Multiparametric Abdominal Ultrasound for Portal Hypertension Assessment in Chronic Liver Disease
- 1. Department of Internal Medicine, “Nicolae Testemi?anu” State University of Medicine and Pharmacy, Republic of Moldova
- 2. ”Victor Babe?” University of Medicine and Pharmacy, Romania
- 3. Regional Center of Advanced Hepatological Reseach Timi?oara, Romanian Academy of Medical Science, Romani
Abstract
The trend in mortality induced by liver disease over the past five years has shown an increasing burden, largely driven by conditions such as non-alcoholic fatty liver disease (MASLD), alcoholic liver disease (ALD), cirrhosis, and hepatocellular carcinoma. Accurate diagnosis of liver disease is crucial for effective management and timely intervention. Portal hemodynamics play a central role in the pathophysiology of cirrhosis due to their close association with disease severity. Ultrasound (US) is a commonly utilized imaging technique in the management of chronic liver disease (CLD), due to its simplicity and minimally invasive nature. Doppler ultrasound enables real-time visualization of blood flow under physiological conditions, while contrast-enhanced US with microbubble contrast agents provides detailed evaluation of peripheral blood flow. Additionally, elastography, originally designed for fibrosis assessment, now has a wide range of applications for the liver and spleen. These advancements are driven by the evolution of digital technologies and the widespread dissemination of information. Elastography is a widely used non-invasive technique for staging CLD and has become increasingly popular. Liver and spleen stiffness are important parameters in the assessment of portal hypertension (PHT) and stratify patients with compensated advanced chronic liver disease (cACLD), clinically significant PHT and monitor disease progression or response to therapy. Endoscopic ultrasound (EUS) provides a detailed view of the entire portal vascular system, enabling close observation of the static response, as indicated by the diameter of the component vessels. Given our inability to cure most primary liver diseases (except for hepatitis C), prevention becomes the key intervention. Preventive strategies can help extend the period before the progression of liver disease by preventing or slowing further damage and addressing comorbidities. Multiparametric ultrasound (MPUS) served as an effective tool for screening compensated advanced chronic liver disease (cACLD) and clinically significant portal hypertension (CSPH). Non-invasive tools play a crucial role in risk stratification and assist healthcare providers in identifying patients who are at the highest risk of liver-related complications.
KEYWORD
- Multiparametric ultrasound; Portal hypertension; Advanced liver disease; Doppler ultrasound; Elastography; EUS; Preventive hepatology
CITATION
Peltec A, Tcaciuc E, Sporea I (2024) Multiparametric Abdominal Ultrasound for Portal Hypertension Assessment in Chronic Liver Disease. JSM Hepat 5(1): 1013.
INTRODUCTION
Liver disease was the 11th leading cause of death worldwide in 2016, but it moved up to the 9 th leading cause by 2022 [1]. Liver disease causes more than two million deaths each year, including deaths from cirrhosis, viral hepatitis, and liver cancer [2]. Out of the 2 billion people who drink alcohol globally, over a third are diagnosed with alcohol use disorders (AUD) and face a higher risk of developing alcohol liver disease (ALD). Approximately 2 billion adults are overweight, and over 400 million have diabetes, both of which are risk factors for MASLD (Metabolic dysfunction Associated Steatotic Liver Disease) and liver cancer. The global prevalence of viral hepatitis remains significant, with an estimated 58 million chronic hepatitis C patients in need of treatment and approximately one-fifth of the 256 million hepatitis B patients eligible for treatment [3,4]. The projected prevalence of liver disease is anticipated to rise by 34% from 2018 to 2033, with the number of patients suffering from MASLD expected to grow by 33% during the same period [5].
Accurate diagnosis of liver disease is crucial for effective management andtimely intervention. Multiparametric ultrasound (MPUS) addresses this challenge by integrating multiple imaging parameters, providing a detailed and comprehensive assessment of liver health. The introduction of MPUS represents a significant shift in liver disease diagnosis. By combining various ultrasound techniques such as B-mode, liver stiffness measurement, fat quantification, dispersion imaging, Doppler ultrasound, and contrast-enhanced ultrasound (CEUS), clinicians gain a holistic diagnostic view in a single examination [6]. This tool can serve as both a screening method and a diagnostic procedure for patients with CLD [7].
Portal hemodynamics play a central role in the pathophysiology of cirrhosis, due to their close association with disease severity. In cirrhosis, portal hypertension (PHT) develops due to increased intrahepatic vascular resistance, resulting from both structural and functional abnormalities. The structural factors include the presence of fibrous tissue within the sinusoids and the formation of regenerative nodules. Functional factors involve dysfunction of the sinusoidal endothelial cells, which leads to vasoconstriction [8]. The formation of collateral vessels reflects a disruption in portal circulation, leading to complications such as gastroesophageal varices, ectopic varices, and hepatic encephalopathy, which are key clinical manifestations of cirrhosis [9]. Portal pressure determines the severity of PHT, with the hepatic venous pressure gradient (HVPG) serving as a surrogate marker for direct measurement. A HVPG greater than 10 mm Hg is regarded as the threshold for CSPH [10]. An HVPG greater than 12 mm Hg is linked to an increased risk of variceal bleeding, while values exceeding 16 mm Hg are associated with higher mortality. An HVPG above 20 mm Hg is a predictor of failure to control variceal bleeding [11]. Consequently, non- invasive markers that can be used repeatedly over the long-term clinical course are preferred. Herein, we aim to provide a brief overview of the advantages of using multiparametric ultrasound for the diagnosis of portal hypertension, along with supporting evidence.
