The Impact of Plant Guttation on Insects
- 1. Department of Entomology, University of Agricultural Sciences, PhD scholar, GKVK, Bengaluru, India
- 2. Department of Food Science and Nutrition, University of Agricultural Sciences, PhD Scholar, GKVK, Bengaluru, India
- 3. Department of Entomology, Jhansi Rani Lakshmi Bai Central Agricultural University, Teaching associate, India
Abstract
Arthropod predators and parasitism, traditionally considered carnivorous, often rely on plant-derived food sources at various stages of their life cycles, influencing their life-history parameters. Plant guttation, a largely unexplored food source, consists of exudates secreted through hydathodes on leaf surfaces, containing a diverse array of organic and inorganic compounds. While plant guttation can serve as a valuable nutritional resource for insects, it can also harbour pesticide residues and microorganisms, influencing insect communities and interactions. Additionally, plants utilize guttation as a mechanism for defence against herbivores and pathogens by accumulating toxic compounds. This review explores the nutritional value of plant guttation for insects, its role in pesticide residue analysis, and its significance in plant defence mechanisms. Understanding the complexities of plant guttation is crucial for maximizing its potential benefits in agricultural and non-agricultural ecosystems, highlighting the need for integrated approaches to enhance biological control and ecological sustainability.
Keywords
Plant Derived Foods; Plant Guttation; Natural Enemies; Pesticide Residue; Biological Control
CITATION
Amogha, Bhavana A, Mishra VK (2024) The Impact of Plant Guttation on Insects. JSM Environ Sci Ecol 12(1): 1089.
INTRODUCTION
Arthropod predators and parasitism are typically thought of as being carnivorous, although they frequently require consuming plant-derived food sources for at least part of their life cycles. The degree to which these plant-provided foods are essential to predators or parasitism varies. Predators and parasitoids’ life-history parameters can be significantly impacted by the plant derived food sources. Plant derived food sources can be considered as alternate food sources. These alternate food sources are floral nectar, extra floral nectar, pollen, and plant guttation. Availability of alternative food sources increases the presence and fitness of natural enemies. For natural enemies, these plant-derived compounds are a vital and abundant supply of proteins and carbohydrates, but their availability can vary greatly [1,2]. Due to these variations, enormous efforts have been made over the past few decades to control plant-derived food supplies in agroecosystems and, as a result, raise the fitness of natural enemies that consume them. Despite these initiatives, plant guttation is a food source generated from plants that is still largely unexplored.
Guttation is the process by which secretion of exudates or fluids containing dissolved components through unique structures known as “Hydathodes,” occasionally referred to as “Water Stomata” or “Water Pores,” which are constantly open and situated on the surfaces and peripheries of leaves [3]. Guttation fluid is composed of both xylem and phloem sap occurs in wide range of plant species belonging to angiosperms, gymnosperms, ferns, algae, and fungi [4]. Exudation of guttation fluid is mainly controlled by root pressure that it is affected by abiotic (ambient and soil temperatures, Relative Humidity (RH) and solar radiation) and biotic (vegetative and reproductive growth) factors.
Guttation is the result of reduced transpiration brought on by elements such as high humidity, wind, and closed stomata. The plant’s root system takes up extra water when the soil is saturated with rainwater and high humidity in the atmosphere. Hydrostatic pressure thus builds up in the roots, pushing the water upward and out through the leaf tip, hydathodes, or water glands to create droplets. The key physiological role of the plant guttation is to promote water flux when there is reduced transpiration. Guttation is often confused with dew droplets that condense from the atmosphere onto the plant’s surface. Guttation fluids showed a complex and varied composition with a wide range of organic and inorganic compounds, varying according to the plant, the environment, and the age of the plant tissues [5]. The significance of organic compounds in guttation droplets has been disregarded in the context of plant guttation serving as a potential food source for natural enemies, primarily due to the prevailing misconception that these droplets solely consist of water [6]. This review highlights the positive as well as negative effects of plant guttation on insects.
The Nutritional Significance of Plant Guttation for Insects
Plant guttation contains several organic and inorganic compounds. But the contents of these droplets can range significantly between plant species, most likely because of variations in their morphology and physiology (e.g. leaf structures, xylem, and phloem composition) (Table 1).
