Phytoplankton Assemblages of Apodu Reservoir, Malete, Nigeria (A Tropical African Reservoir)
- 1. Department of Zoology, University of Ilorin, Ilorin, Nigeria
Abstract
Qualitative sampling and relative abundance of phytoplankton of Apodu reservoir, an important tropical African drinking water reservoir in Malete, Nigeria was analysed monthly from the three stations for one year between January 2018 and December 2018 spanning both rainy and dry seasons, using a plankton net. Identification of the phytoplankton was done under a digital binocular compound microscope equipped with a camera. The phytoplankton were identified to generic level using standard taxonomic identification keys. Six classes of phytoplankton namely, Bacillariophyceae, Chlorophyceae, Cyanophyceae, Zygnematophyceae, Coscinodiscophyceae, Xanthophyceae were recorded in the reservoir. Thirty-five (35) genera were found in the phytoplankton classes with Chlorophyceae having the highest of thirteen genera, while Coscinodiscophyceae was the least with one genus. Microspora had the highest relative abundance of the total phytoplankton, while Stephanodiscus had the least relative abundance of the total phytoplankton population. The phytoplankton classes were present in all the three stations and highly abundant in the rainy season. The assemblages of the various classes of phytoplankton in the reservoir was attributed to the optimal ranges of the physico- chemical factors of the water. The heterogeneity of the phytoplankton assemblage enabled high primary production in the reservoir and this will support a high fish production in the reservoir.
Keywords
• Phytoplankton
• Apodu Reservoir
• Bacillariophyceae
• Chlorophyceae
• Cyanophyceae
CITATION
Mustapha MK, Abdulkareem JA (2024) Phytoplankton Assemblages of Apodu Reservoir, Malete, Nigeria (A Tropical African Reservoir). JSM Environ Sci Ecol 12(3): 1100.
INTRODUCTION
Reservoirs provide valuable ecosystem services, such as water supply, irrigation, hydroelectric power generation, fish production, recreation and aesthetics [1]. The knowledge of the limnology, abundance, composition, seasonal succession, maturation and changes in a reservoir is a prerequisite for a long-term management of its aquatic resources. The limnology of reservoirs has been used in estimating the water quality [2], productivity and fisheries potentials [1-3], management [4], climate change impact, among several other uses of the discipline. Reservoirs are considered favorable environments to the development of phytoplankton communities, which may establish diverse assemblages in relatively short periods of time after impoundment [5]. Several factors usually contribute to the establishment of plankton communities in a reservoir, among which are good water quality, presence of nutrients, physico- chemical factors of water, hydrological characteristics of the reservoirs and reservoir ageing [6].
Phytoplankton are usually at the base of aquatic food web and are the most important factor for production of organic matter in aquatic eco system. Most reservoirs will require significant number of plankton to have productive and sustainable fisheries [7]. The limnology or interplay of physical, chemical and biological properties of water most often lead to the production of phytoplankton, while their assemblages are structured by these factors. Thus, any perturbations in these factors may affect their assemblage which could have a significant impact on water quality and fisheries of reservoirs. Apart from primary production, phytoplankton plays an important role as food for herbivorous animals and act as biological indicator of water quality [8]. Phytoplankton are free-floating microscopic plants that are mostly unicellular and produce chemical energy from light by the process of photosynthesis which leads to primary production in aquatic ecosystems. Phytoplankton has a critical role in primary production, nutrient cycling, and food webs and make up a significant proportion of the primary production in aquatic systems [9]. The study of phytoplankton composition and physico-chemical parameters has become the subject of much research because of their importance in energy production and as long-term indicators of water quality [10]. Although, studies have been conducted on phytoplankton assemblages of many lakes in Nigeria, there is none about Apodu reservoir, Malete, Nigeria an important tropical African drinking water reservoir. This study will therefore serve as a baseline for the phytoplankton assemblages of Apodu reservoir, Malete, Nigeria.
