Drug Eluting Stent Restenosis
- 1. Department of Cardiovascular Medicine, Beni Suef University, Egypt
Abstract
n-stent restenosis has been a longstanding problem after percutaneous coronary intervention. Randomized trials comparing drug-eluting stents (DES) with bare-metal stents (BMS) have shown that the former significantly reduce the incidence of angiographic and clinical restenosis. The introduction of the drug-eluting stent (DES) successfully reduced the rate of restenosis; however, it is not completely diminished. Multiple factors may be involved in the mechanism of DES restenosis. Next to incomplete coverage with DES of the vessel segment injured by balloon angioplasty, factors such as stent under expansion, stent overexpansion, and non-uniform distribution of stent struts have been associated with DES restenosis. In addition, worse outcomes after repeat revascularization compared to BMS restenosis are reported in DES restenosis. Management of DES restenosis is an emerging issue, which requires careful evaluation of the restenosed lesion, together with determination of therapeutic strategy. There is no consensus at present on how to treat post-DES restenosis.
Keywords
In-Stent Restenosis , Drug-Eluting Stent, Intravascular ultrasound, Zotralimus Eluting Stents, Acute coronary syndrome
Citation
el Mokadem M (2018) Drug Eluting Stent Restenosis. JSM Heart Surg Case Images 2(1): 1009.
INTRODUCTION
Bare metal stent (BMS) restenosis is expected to occur within 6 months of intervention [1]. Drug-eluting stents (DES) proved to be superior to bare metal stents (BMS) in reducing restenosis rate and the need for repeat revascularization compared with bare metal stents [2-3].In spite of low rate of drug eluting stent restenosis, its management looks to be challenging. The clinical presentation of drug eluting stent restenosis is usually effort angina but some patients may present with acute coronary syndrome.
DEFINITION
Angiographic in stent restenosis is defined as reduction of lumen diameter after percutaneous intervention (PCI), it looks to be of clinical significance if more than 50%. Use of Fractional flow reserve (FFR) and intravascular ultrasound (IVUS) can be helpful during evaluation of intermediate lesions [4-5].
Intravascular ultrasound (IVUS) provides detailed information about reference vessel diameter, minimal lumen diameter before and after stenting, percent lumen stenosis, distribution of intimal tissue and presence of stent under expansion with possible need for post stent dilatation with non-compliant balloon. Clinical restenosis is defined as presence of angiographic stenosis more than 50% plus one of the following: a) recurrent anginal pain related to restenotic lesion b) objective evidence of ischemia either during resting or exercise ECG c) abnormal results of functional diagnostic test e.g. FFR less than 0.8 [6]. The clinical effect of a DES is dependent on its components: stent platform, active pharmacologic compound, and drug carrier. The new generations of DES, such as everolimus eluting stents (EES), zotralimus eluting stents (ZES) and biolimus A9 eluting stents are characterized by improvements in stent platform (thin-strut cobalt chromium vs. thick-strut stainless steel), polymer (thinner and/or biodegradable or even polymer free in other DES), and drug (biolimus A9, zotralimus designed for coronary stents). Second generation DES proved to be superior to first generation DES especially paclitaxel eluting stent regarding the need for target lesion revascularization and stent thrombosis [7].
INCIDENCE
DES restenosis rates are related to degree of lesion complexity in addition to clinical risk factors. In simple lesions, restenosis rate is expected to be less than 5% at 1 year [8]. However, in more complex lesions, restenosis rate is expected to be 10% at 2 years [9]. The exact mechanism of drug eluting stent restenosis is to some extent controversial. Biological, mechanical, and technical factors may be involved.
Biological factors
Limus based drugs like sirolimus have cytostatic effect. They suppress smooth muscle cell migration and proliferation by arresting the cell cycle in the G1 phase [10]. Paclitaxel has a cytotoxic effect, binding specifically to the beta-tubulin subunit of microtubules; interfering with microtubule dynamics, preventing their depolymerization [10]. Genetic mutations affect the sensitivity to these drugs, leading to resistance to sirolimus, its analogs, or paclitaxel [11].
Hypersensitivity:
The predominant stent platform for BMS and first-generation DES is 316L stainless steel. Allergic reactions to nickel and molybdenum released from 316L stainless steel stents may be the mechanism for in stent restenosis [12]. The stent platform used in new generation DES like everolimus eluting stents (EES) is cobalt chromium, which has lower nickel content than 316L stainless steel, and does not appear to trigger proliferative response and hypersensitivity reaction as compared with BMS and older generations of DES. However hypersensitivity
reactions may occur in response to other components of DES like drug or and polymer. Use of polymer free DES seems to reduce hypersensitivity and inflammatory reactions incriminated in instent restenosis and thrombosis.