Table 1: Major organic and inorganic compounds present in the guttation fluids.
|
S No. |
Crops |
Compounds Present |
References |
|
1 |
Maize |
Amino acids, sugars, auxin, ABA |
[6] |
|
2 |
Wheat |
Aminoacids, harmones, vitamins |
[7] |
|
3 |
Pear |
Peroxidases, proteins |
[8] |
|
4 |
Highbush blueberry |
Sugars, aminoacids |
[9] |
|
5 |
Peach |
Peroxidases, proteins |
[8] |
|
6 |
Castor |
Fatty acids, terpinoids, organic acids |
[10] |
|
7 |
Oat |
Aminoacids, sugars, auxin, enzymes |
[11] |
|
8 |
Rye |
Aminoacids, harmones |
[7] |
|
9 |
Cabbage |
Peroxidase, lignin |
[12] |
Plant guttation can be consistent and nutrient rich food source for insects with effect on their communities [9]. Highbush blueberries guttation droplets are rich in carbohydrates and protein. Longevity and fecundity of insects, including predators and parasitism increased, that feed on them [9].
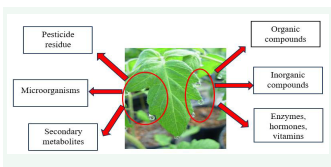
For insects, guttation can be a rich source of food, but systemic pesticides, such as neonicotinoids, can contaminate it. Using systemic insecticides can also result in the build-up of insecticide residue in guttation drops, which can kill pollinators and insect predators that feed on it [13]. Guttation fluid also contains microorganism. Guttation fluids promote the growth of microorganisms, and the fluids from the youngest leaves stimulate faster growth, probably because they contain more carbohydrates, vitamins, amino acids, and ions [5]. Guttation fluid from cereals can also promote germination and growth of spores from ascomycete fungi [14]. But presence of microorganisms in guttation fluid might affect the nutritional value for insects, similar to other plant derived food sources [15]. In case of other plant derived food sources like nectar contains microorganism, which influence the nectar volatile composition and attractiveness to a generalist pollinator. The variable occurrence of microorganisms could potentially release chemical signals that affect the interactions between plants and pollinators [16].(Figure 1).
Figure 1: Plant guttation containing various compounds like organic and inorganic compounds including enzymes, hormones, vitamins, secondary metabolites and pesticide residues.
Guttation as an Assessment Tool for Pesticide Residue Analysis
Using systemic insecticides can also result in the build-up of pesticides in guttation drops, which might kill pollinators and insect predators that eat them. Systemic insecticides can reach other plant derived food sources like nectar, extra floral nectar, pollen and honey dew and can cause antagonistic effect on natural enemies. Study conducted on nectar from directly sprayed clover blooms had higher concentration of imidacloprid and was acutely toxic to Orius insidiosus (Hemiptera: Anthocoridae) [17]. On the other hand, imidacloprid levels in nectar from blooms that opened 1-2 weeks following the imidacloprid treatment did not have an impact on predator survival. Additionally, authors found that one-week following imidacloprid treatment, the generalist predator Orius insidiosus showed reduced survival when exposed to guttation from creeping-bent grass. Nevertheless, three weeks after treatment, there were no detrimental effects of guttation on O. insidiosus survival in creeping bent grass treated with imidacloprid [17].
Comparably, studies on pollinators found that guttation could be potential route for pesticide exposure. Whether bees should be concerned about plants’ active water secretions, or guttation, due to potential neonicotinoid pesticide residues, is a topic of current debate. Given its importance to agriculture, it was investigated whether guttation water and dew from winter rape plants treated with the insecticide Nurelle D® (a.i., chlorpyriphos+cypermethrin) could be acutely or chronically toxic to honeybees (Apis mellifera). Divergent opinions were expressed, though [18].
The neonicotinoids used for seed coating could be translocated in guttation drops and reach concentrations toxic to bees [19]. The authors measured neonicotinoid residues in guttation water samples from maize seedlings grown from neonicotinoid-treated seeds and fed the guttation water (spiked with sugar) to honey bees in the laboratory due to concerns about potential exposure to systemic pesticides through water foraging. Concerns have been raised that guttation water from treated crops may be a potential source of exposure to systemic insecticides, as neonicotinoid concentrations in the samples reached levels that were hazardous to honey bees.
The residue of thiamethoxam were also found in the guttation fluid from seed-coated winter oilseed rape plants and concentration was toxic to honey bees [20]. Proof for the uptake of guttation fluid from seed-coated winter oilseed rape by honeybees was provided by measuring residues in individual honey-sac contents. This confirms that the translocation of systemic active substances is not only limited to pollen and nectar [20]. The potential risk of guttation mainly depends on the distance between the colonies and treated crops, because the distance between colony and crop and the availability of other water sources mainly determine uptake of guttation droplets. The risk of uptake of contaminated water is higher if the colonies are located in closer proximity to the crop, and lower with increasing distance [21].