COMPONENTS OF MULTIPARAMETRIC ULTRASOUND
Ultrasound (US) is a commonly utilized imaging technique in the management of CLD due to its simplicity and minimally invasive nature. Doppler ultrasound enables real-time visualization of blood flow under physiological conditions, while Contrast Enhanced Ultrasound (CEUS) with microbubble contrast agents provides detailed evaluation of peripheral blood flow. Additionally, elastography, originally designed for fibrosis assessment, now has a wide range of applications for the liver and spleen. These advancements are driven by the evolution of digital technologies and the widespread dissemination of information.
B-Mode Sonography
B-mode sonography, despite its simplicity, remains essential for obtaining fundamental tissue images. In the context of PHT, it plays a key role in characterizing cirrhosis, measuring vessel diameters and spleen size, and detecting ascites and abnormal collateral pathways. PHT can be evaluated using B-mode ultrasound, which detects several key indicators. Common findings in PHT include an enlarged portal vein, typically measuring over 13 mm in diameter, a splenic vein with a diameter exceeding 10–12 mm, and a superior mesenteric vein generally measuring more than 10–12 mm. Splenomegaly is often a crucial indicator of PHT, while collateral vessels such as gastroesophageal varices and the presence of ascites also reflect abnormal blood flow associated with this condition. Together, these findings contribute to the diagnosis of PHT and aid in monitoring its progression.
The most common types of portosystemic shunts that may appear on ultrasound include gastroesophageal varices, which are dilated veins in the esophagus and stomach, seen as large, serpentine vessels near these regions. Paraumbilical collaterals may also form in severe PHT, with the umbilical vein reopening and creating abnormal vessels near the umbilicus. The umbilical vein with diameter greater than 5 mm is considered indicative of a patent umbilical vein, suggesting the development of portosystemic collateral circulation. A spleno-renal shunt, which connects the splenic vein to the left renal vein, can be identified by abnormal flow between these two vessels.
Screening tests should focus on identifying both direct signs of PHT (e.g., varices and portosystemic collaterals) and indirect markers (e.g., splenomegaly, thrombocytopenia, and ascites). The ability of B-mode ultrasound to accurately predict portal pressure or determine the severity of PHT is limited, as it mainly assesses indirect signs of the condition.
Doppler Ultrasound
Doppler Ultrasound is gaining recognition as a non-invasive approach to assess blood flow changes in chronic liver disease, including PHT. Doppler ultrasound is essential for assessing hemodynamic changes in cirrhotic livers, providing valuable insights into both the morphological and functional aspects of the portal vein, hepatic veins, and overall hepatic vasculature. In liver cirrhosis, hepatic hemodynamics are characterized by enlarged portal and splenic vein diameters and reduced portal vein velocity in affected patients. Specific Doppler indices, such as the shift of hepatic vein waveform from triphasic to monophasic or biphasic patterns in cirrhotic patients, have been suggested as indicators for PHT. Higher hepatic artery resistive index levels are seen in patients with advanced liver fibrosis, as these values are affected by hepatic inflammation and the accumulation of fibrous tissue within the liver parenchyma [12].
These portosystemic shunts are important markers of advanced PHT, and their presence, size, and blood flow characteristics are crucial for assessing and managing complications associated with the condition. Additionally, portocaval shunts, forming a direct connection between the portal vein and the inferior vena cava, and mesocaval shunts, forming between the superior mesenteric vein and the inferior vena cava, can also be detected using Doppler ultrasound. The left gastric vein, also known as the coronary vein, plays a critical role in draining blood from the stomach and esophagus into the portal venous system. In PHT, this vein can become dilated due to increased pressure in the portal system, contributing to the formation of gastroesophageal varices. On B-mode ultrasound, the left gastric vein may appear enlarged, and Doppler ultrasound can reveal altered flow patterns or retrograde flow, which are indicative of PHT. The azygos vein is part of the venous system that drains the thoracic wall and abdomen, including the esophagus and the mediastinum, into the superior vena cava. In PHT, the azygos vein can become dilated due to the formation of portosystemic collaterals, which redirect blood away from the liver. In summary, the left gastric vein and azygos vein are crucial in the evaluation of PHT. Their size, flow patterns, and potential for retrograde flow can be assessed using B-mode and Doppler ultrasound, helping to monitor the severity of PHT and detect complications like varices and shunting.
