy efficiency is improved, there are no net emissions of greenhouse gases and the direct heat gain from the inevitable energy dissipation resulting from the second law of thermodynamics is minimized. Environmental temperature rises can be controlled. However, we must bear in mind the limitations imposed by (see Figure 7).
Figure 7: Measures to avoid global warming negative consequences.
AUTHOR CONTRIBUTIONS
Conceptualization, ZK and JD; methodology, ZK, JD and JS; resources, ZK, JD, JS and AT; visualisation, JD; writing-review and editing, JD
ACKNOWLEDGMENTS
The present research was supported by the Polish National Agency for Academic Exchange (NAWA), within the Strategic Partnerships Programme, Project No. BPI/PST/2021/1/00023. The numerical results were obtained by computational power financially supported by the program “Excellence Initiative – Research University” for the AGH University of Krakow, Poland.
REFERENCES
- Harari YN. Homo Deus; a brief history of tomorrow. Clitheroe: Joosr. 2016; 448.
- Chaisson EJ. EOS, Trans Am Geophys. Union. 2011; 89: 253-260.
- BP Statistical Review of World Energy 2022, 71st Edition.
- Knoema BP Energy Outlook to 2050.
- Nikolenko AP. Concept of planetary thermal balance and global warming. J Gephysical Research. 2009; 114: 1-4.
- Climate Change 2021; The Physical science Basis, Contribution of Working Group I to the Sixth Assessment Report of the IPCC.
- Nordel BO, Garvet B. Global energy accumulation and heat emission. Int J Global Warming. 2009; 1: 378-391.
- Flanner MG. Integrating anthropogenic heat flux with global climate models. Geopyc Res Lett. 2009; 36: 1-5.
- Forman C, Muritala IK, Pardemann R, Meyer B. Estimating the global waste heat potential, Renew Sustain Energy Rev. 2016; 57: 1568- 1579.
- 10. Penner SS. Energy. 1976; 1: 407-412.
- Kondepudi D, Prigogine I. Modern Thermodynamics From Heat Engines to Dissipative Structures. John Wiley and Sons Ltd. 2014.
- Bejan A. Entropy Generation Minimization. CRC Press. Boca Raton. 1996; 400.
- Gouy G. About available energy (in French). J de Physique II. 1889; 8: 501-518.
- Stodola A. The Cyclic Processes of the Gas Engine (in German). Zeitschrift der VDI. 1898; 23: 1086-1091.
- Rose D. Learning about Energy. Plenum Press New York. 1986.
- Szargut J, Morris JDR, Steward FR. Exergy Analysis of Thermal, Chemical and Metallurgical Processes. Hemisphere Publ Corp New York. 1988, 332.
- Keenan JH. Availability and Irreversibility in Thermodynamics. British J Appl Physics. 1951; 2: 183-192.
- Kleidon A, Lorenz RD. Non-equilibrium Thermodynamics and the Production of Entropy. Springer. 2005.
- Peixoto JP, Oort AH. Physics of Climate. American Institute of Physics New York. 1992.
- Budyko MJ. Climate and Life. Academic Press. 1974.
- Hafele W. In Proceedings of IIASA Planning Conference Energy Systems. Int Inst Appl Systems Analysis. 1973, 9-78.
- Gates B. How to Avoid a Climate Disaster: The Solution We Have and the Breakthroughs We Need Penguin Random House. 2021.
![Primary energy consumption [3,4].](https://www.jscimedcentral.com/public/assets/images/uploads/image-1721718543-1.PNG)
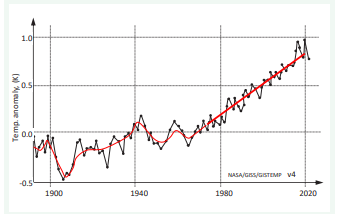
![Principal energy flow in United States, 1979 (Quads) [15].](https://www.jscimedcentral.com/public/assets/images/uploads/image-1721718606-1.PNG)