Chemicals Found In Guttation Serve As A Form Of Defence For Plants Against Herbivores
Plants have the ability to generate a diverse array of substances that may initially appear to be excretory products. Nevertheless, extensive research has revealed that many of these substances actually serve a purpose, such as defending against herbivores and parasites. By accumulating toxic compounds on their surface, plants are able to directly interact with insects, pathogens, and herbivores [22]. In the case of various grasses, they form a symbiotic relationship with fungi (Neotyphodium spp. endophytes) that reside in the intercellular spaces of the grass. These endophytes produce a variety of alkaloids that act as protective agents against grazing by mammals and insects. A comprehensive study has been conducted to demonstrate the transfer of fungal alkaloids from the grass-endophyte associations into the plant fluids by the host plant [23]. One of the alkaloids found in this study is peramine, which is an uncommon pyrrolopyrazine. Peramine is continuously synthesized by the endophyte, but it does not accumulate progressively. The process by which peramine is eliminated through further metabolism or any other means has not yet been documented. The presence of peramine has been observed in both the cut leaf fluid and the guttation fluid in all grass-endophyte associations. In certain associations, other alkaloids such as lolines and ergot peptides have also been detected [23]. Hence, it can be inferred that the guttation and trichome exudates play a crucial role in providing an initial defence against attacking organisms, potentially allowing for the activation of induced defences [24]. The presence of the physical structures and biochemical of the phyllo plane and protein-based surface provide defences against animals [25]. Authors have also reviewed the emerging evidence pertaining to antimicrobial phyllo plane proteins and mechanisms by which proteins can be released to the phyllo plane, including biosynthesis (e.g. phylloplanins) by specific trichomes and delivery in guttation fluid from hydathodes [25]. Hence, guttation fluid can also accumulate various secondary compounds, which can be detrimental for herbivores.
CONCLUSION
Among several biological phenomena, guttation remains one of the most powerful engines for the mobility of organic and inorganic chemicals in plants. Although the phenomenon of plant guttation has the potential to increase the abundance of natural enemies in agricultural fields, the effectiveness of these natural enemies as agents of biological control will likely be influenced by various factors. These factors include the composition of the natural enemy communities and their attraction to guttation, the response of pests to plant guttation, and the prevailing environmental conditions. Therefore, in order to maximize the benefits of plant guttation for biological control in agricultural ecosystems, it is necessary to integrate this phenomenon with other appropriate agricultural practices. This review has given the insight of both positive as well as negative consequences of plant guttation on insects. It further emphasizes the necessity to take into account guttation as a significant plant derived source nutrients for insects in agricultural systems, as well as in non agricultural communities and ecosystems.
REFERENCES
- Tena A, Wäckers FL, Heimpel GE, Urbaneja A, Pekas A. Parasitoid nutritional ecology in a community context: the importance of honeydew and implications for biological control. Curr Opin Insect Sci. 2016; 14: 100-104. doi: 10.1016/j.cois.2016.02.008. Epub 2016 Feb 22. PMID: 27436654.
- Heil M. Nectar: generation, regulation and ecological functions. Trends Plant Sci. 2011; 16(4): 191-200. doi: 10.1016/j.tplants.2011.01.003. Epub 2011 Feb 21. PMID: 21345715.
- Dixon HH, Dixon GJ. The exudation of water from the leaf tips of Colocasia antiquorum. Schott. Proc. Roy. Soc. Dublin. 1931; 20: 7-10.
- Singh S. Guttation: New Insights into Agricultural Implications. Advances in agronomy. 2014; 128: 97-135.
- Cerutti A, Jauneau A, Laufs P, Leonhardt N, Schattat MH, Berthomé R, et al. Mangroves in the Leaves: Anatomy, Physiology, and Immunity of Epithemal Hydathodes. Annu Rev Phytopathol. 2019; 57: 91-116. doi: 10.1146/annurev-phyto-082718-100228. Epub 2019 May 17. PMID: 31100996.
- Urbaneja-Bernat P, Tena A, González-Cabrera J, Rodriguez-Saona C. An insect’s energy bar: the potential role of plant guttation on biological control. Current Opinion in Insect Science. 2024; 61: 101140.
- Goatley JL, Lewis RW. Composition of guttation fluid from rye, wheat, and barley seedlings. Plant Physiol. 1966; 41(3): 373-375. doi: 10.1104/pp.41.3.373. PMID: 16656266; PMCID: PMC1086351.