CEUS
CEUS offers significant benefits in the practical management of patients with PHT, including predicting HVPG, managing complications, and diagnosing both cirrhosis and non-cirrhotic PHT. It provides a simpler and less invasive alternative to liver biopsy or hepatic venous catheterization. Evidence suggests that hepatic vein acceleration time is the key parameter to assess significant liver disease in routine clinical practice, due to its excellent reproducibility and diagnostic performance [13]. The area under the receiver operating characteristic curve (AUROC) for intrahepatic transit time, defined as the difference between hepatic vein arrival time and hepatic artery arrival time (HV–HA), was 0.883 (the cutoff values were 8.2 seconds) for the presence of esophageal varices (EVs) and 0.915 (the cutoff values were 7 seconds) for high-risk EVs [14]. In addition to its use in assessing PHT and varices, intrahepatic transit time could potentially serve as a non-invasive biomarker for evaluating the severity of liver disease and predicting complications related to cirrhosis. The ability to detect high-risk EVs early could improve management strategies, allowing for timely interventions that reduce the risk of bleeding and other related complications.
Investigators have highlighted the advantages of CEUS in managing patients with portal vein thrombosis, particularly in differentiating between bland and tumor thrombus. CEUS has also been used to predict anticoagulation outcomes [15]. Additionally, CEUS is valuable for detecting portal hypertensive gastropathy through quantitative analysis of the contrast effect in the stomach wall, especially when using Sonazoid [16]. Furthermore, CEUS can be employed to estimate prognosis and the likelihood of hepatocellular carcinoma in patients with cirrhosis by analyzing the time between hepatic arterial enhancement and the maximum enhancement of the liver parenchyma, known as the “hepatic filling rate.”
Although conventional color Doppler has been used to assess flow in stents, recent studies have demonstrated the effectiveness of CEUS in evaluating shunt dysfunction. CEUS offers the advantage of avoiding additional radiation exposure, making it a safer alternative for monitoring shunt performance. The practical value of CEUS for assessing portal venous pressure needs to be further evaluated.
Elastography
Elastography is a widely used non-invasive technique for staging cACLD (compensated Advanced Chronic Liver Disease) and has become increasingly popular. However, its accuracy can be influenced by factors such as body mass index, inflammation or cholestasis. Three main types of elastography used in the field of hepatology include: Vibration Controlled Transient Elastography (VCTE), Shear Wave Elastography (SWE) and Magnetic Resonance Elastography (MTE).
VCTE has been validated for diagnosing liver disease progression in various populations. The FibroScan 630 Expert is an innovative device capable of measuring both liver stiffness at 50Hz and spleen stiffness (SS) at 100Hz. Approved by the United States Food and Drug Administration, it is designed for routine use in detection, surveillance, and prioritizing treatment for liver disease [17].
SWE is a technique that measures the propagation of shear waves in hepatic parenchyma and is regarded as one of the most important non-invasive methods for assessment liver stiffness. In ultrasound-based systems, shear waves are produced using an acoustic radiation force impulse (ARFI). ARFI techniques also offer the advantage of real-time imaging, enabling precise guidance of the probe to the targeted region of interest. There are two primary techniques based on high-intensity ultrasound waves, both of which combine imaging with elastography and are generally referred to as SWE: point SWE (pSWE) and two- dimensional SWE (2D-SWE). SWE is integrated into advanced ultrasound devices, allowing the examiner to select a region of interest, with a high-frame-rate B-mode image. ARFI techniques enable liver stiffness measurement (LSM) even in patients with advanced liver cirrhosis who have ascites (that is not possible with VCTE). Additionally, ARFI methods have shown outstanding diagnostic accuracy in predicting CSPH and detecting the presence of EVs [18,19].
Liver stiffness
In 2015, the Baveno VI consensus suggested replacing the term “cirrhosis,” which is based on histological criteria, with “advanced chronic liver disease” for patients in the later stages of CLD [20]. Recognizing the distinction between severe liver fibrosis and compensated cirrhosis in asymptomatic patients is often challenging in routine clinical practice, and the term cACLD more effectively reflects this continuum.
The Baveno VII consensus suggests applying the rule-of-five for LSM cut-offs by VCTE (5-10−15−20−25 kPa), in conjunction with platelet count, to quickly assess the risk of liver-related complications and mortality, independent of the underlying cause. This is a simple classification system for liver stiffness values: a value of < 5 kPa indicates a normal liver, less than 10 kPa excludes cACLD, values between 10 and 15 kPa are suggestive of cACLD, more than 15 kPa are highly consistent with cACLD, and values exceeding 25 kPa are indicative of CSPH. A positive diagnosis of CSPH in patients with an LSM ranging from 15 to 25 kPa requires the presence of additional signs of PHT, such as a low platelet count. The probability of CSPH is very low when LSM < 15 kPa and platelet count ≥ 150 x10 9/L [21].