Stent under expansion: Stent under expansion results from suboptimal expansion during implantation rather than from stent recoil [13]. Stent under expansion usually occurs in setting of tight markedly calcified lesions, so adequate lesion preparation before stenting either by simple balloon pre dilatation or use of rotational atherectomy device in setting of marked calcifications seems to be crucial. Post stent dilatation with non compliant balloon can also correct any residual under expansion or malapposition.
The use of IVUS can be useful to detect under expansion despite good apposition of the stent struts to the vessel wall with smaller stent cross-sectional area compared with vessel crosssectional area in the same site and also with reference vessel area. Excellent expansion is evident when the minimum lumen area in the stent is ≥ 90% of the average reference lumen area [14].
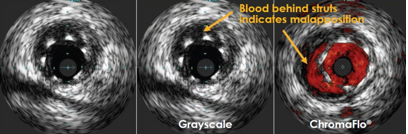
A condition that needs to be differentiated from under expansion is stent malapposition; unlike under expansion, there are stent struts not opposed to the vessel wall (i.e. space occupied by blood can be detected between the stent struts and the arterial intima) (Figure 1).
Figure 1 Showing mal apposition with blood accumulation behind stent struts
Malapposition is considered an important predisposing factor for stent thrombosis and restenosis. It can be diagnosed by IVUS or OCT. In IVUS malapposition is defined as a separation of at least one stent strut from the intimal surface of the arterial wall that is not overlapping a side branch and has evidence of blood flow behind the strut [15]. In contrast to IVUS, Optical coherence tomography (OCT) has a resolution of 10-20 µm, which is about 10 times higher than that of IVUS (80-120 µm). Malapposition is defined by OCT as a distance between the strut marker and lumen contour greater than the strut thickness plus the axial resolution of OCT [16].
Stent fracture: A stent fracture is defined as complete or partial separation of a stent at follow-up that was contiguous after the original stent implantation [17]. The reported incidence of stent fracture ranges between 0.8 and 19% [18]. Stent fracture is usually associated with binary restenosis, thrombosis, aneurysm, embolization, ischemic events, and target lesion revascularization (TLR) and could thereby increase morbidity and mortality [19]. Several studies have reported a rise in in-stent restenosis (ISR) with stent fracture [20]. It has been suggested that stent fracture leads to impaired and unequal local drug delivery at the fractured site [21].
Stent fracture can be diagnosed by IVUS where partial stent fracture is characterized by the absence of at least one-third or 120° of stent struts for at least 1 frame while complete stent fracture is characterized by complete absence of stent struts within the stented segment for at least 1 frame [17]. Multi slice CT is another diagnostic modality for detection of stent fracture [22]. Excessive tortuosity, angulation and torsion of the vessel, overlapping stents, longer stents, and SES (owing to its rigid closed-cell structure) are associated with increased risk of stent fracture [23-25].
Technical factors
As known that balloon is slightly 1-2 mm longer than stent itself. This leads to presence of area of vessel wall exposed to injury effect of balloon inflation but not covered by the stent i.e. not exposed to drug antiestenotic effect. Subgroup analyses from an early SES randomized clinical trial showed instent estenosis may occur predominantly at the proximal stent margin after SES implantation [26].
This was decreased in subsequent studies that with recommended technique of pre-dilation with shorter balloons use of a single long stent enough to cover whole diseased segment.
Stent Gap is another predisposing factor for instent restenosis similar to stent fracture where drug delivery in the vessel wall is minimal at the gap site. So overlapping DES with avoidance of short stent gaps is extremely important where there is defective drug delivery, this should be followed by post stent dilatation at overlapping segment preferably by using non compliant balloon [27].
CONCLUSION
DES proved to be superior to BMS in reducing the risk of instent restenosis and need for target lesion revascularization.
DES restenosis is related to degree of lesion complexity in addition to clinical risk factors where in simple lesions restenosis rate is e less than 5% at 1 year. However, in more complex lesions, restenosis rate increased to be 10% at 2 years. The exact mechanism of drug eluting stent restenosis is to some extent controversial. Biological, mechanical, and technical factors may be involved. Understanding the mechanism of restenosis in every case is important as it will be guide for optimal modality of treatment.