MATERIALS AND METHODS
Site Description
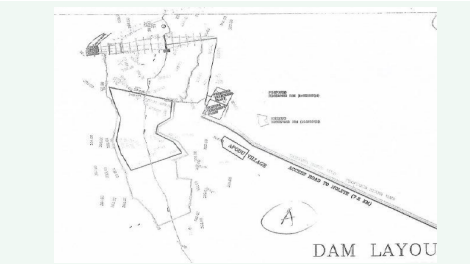
The study site is Apodu reservoir, in Apodu village, about 7km away from Malete town in Moro Local Government Area of Kwara State, Nigeria. The reservoir was constructed some years back and the re-impoundment of the reservoir was carried out in 2016 (Figure 1).
Figure 1: Map showing Apodu Reservoir, Malete, Nigeria.
It lies between the longitude 8?45”2.59” N, 8?, 45? 27.7” N and latitude 4? 27? 41.4” E, 4?27? 35.5” E. During dry season, the water does not flow to the other end of the reservoir but flows continuously during rainy season. The main purpose of the reservoir is to provide drinking water and water for irrigation for Apodu, Malete (a University town), and other nearby communities in Moro Local Government Area of Kwara State, Nigeria (Table 1).
|
Table 1: Morphometric Features of Apodu Reservoir, Malete, Nigeria. |
|
|
Features |
Measurements |
|
Storage capacity |
0.75MCM |
|
Surface area |
15 hectares |
|
Earth embankments |
|
|
Length |
360m |
|
Crest width |
4m |
|
Upstream slope |
01:03 |
|
Downstream slope |
01:02:05 |
|
Spill ways |
|
|
Length |
30m |
|
Height |
2.6m |
|
Maximum discharge |
10.8m3/s |
|
Free board |
1 |
Phytoplankton Sampling and Analysis
Qualitative sampling and relative abundance of the phytoplankton were analysed monthly from the three stations for a year between January 2018 and December 2018 spanning both rainy and dry seasons between the hours of 08.00 and 10.00 am by filtering 1L of water sample through a standard Lamotte plankton sieve net of bottling silk with mesh size 153μ. The samples were fixed on the field with Lugol solution. The sample was stored in a dark compartment in the laboratory for overnight sedimentation. In the morning, the upper 900ml was siphoned out and the remaining 100ml homogenized samples were retained from which 1ml subsample was collected for phytoplankton identification. Duplicate replicate samples of the plankton were taken from all the sampling points. Identification of the plankton was done under an Amscope digital binocular compound microscope (100×–400×) B-120 series equipped with a camera. The plankton was identified to generic level using standard taxonomic identification keys, compiled by Edmonson and Withford & Schumacher [11,12] and genera were arranged as per AlgaeBase [13].
RESULTS
Phytoplankton Species Composition and Relative Abundance
Six classes of phytoplankton namely Bacillariophyceae (diatoms), Chlorophyceae (green algae), Cyanophyceae (blue green algae), Zygnematophyceae (green algae), Coscinodiscophyceae (diatoms), Xanthophyceae (yellow-green algae) were recorded in the reservoir. A total of thirty-five (35) genera were found in the phytoplankton classes with Chlorophyceae having the highest of thirteen genera, while Coscinodiscophyceae was the least with one genus (Table 2).
|
Table 2: Phytoplankton species composition and relative abundance in Apodu reservoir, Malete, Nigeria. |
||
|
Class |
Genera |
Relative abundance |
|
Chlorophyceae |
Microspora |
++++ |
|
Scenedesmus |
++++ |
|
|
Coelastrum |
+++ |
|
|
Protococcus |
+++ |
|
|
Netrium |
+++ |
|
|
Straurastrum |
+ |
|
|
Closterium |
++++ |
|
|
Cladophora |
++ |
|
|
Spirogyra |
++ |
|
|
Ulothrix |
++ |
|
|
Mougeotia |
+ |
|
|
Draparnaldra |
+ |
|
|
Dictysophaeriaum |
+ |
|
|
Bacillarophyceae |
Nitzchia |
+++ |
|
Surirella |
++ |
|
|
Cymbella |
+ |
|
|
Gomphonemia |
+ |
|
|
Cyclotella |
+++ |
|
|
Synedra |
+++ |
|
|
Diatoma |
+++ |
|
|
Navicula |
+++ |
|
|
Cerataulina |
+ |
|
|
Zygnematophyceae |
Zygnema |
+ |
|
Gonatozgon |
+ |
|
|
Genicularia |
+ |
|
|
Cylindrocystis |
+ |
|
|
Coscinodiscophyceae |
Stephanodiscus |
+ |
|
Xanthophyceae |
Tribonema |
+ |
|
Ophiocytium |
+ |
|
|
Cyanophyceae |
Phormidium |
++ |
|
Aphanocapsa |
++ |
|
|
Nostoc |
+++ |
|
|
Anabeaena |
+++ |
|
|
Coelosphaerium |
+++ |
|
|
Oscillatoria |
+++ |
|
|
+ = Sparse + + = Many + + + = Very many + + + + = Highly abundant |
||
Among phytoplankton genera, Microspora had the most relative abundance of the total phytoplankton and was also the predominant in the class Chlorophyceae while Stephanodiscus, a Coscinodiscophyceae had the least relative abundance of the total phytoplankton population.