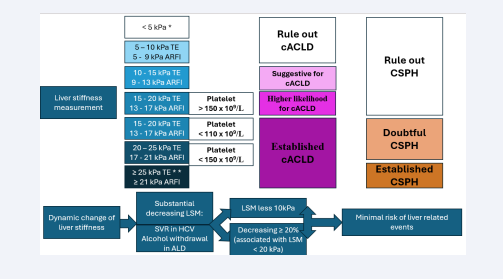
The “Rule of 4” (5-9-13-17) for interpreting liver stiffness by ARFI (for pSWE and 2D-SWE) classifies liver disease severity and PHT risk based on stiffness values. Values of less than 5 kPa suggest normal liver stiffness. Between 5 and 9 kPa, it rules out cACLD. Values between 9 and 13 kPa are suggestive for early cACLD. Values between 13 and 17 kPa indicate a higher likelihood of cACLD with increased risk of complications, but without CSPH. Values above 17 kPa are indicative of CSPH, suggesting a higher risk of complications such as variceal bleeding or ascites. This system helps in assessing both liver disease severity and PHT risk [22]. This rule is useful in daily practice for purposes like diagnosing cACLD or CSPH. If liver stiffness is below 20 kPa (for VCTE) and platelet count is ≥ 150 x 109/L, screening upper endoscopy may be unnecessary. Patients who can avoid endoscopy should be monitored with annual repeat VCTE and platelet count to track disease progression or changes in PHT. Baveno VII also highlighted the prognostic significance of LSM dynamics over time for liver-related events and deaths in patients with cACLD. The conference defined a significant change as any decrease in LSM of ≥20%, especially when associated with an LSM of <20 kPa, or any decrease resulting in an LSM <10 kPa. Additionally, a recent study has demonstrated that a 20% increase (or decrease) in LSM at any time is associated with a greater than 50% increased (or decreased) risk of hepatic decompensation or death [23] [Figure 1].
Figure 1: Liver stiffness measurement and dynamic change of liver stiffness. Use of LSM according to rule 5 (for TE) and rule 4 (for ARFI methods) to determine cACLD and CSPH. Patients having a LSM < 10 kPa rules out cACLD in the absence of other clinical/imaging signs. LSM values between 10 kPa and 15 kPa for TE (9kPa and 13 kPa for ARFI) are suggestive of cACLD. LSM measured by VCTE > 15 kPa (> 13 kPa by ARFI) are considered as a high likelihood of cACLD in all etiologies. LSM ≤ 15 kPa for VCTE (≤ 13 kPa for ARFI) plus platelets ≥ 150 × 109/L rule out CSPH in most of etiologies. Patients with intermediate values of LSM between 15 kPa and 25 kPa for VCTE (13 kPa and 21 kPa for ARFI) are in a “gray zone” of CSPH. The best cutoff to determine the presence of CSPH was an LSM ≥ 25 kPa (specificity and positive predictive value > 90%) in alcoholic liver disease, chronic hepatitis B, chronic hepatitis C, and non-obese patients with NAFLD. A dynamic change in liver stiffness is considered significant if there is a reduction of ≥20% in LSM, particularly when accompanied by an LSM of <20 kPa, or any decrease that brings the LSM below 10 kPa.
Abbreviation: TE: Transient Elastography; ARFI: Acoustic Radiation Force Impulse; cACLD: Compensated Advanced Chronic Liver Disease; CSPH: Clinically Significant Portal Hypertension; LSM: Liver Stiffness Measurement; SVR: Sustained Virological Response; HCV: Hepatic C Virus; ALD: Alcoholic Liver Disease.
Spleen Stiffness
Increasing efforts have been made to evaluate the accuracy of spleen stiffness measurement (SSM) and establish optimal SSM values for diagnosing PHT (PHT).
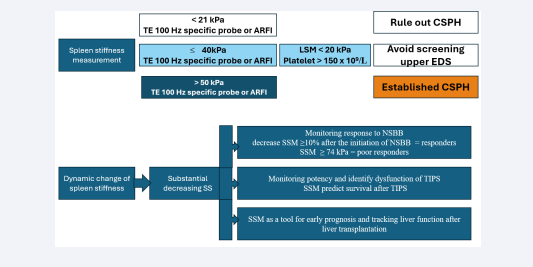
Recently, various elastography techniques have demonstrated the utility of SSM in assessing PHT [24,25]. Notable technical advancements have occurred in elastography, which is increasingly used to detect changes in spleen elasticity as a non- invasive method. The superficial location of the spleen allows for reliable results [26]. SSM has been proposed as an effective tool for monitoring and predicting PHT and the presence of EVs in cirrhotic patients. According to the Baveno VII guidelines, a VCTE-SSM value of <21 kPa can be used to rule out CSPH, while a value of >50 kPa can be used to rule in CSPH in patients with viral hepatitis-related cACLD [27]. Incorporating SSM into the algorithm enhanced accuracy and more effectively identified patients with PHT [28]. It is important to note that SS may increase earlier in patients with hepatitis B or C virus infections than liver stiffness, even when liver fibrosis is not present [29]. These findings support the use of SS as a more dynamic parameter for predicting PHT, demonstrating high diagnostic performance. SSM demonstrated better performance than LSM in detecting EVs, with a sensitivity of 90% compared to 85%, specificity of 73% versus 64%, and an AUROC of 0.90 versus 0.82 [30]. Furthermore, SSM may play a crucial role in monitoring treatment response and stratifying risk following therapy for PHT. A change in SS measurement of ≥10% after the initiation of non-selective beta-blockers demonstrated excellent accuracy in identifying HVPG responders, with an AUROC of 0.973 [31] [Figure 2].
Figure 2: Spleen stiffness measurement and dynamic change of spleen stiffness: TE-SSM value of 50 kPa can rule in CSPH in patients with viral hepatitis-related cACLD. An SSM of ≤40 kPa, an LSM of <20 kPa, and a platelet count of ≥150 × 10?/L allow for the omission of screening upper EDS. A change in SS measurement of ≥10% after the initiation of NSBB demonstrated excellent accuracy in identifying NSBB responders. An SSM of ≥74 kPa, measured by TE, demonstrated outstanding performance in predicting a poor response to NSBB. SSM is essential for non-invasively monitoring TIPS patency and identifying dysfunction. SSM is a useful tool for early prognosis and tracking liver function after liver transplantation.