- Biles CL, Abeles FB. Xylem sap proteins. Plant Physiology. 1991; 96: 597-601.
- Urbaneja-Bernat P, Tena A, González-Cabrera J, Rodriguez-Saona C. Plant guttation provides nutrient-rich food for insects. Proc Biol Sci. 2020; 287: 20201080. doi: 10.1098/rspb.2020.1080. Epub 2020 Sep 16. PMID: 32933440; PMCID: PMC7542811.
- Venkateswaran V, Muralidharan S, Gopalakrishnan AV, Krishnan N, Velmurugan D, Raman P. GC-MS analysis of guttation fluids from selected crop plants. Journal of Crop Improvement. 2022; 36: 801- 815.
- Sheldrake AR, Northcote DH. Some constituents of xylem sap and their possible relationship to xylem differentiation. Journal of Experimental Botany. 1968; 19: 681-689.
- Gay PA, Tuzun S. Temporal and spatial assessment of defense responses in resistant and susceptible cabbage varieties during infection with Xanthomonas campestris pv. campestris. Physiological and Molecular Plant Pathology. 2000; 57: 201-220.
- Schmolke A, Kearns B, O’neill B. Plant guttation water as a potential route for pesticide exposure in honeybees: a review of recent literature. Apidologie. 2018; 49: 637-646.
- Lewis RW. Guttation Fluid: Effects on Growth of Claviceps purpurea in vitro. Science. 1962; 138: 690-691. doi: 10.1126/ science.138.3541.690. PMID: 17829704.
- Lenaerts M, Goelen T, Paulussen C, Herrera-Malaver B, Steensels J, Van den Ende W, et al. Nectar bacteria affect life history of a generalist aphid parasitoid by altering nectar chemistry. Functional Ecology. 2017; 31: 2061-2069.
- Rering CC, Beck JJ, Hall GW, McCartney MM, Vannette RL. Nectar- inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2018;220(3): 750-759. doi: 10.1111/nph.14809. Epub 2017 Sep 28. PMID:28960308.
- Larson JL, Redmond CT, Potter DA. Mowing mitigates bioactivity of neonicotinoid insecticides in nectar of flowering lawn weeds and turfgrass guttation. Environ Toxicol Chem. 2015; 34(1): 127-132. doi: 10.1002/etc.2768. Epub 2014 Nov 12. PMID: 25319809.
- Shawki MA, Tit?ra D, Kazda JA, Kohoutkova J, Taborsky V. Toxicity to honeybees of water guttation and dew collected from winter rape treated with Nurelle D®. Plant Protection Science. 2006; 42: 9-14.
- Girolami V, Mazzon L, Squartini A, Mori N, Marzaro M, Di Bernardo A, et al. Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: a novel way of intoxication for bees. J Econ Entomol. 2009; 102(5): 1808-1815. doi: 10.1603/029.102.0511. PMID: 19886445.
- Reetz JE, Schulz W, Seitz W, Spiteller M, Zühlke S, Armbruster W, et al. Uptake of Neonicotinoid Insecticides by Water-Foraging Honey Bees (Hymenoptera: Apidae) Through Guttation Fluid of Winter Oilseed Rape. J Econ Entomol. 2016; 109(1): 31-40. doi: 10.1093/jee/tov287. Epub 2015 Oct 29. PMID: 26516090.
- Pistorius J, Brobyn T, Campbell P, Forster R, Lortsch JA, Marolleau F, et al. Assessment of risks to honey bees posed by guttation. Julius- Kühn-Archiv. 2012; 4: 199-208.
- Singh S, Singh TN. Guttation 1: chemistry, crop husbandry and molecular farming. Phytochemistry Reviews. 2013; 12: 147-72.
- Koulman A, Lane GA, Christensen MJ, Fraser K, Tapper BA. Peramine and other fungal alkaloids are exuded in the guttation fluid of endophyte-infected grasses. Phytochemistry. 2007; 68(3): 355-360. doi: 10.1016/j.phytochem.2006.10.012. Epub 2006 Nov 28. PMID: 17126863.
- Wagner GJ, Wang E, Shepherd RW. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot. 2004; 93(1): 3-11. doi: 10.1093/aob/mch011. PMID: 14678941; PMCID: PMC4242265
- Shepherd RW, Wagner GJ. Phylloplane proteins: emerging defenses at the aerial frontline?. Trends in Plant Science. 2007; 12: 51-56.