Seasonal Abundance and Distribution of Phytoplankton
The phytoplankton classes were present in all the three stations and highly abundant in the rainy season especially between May and October with high relative abundance in August, while in the dry season the phytoplankton relative abundance was highly reduced. Station 1 had a higher genera diversity with 35 genera recorded, Station 1 recorded 32 genera, while 30 genera were observed from Station 3. Chlorophyceae, Cyanobacteria and Bacillariophyceae were highly abundant in Station 1 with all the genera found in the station.
DISCUSSION
The phytoplankton assemblage of Apodu reservoir was highly diverse, heterogenous and abundant. This could be due to the presence of large amount of nutrients especially nitrate, phosphate, silica and sulphate as well as the shallow nature of the reservoir, which greatly exposes the surface to light for algal productivity. The phytoplankton were highly abundant during the rains which corresponded to the period when the ions were highly concentrated in the reservoir. Thomas et al. [14] has reported that high primary productivity in tropical reservoir is usually rain induced. The assemblage of Bacillariophyceae and Coscinodiscophyceae (diatoms) was attributed to the high concentration of silica, while the assemblage of Chlorophyceae (green algae), Cyanophyceae (blue green algae), Zygnematophyceae (green algae), and Xanthophyceae (yellow- green algae) could be attributed to high amount of nitrate, phosphate and sulphate. These nutrients have been known to be limiting in phytoplankton growth [15].
The order of abundance and assemblage of the phytoplankton of Apodu reservoir was similar to the observations of Brooklemma [16] in tropical lakes. The high assemblage of Chlorophyceae, Cyanobacteria and Bacillariophyceae in Station 1 especially during the rains was due to the station’s high nutrient load probably on the account of its eutrophication. The effects of these high nutrient loads in the reservoir and favourable environmental conditions led to the production of large algal bloom with the occurrence of 35 genera of phytoplankton in the station. Station 1 where eutrophication was high and pronounced had some genera of toxic blue-green algae which could impair the water quality, making the water unfit for drinking, disrupt the recreational and aesthetic nature of the reservoir. .
Plate 1: Genicularia.
Plate 2: Closterium.
Plate 3: Cymbella
Plate 4: Microspore.
Plate 5: Spirogyra.
Plate 6: Gonatozygon.
The impacts of high algal biomass and the deleterious effects of blue-green algae on the ecology of freshwater ecosystems are well known [17]. All these negative effects of eutrophication will affect the composition, diversity and abundance of fish species in the reservoir once the eutrophication becomes intense, moving from slow to a fast one if the process is not checked in time. The relatively low assemblage of the phytoplankton in Station 3 was attributed to factors such as patchiness and low concentration of dissolved salts in the station.
The phytoplankton assemblage of Apodu reservoir was similar to other tropical reservoirs [5]. Factors such as high temperature, high transparency, short water residence time, high flushing rate of nutrients in the dry season and grazing effects by zooplankton and fishes could have caused the reductions in numbers of the phytoplankton in the reservoir [7]. The phytoplankton assemblage also shows the reservoir to be eutrophic and it fluctuation is in response to seasonal concentration of nutrients, grazing pressure by biotic organisms and the reservoir hydrology. The heterogeneity of the phytoplankton assemblage enabled high primary production in the reservoir and this will support a high fish production, thereby contributing to food security of the people. The phytoplankton community composition, diversity and abundance of this reservoir could be used as the main controlling variable for predicting fish yields in the reservoir. Knosche & Barthemes [18] have used similar model of phytoplankton composition to predict fish yields in lakes and reservoirs and reported this model resulted in more successful predictions than. Cushing [19] also observed a strong relationship between phytoplankton primary production and fish production and concluded that a long-term fluctuation in fish stocks was due to this relationship.