Abbreviation: TE: Transient Elastography; ARFI, Acoustic Radiation Force Impulse; CSPH, Clinically Significant Portal Hypertension; EDS, Endoscopy; LSM, Liver Stiffness Measurement; SS, Spleen Stiffness; NSBB, Nonselective Betablockers; SSM, Spleen Stiffness Measurement; TIPS, Transjugular Intrahepatic Portosystemic Shunt; cACLD, Compensated Advanced Chronic Liver Disease; PHT, Portal Hypertension.
An SSM of ≥74 kPa, measured by VCTE, demonstrated outstanding performance in predicting a poor acute response to beta-blockers (100% sensitivity, 60% specificity, and 100% negative predictive value) as well as a poor chronic response (87% sensitivity, 71% specificity, and 71% negative predictive value) [32]. The changes in portal pressure gradient before and after transjugular intrahepatic portosystemic shunt (TIPS) were positively associated with SSM. An SSM value of 3.60 m/s has been proposed as a threshold to predict survival [33]. Importantly, elevated SSM values can act as an independent prognostic factor for survival following TIPS and are essential for non-invasively monitoring TIPS patency and identifying dysfunction [34].
Following liver transplantation, SSM significantly decreases as PHT improves, making SSM a useful tool for early prognosis and tracking liver function after the procedure [35] [Figure 2].
Endoscopic Ultrasound
Endoscopic ultrasound (EUS) provides a detailed view of the entire portal vascular system, enabling close observation of the static response, as indicated by the diameter of the component vessels. It is also effective in detecting collateral vessels, such as varices, and identifying intravascular complications, including portal vein thrombosis (PVT).
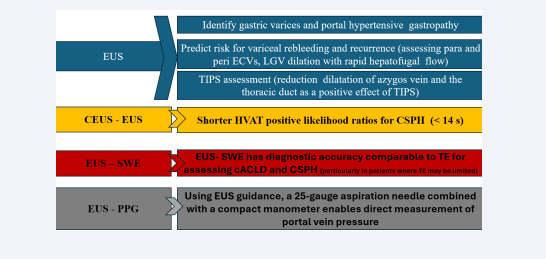
EUS demonstrates equivalence to conventional upper endoscopy in the detection of esophageal varices, while it is considered more effective in identifying gastric varices. The capacity to differentiate between periesophageal collateral veins (peri-ECVs) and paraesophageal collateral veins (para- ECVs) using EUS is crucial for managing PHT. A higher number or larger size of peri-ECVs indicates an elevated risk of variceal recurrence. Additionally, para-ECVs may link to esophageal varices via perforating veins, further increasing the risk of recurrence and bleeding [36]. A gastric cardia perforating vein diameter greater than 3 mm was associated with a higher likelihood of recurrence of esophageal varices in 3 months and presence of para-ECVs larger than 4 mm after band ligation was shown to predict variceal recurrence in 1 year with a sensitivity and specificity of 70.6% and 84.6%, respectively [37,38]. Rapid hepatofugal left gastro vein (LGV) flow and anterior branch dominant LGV pattern appear to be associated with increased odds of post-eradication esophageal varices recurrence in one year. Post-TIPS, EUS might monitor the reduction in collateral vessel dilation and flow, including the thoracic duct and azygos vein, which could help evaluate the effectiveness of the shunt in alleviating PHT. Rapid hepatofugal flow in the left gastric vein (LGV) and anterior branch dominant LGV pattern are linked to a higher likelihood of esophageal varices recurrence within one year following eradication [39]. After TIPS placement, EUS could play a role in monitoring reductions in collateral vessel dilation and blood flow, including the thoracic duct and azygos vein. This approach may help assess the shunt’s effectiveness in mitigating portal hypertension [40].
CEUS used in combination with EUS is a valuable tool in assessing PHT. Hepatic vein arrival time (HVAT), measured using microbubble CEUS, is inversely related to the histological grade of liver fibrosis due to the resulting hemodynamic changes. HVAT less than 14 second with positive likelihood ratios for CSPH was 6.95 [41]. EUS-SWE offers diagnostic accuracy comparable to TE for evaluating cACLD and CSPH, especially in cases where TE has limitations. [42]. EUS-guided portal pressure gradient measurement (EUS-PPG) is a recently developed advanced diagnostic method that allows for direct measurement of portal vein pressure. This is achieved using a 25-gauge aspiration needle in conjunction with a compact manometer connected via non-compressible tubing [43] [Figure 3].
Figure 3: Endoscopic ultrasound in assessment of portal hypertension: EUS is equivalent to conventional upper endoscopy for detecting esophageal varices but is superior for identifying gastric varices and portal hypertensive gastropathy. Evaluating para-ECVs and peri-ECVs and LGV can help predict the risk of variceal rebleeding and recurrence. EUS has potential utility in monitoring the effectiveness of TIPS. When combined with CEUS, it becomes a valuable tool for assessing PHT. EUS-SWE provides diagnostic accuracy comparable to TE for evaluating cACLD and CSPH. EUS-PPG enables direct measurement of portal vein pressure.