REFERENCES
- Mustapha MK. Perspectives in the limnology of shallow tropical African reservoirs in relation to their fish and fisheries. J Trans Environ Stud. 2011. 10: 16-23.
- Mustapha MK. Assessment of the water quality of Oyun Reservoir Offa, Nigeria, using selected physico-chemical parameters. Turk J Fish Aquat Sci. 2008. 8: 309-319.
- Mustapha MK. Limnological evaluation of the fisheries potentials and productivity of a small shallow tropical African reservoir. Rev Biol Trop. 2009; 57: 1093-1106.
- Mustapha MK. Problems, challenges and management of small, shallow tropical African reservoirs – a case study of Oyun Reservoir, Offa, Nigeria. Int J Lakes Rivers. 2009b. 2: 163-174.
- Rocha O, Matsumura-Tundisi T, Espindola ELG, Roche KF, Rietzler AC. Ecological theory applied to reservoir zooplankton. In Tundisi, J.G & M. Straskraba, (eds.). Theoretical Reservoir Ecology and its Applications. International Institute of Ecology, Brazilian Academy of Sciences. Backhuys Publishers, Leiden, Netherlands. 1999: 29-51.
- Mustapha MK. Seasonal influence of limnological variables on plankton dynamics of a small shallow, tropical African reservoir. Asian J Exp Biol Sci. 2010. 1: 60-79.
- Mustapha MK. Phytoplankton assemblage of a small, shallow, tropical African reservoir. Rev Biol Trop. 2009; 57: 1009-1025.
- Khatib S, Nandita S. Phytoplankton Dynamics of Fresh Water Lake Varhala in Thane District, Maharashtra Glo J Sci Fron Res: H Environ Earth Sci. 2017; 17: 1-7.
- Babu JM, Getabu AM, Nyaundi K jembe D, Boera P, Sitoki L, Mwayuli GA, Omondi RO, Olilo CO. Phytoplankton distribution and abundance in small water bodies of the Lake Victoria Basin of Kenya. IJFAS. 2015; 2: 166-169.
- Kozak A, Go?dyn R, Dondajewska R. Phytoplankton Composition and Abundance in Restored Malta?ski Reservoir under the Influence of Physico-Chemical Variables and Zooplankton Grazing Pressure. PLoS One. 2015; 10: e0124738.
- Edmonson WT. Freshwater Biology. Wiley, New York. 1959.
- Withford LA, Schumacher GJ. A manual of freshwater algae. Sparks, Raleigh, North Carolina, USA. 1973.
- Guiry MD, Guiry GM. AlageBase. World-wide electronic publication. University of Galway. 2024.
- Thomas S, Cecchi P, Corbin D, Lemoalle J. The different primary producers in a small African tropical reservoir during a drought: temporal changes and interactions. Freshw Biol. 2000; 45: 43-56.
- Talling JF, Lemoalle J. Ecological dynamics of tropical inland waters, In J.F. Talling & J. Lemoalle (eds.). Resource utilization and biological production- primary utilization: energy. Cambridge, Cambridge, England. 1998: 82-117.
- Brook L. Seasonal limnological studies on Lake Alemaya: a tropical African lake, Ethiopia. Arch. Hydrobiol. 1995; 107: 263-285.
- Newton AR, Melaram R. Harmful algal blooms in agricultural irrigation: risks, benefits, and management. Environmental Water Quality. 2023; 5: 1325300.
- Knosche, R, Barthelmes D. A new approach to estimate lake fisheries yields from limnological basic parameters and first results. Limnologica. 1998; 28: 133-144.
- Cushing DH. Climate and Fisheries. Cambridge, England. 1982.