Abbreviation: EUS, Endoscopic Ultrasound; ECVs, Esophageal Collateral Veins; TIPS, Transjugular Intrahepatic Portosystemic Shunt; CEUS, Contrast-Enhanced Ultrasound; HVAT, Hepatic Venous Arrival Time; CSPH, Clinically Significant Portal Hypertension; SWE, Shear Wave Elastography; cACLD, Compensated Advanced Chronic Liver Disease; PPG, Portal Pressure Gradient.
PREVENTIVE HEPATOLOGY
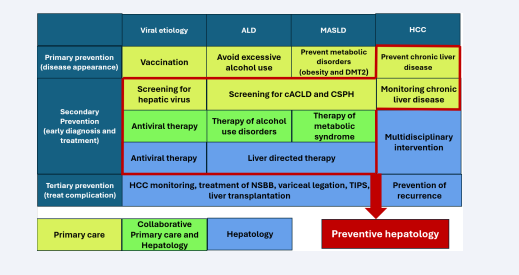
Since most primary liver diseases cannot be cured, apart from hepatitis C, prevention stands as the cornerstone of effective intervention. Preventive hepatology seeks to mitigate the impact of liver diseases by targeting risk factors and managing complications across various stages. It encompasses three tiers of prevention: primary, secondary, and tertiary [44]. Primary prevention aims to avert the development of liver diseases through strategies such as vaccinating against hepatitis A and B, encouraging healthy lifestyle choices to combat obesity and fatty liver, and minimizing alcohol intake. Secondary prevention focuses on the early detection and effective management of liver diseases to halt their progression. This involves routine screening for hepatitis B and C, conducting liver function tests, and using imaging techniques for individuals at risk. Key approaches include initiating antiviral treatments for chronic hepatitis, implementing lifestyle modifications for managing NAFLD, and supporting efforts to reduce alcohol consumption. Additionally, secondary prevention emphasizes consistent monitoring of high-risk individuals to identify complications like hepatocellular carcinoma at an early stage. It is crucial to stratify patients for cACLD and CSPH. Stratification helps in monitoring for varices, guiding decisions about NSBB or endoscopic therapy, and optimizing follow-up strategies. Early recognition of cACLD and CSPH is a key step in reducing the risk of decompensation and improving long-term outcomes in patients with liver disease. Tertiary prevention is centered on reducing complications and improving the quality of life for individuals with advanced liver disease. Once cirrhosis develops, no strategies have been identified to reverse the condition. Preventive strategies can help extend the period before the progression of liver disease by preventing or slowing further damage and addressing comorbidities [Figure 4].
Figure 4: Preventive hepatology encompasses three tiers of prevention: primary, secondary, and tertiary: Primary prevention aims to avert the development of liver diseases. Secondary prevention focuses on the early detection and effective management of liver diseases. Tertiary prevention is centered on reducing complications and improving the quality of life for individuals with advanced liver disease. All the cells outlined in red represent the actions of preventive hepatology that can be performed at the primary care settings, by hepatologist, or in collaboration. Screening for cACLD and CSPH can be conducted in primary care settings using MPUS.
Abbreviation: ALD, Alcoholic Liver Disease; MASLD, Metabolic Associated Steatotic Liver Disease; HCC, Hepatocarcinom; DMT2, Diabetes Mellites Type 2; cACLD, Compensated Advanced Chronic Liver Disease; CSPH, Clinically Significant Portal Hypertension; NSBB, Nonselective Betablockers; TIPS, Transjugular Intrahepatic Portosystemic Shunt.
MPUS is an emerging, non-invasive imaging modality with significant potential as a tool in preventive hepatology. In the context of primary prevention, MPUS can assess LS and fat content, enabling early detection of conditions like NAFLD and early fibrosis in at-risk populations. This facilitates timely lifestyle interventions to prevent disease progression. For secondary prevention, MPUS is particularly valuable in diagnosing and monitoring ACLD and assessing the presence of CSPH. Quantitative measurements of LS help stratify patients, guide clinical management, and optimize surveillance for complications such as varices or HCC. In tertiary prevention, it serves as a tool to monitor disease progression in patients with cirrhosis and assess responses to therapeutic interventions. These tools assist healthcare providers in identifying patients who are at the highest risk of liver-related complications
The assessment of portal hypertension using MPUS plays a crucial role in tertiary prevention by enabling the non-invasive evaluation and monitoring of this complication in advanced liver disease. MPUS combines techniques such as elastography to measure liver stiffness, Doppler imaging to assess portal vein hemodynamics, and spleen size evaluation, all of which provide insights into the presence and severity of portal hypertension.
This approach helps identify clinically significant portal hypertension (CSPH) without the need for invasive procedures like hepatic venous pressure gradient (HVPG) measurement. Early detection of CSPH through MPUS supports timely interventions, such as initiating non-selective beta-blockers, managing varices, or addressing other complications. Additionally, regular monitoring with MPUS allows for tracking disease progression and the effectiveness of therapeutic measures, improving overall patient outcomes in advanced liver disease.
CONCLUSION
The prevalence of liver disease is expected to rise significantly in the coming years, while the availability of hepatologists is decreasing (the modelling analysis predicts a shortage of adult hepatology providers of 23% in 2028, and 35% in 2033) [45]. This highlights the importance of preventive hepatology, particularly in primary care settings. MPUS offers a valuable tool for early detection and monitoring of liver disease, providing a non-invasive and accessible approach. By integrating MPUS into primary care, preventive strategies can be more effectively implemented, reducing the burden on specialists and improving overall patient care. Overall, preventive hepatology emphasizes early intervention, continuous care, and a holistic approach to reduce the impact of liver diseases and improve outcomes.
REFERENCES
- Chronic Liver disease and Cirrhosis. National Vital Statistic System via CDC WONDER.
- Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023; 79: 516-537.
- Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021; 6: 106-119.
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Vol. 53, World Health Organization; 2021; 1689: 99.
- Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modelling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018; 67: 123-133.
- Peltec A, Sporea I. Multiparametric ultrasound as a new concept of assessment of liver tissue damage. World J Gastroenterol. 2024; 30: 1663-1669.
- Sporea I, Mare R, Popescu A, Nistorescu S, Baldea V, Sirli R, et al. Screening for Liver Fibrosis and Steatosis in a Large Cohort of Patients with Type 2 Diabetes Using Vibration Controlled Transient Elastography and Controlled Attenuation Parameter in a Single- Center Real-Life Experience. J Clin Med. 2020; 9: 1032.
- Mendizabal M, Cançado GGL, Albillos A. Evolving portal hypertension through Baveno VII recommendations. Ann Hepatol. 2024; 29: 101180.
- Maruyama H, Yokosuka O. Ultrasonography for Noninvasive Assessment of Portal Hypertension. Gut Liver. 2017; 11: 464-473.
- Lebrec D, De Fleury P, Rueff B, Nahum H, Benhamou JP. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology 1980; 79:1139-1144.
- Lunova M, Frankova S, Gottfriedova H, Senkerikova R, Neroldova M, Kovac J, et al. Portal hypertension is the main driver of liver stiffness in advanced liver cirrhosis. Physiol Res. 2021; 70: 563-577.
- Ellaban HS, Afify SAM, Abdelgawad MS. Doppler ultrasound compared to shear wave elastography for assessment of liver cirrhosis. Egypt J Intern Med. 2024; 36: 61.
- Maruyama H, Tobari M, Nagamatsu H, Shiina S, Yamaguchi T. Contrast-enhanced ultrasonography for the management of portal hypertension in cirrhosis. Front Med (Lausanne). 2022; 9: 1057045.
- Li J, Feng JC, Peng XY, Wu XW, Du TT, Wang JJ, et al. Usefulness of contrast-enhanced ultrasonography for predicting esophageal varices in patients with Hepatitis B virus (HBV)-related cirrhosis. Med Sci Monit. 2017; 23: 2241-2249.
- Maruyama H, Takahashi M, Shimada T, Yokosuka O. Emergency anticoagulation treatment for cirrhosis patients with portal vein thrombosis and acute variceal bleeding. Scand J Gastroenterol. 2012; 47: 686-691.
- Kiyono S, Maruyama H, Kobayashi K, Kondo T, Sekimoto T, Shimada T, et al. Non-invasive diagnosis of portal hypertensive gastropathy: quantitative analysis of microbubble-induced stomach wall enhancement. Ultrasound Med Biol. 2016; 42: 1792-1799.
- Uzlová N, Mejzlíková N, Fra?ková S, Libicherová P, Nosek D, Rychlík I. Transient elastography - role in the assessment of the liver disease development. Vnitr Lek. 2018; 64: 916-922.
- Roccarina D, Rosselli M, Genesca J, Tsochatzis EA. Elastography methods for the non-invasive assessment of portal hypertension. Expert Rev Gastroenterol Hepatol. 2018; 12: 155-164.
- Thiele M, Hugger MB, Kim Y, Rautou PE, Elkrief L, Jansen C, et al. 2D shear wave liver elastography by Aixplorer to detect portal hypertension in cirrhosis: An individual patient data meta-analysis. Liver Int. 2020: 40; 1435-1446.
- de Franchis R. Expanding consensus in portal hypertension report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015; 63: 743-752.
- Vuille-Lessard É, Rodrigues SG, Berzigotti A. Noninvasive detection of clinically significant portal hypertension in compensated advanced chronic liver disease. Clin Liver Dis. 2021; 25: 253-289.
- Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020; 296: 263-274.
- Semmler G, Yang Z, Fritz L, Kock F, Hofer BS, Balcar L, et al. Dynamics in Liver Stiffness Measurements Predict Outcomes in Advanced Chronic Liver Disease. Gastroenterol. 2023; 165: 1041-1052.
- Liu Y, Tan HY, Zhang XG, Zhen YH, Gao F, Lu XF. Prediction of high-risk esophageal varices in patients with chronic liver disease with point and 2d shear wave elastography: a systematic review and meta- analysis. Eur Radiol. 2022; 32: 4616-4627.
- Fofiu R, Bende F, Popescu A, ?irli R, Lupu?oru R, Ghiuchici AM, et al. Spleen and Liver Stiffness for Predicting High-Risk Varices in Patients with Compensated Liver Cirrhosis. Ultrasound Med Biol. 2021; 47: 76-83.
- Yuldashev RZ, Aliev MM, Shokhaydarov SI, Tursunova DB. Spleen stiffness measurement as a non-invasive test to evaluate and monitor portal hypertension in children with extrahepatic portal vein obstruction. Pediatr Surg Int. 2020; 36: 637-641.
- de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022; 76: 959-974.
- Dajti E, Ravaioli F, Zykus R, Rautou PE, Elkrief L, Grgurevic I, et al. Spleen Stiffness-IPD-MA Study Group. Accuracy of spleen stiffness measurement for the diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease: a systematic review and individual patient data meta- analysis. Lancet Gastroenterol Hepatol. 2023; 8: 816-828.
- Pawlu? A, Inglot M, Chabowski M, Szyma?ska K, Inglot M, Patyk M, et al. Shear wave elastography (SWE) of the spleen in patients with hepatitis B and C but without significant liver fibrosis. Br J Radiol. 2016; 89: 20160423.
- Manatsathit W, Samant H, Kapur S, Ingviya T, Esmadi M, Wijarnpreecha K, et al. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J Gastroenterol Hepatol. 2018; 33: 1696-1706.
- Marasco G, Dajti E, Ravaioli F, Alemanni LV, Capuano F, Gjini K, et al. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hepatol Int. 2020; 14: 850-857.
- Llop E, Perelló C, Fontanilla T, de la Revilla J, Conde MH, López M, et al. Spleen Transient Elastography and Damping Index Identify a Subgroup of Patients Without an Acute or Chronic Response to Beta- Blockers. Front Med (Lausanne). 2022; 9: 900073.
- Zhu H, Guo H, Yin X, Yang J, Yin Q, Xiao J, et al. Spleen Stiffness Predicts Survival after Transjugular Intrahepatic Portosystemic Shunt in Cirrhotic Patients. Biomed Res Int. 2020; 2020: 3860390.
- Han H, Yang J, Jin WK, Li X, Zhang F, Zhuge YZ, et al. Diagnostic value of conventional ultrasound and shear wave elastography in detecting transjugular intrahepatic portosystemic shunt dysfunction. Acta Radiol. 2021; 62: 1575-1582.
- Bayramov N, Yilmaz S, Salahova S, Bashkiran A, Zeynalov N, Isazade E, et al. Liver Graft and Spleen Elastography After Living Liver Transplantation: Our First Results. Transplant Proc. 2019; 51: 2446-2450.
- Nagashima K, Irisawa A, Tominaga K, Kashima K, Kunogi Y, Minaguchi T, et al. The Role of Endoscopic Ultrasound for Esophageal Varices. Diagnostics (Basel). 2020; 10: 1007.
- Konishi Y, Nakamura T, Kida H, Seno H Okazaki K, Chiba T, “Catheter US Probe EUS Evaluation of Gastric Cardia and Perigastric Vascular Structures to Predict Esophageal Variceal Recurrence”. Gastrointest Endosc. 2002; 55: 197-203.
- Lesmana CRA, Paramitha MS, Gani RA. The Role of Interventional Endoscopic Ultrasound in Liver Diseases: What Have We Learnt? Can J Gastroenterol Hepatol. 2021; 2021: 9948979.
- Kuramochi A, Imazu H, Kakutani H, Uchiyama Y, Hino S, Urashima M. Color Doppler endoscopic ultrasonography in identifying groups at a high-risk of recurrence of esophageal varices after endoscopic treatment. J Gastroenterol. 2007; 42: 219-224.
- Termite F, Borrelli de Andreis F, Liguori A, Gasbarrini A, Attili F, Spada C, et al. The Role of Endoscopic Ultrasound in Assessing Portal Hypertension: A State-of-the-Art Literature Review and Evolving Perspectives. Liver Int. 2024.
- Kim MY, Suk KT, Baik SK, Kim HA, Kim YJ, Cha SH, et al. Hepatic vein arrival time as assessed by contrast-enhanced ultrasonography is useful for the assessment of portal hypertension in compensated cirrhosis. Hepatology. 2012; 56: 1053-1062.
- Kohli DR, Mettman D, Andraws N, Haer E, Porter J, Ulusurac O, et al. Comparative accuracy of endosonographic shear wave elastography and transcutaneous liver stiffness measurement: a pilot study. Gastrointest Endosc. 2023; 97: 35-41.
- Dhindsa BS, Tun KM, Fiedler A, Deliwala S, Saghir SM, Scholten K, et al. Endoscopic ultrasound-guided portal pressure gradient measurement: a systematic review and meta-analysis. Ann Gastroenterol. 2024; 37: 356-361.
- Bhadoria AS, Mohapatra A, Gupta R, Chawla YK, Kant R, Nundy S. Preventive hepatology: An ounce of prevention or pounds of cure to curb liver diseases. J Family Med Prim Care. 2023; 12: 419-421.
- Russo MW, Fix OK, Koteish AA, Duggan K, Ditmyer M, Fuchs M, et al. Modelling the Hepatology Workforce in the United States: A Predicted Critical Shortage. Hepatol. 2020; 72: 1444-1454.