Enhancement of Human T Lymphocyte Proliferation by Nanodiamond and Nanoplatinum in Liquid, DPV576
- 1. Departments of Otolaryngology, Charles Drew University of Medicine and Science USA
- 2. Departments of Internal Medicine, Charles Drew University of Medicine and Science USA
- 3. Department of Regenerative Medicine, Tokai University School of Medicine, Japan
Abstract
Despite increased nanoresearch, the influence of nanoparticles on the immune system is still poorly understood. Recently we demonstrated ex vivo modulation of T lymphocytes from aged mice post exposure to a mixture of nanodiamond (ND) and nanoplatinum (NP) coated material (DPV576-C). Here, we extend our study to examine the direct effect of a dispersed aqueous mixture of ND/NP (DPV576) in modulating human T lymphocytes in vitro. Venous blood drawn from 12 healthy volunteers was diluted 1:4 with AIM V medium, cultured with a polyclonal T cell mitogen, PHA, (10 µg/ml), and then exposed to DPV576 at various concentrations (1.25, 2.5 and 5%v/v) for 3 days. T cell proliferation was examined by thymidine uptake assay. CD4+ and CD8+ T cell proliferation and expression of activation markers (CD69 and CD25) was studied by CFSE dye dilution technique and dual color flow cytometry. Interleukin-2 (IL-2) production was determined by ELISA. DPV576 treatment resulted in a significant increase in T cell proliferation in a dose-dependent manner that was maximized at 5%v/v (p<0.001). DPV576 was effective in enhancing proliferation of both CD4+ and CD8+ T cells. This was associated with an increase in the percentage of cells expressing CD69 (an early activation marker) and CD25, as well as a significant increase in IL-2 production. We conclude that DPV576, a solution containing ND/NP, activates human T cell proliferation and enhances IL-2 production, indicating its possible usefulness in the treatment of immune compromised patients with T cell defects.
Keywords
DPV576; T cells; CD69; CD25
Citation
Ghoneum M, Pan D, Katano H (2014) Enhancement of Human T Lymphocyte Proliferation by Nanodiamond and Nanoplatinum in Liquid, DPV576. JSM Nanotechnol Nanomed 2(2): 1031.
INTRODUCTION
Nanoparticles possess novel physical and chemical proper ties and show great potential in biomedical applications, includ ing cancer therapeutic drug/gene delivery and imaging. The in fluence of nanoparticles on the immune system is increasingly becoming a focus of research; however, it is still poorly investi gated. Nanoparticles that promote host immune responses are of great use in the development of therapeutic strategies for the treatment of cancer and infections in an immunocompromised host. Several studies have reported that nanoparticles are taken up by certain cells of the immune system [1, 2], and that the cel lular uptake of nanoparticles could be affected by several fac tors, including particle size, surface charge, and dispersing am phiphiles [1, 2]. In addition, several studies have also reported immunomodulatory function by nanoparticles, including an increased production of IL-1β in human monocytes post expo sure to silver nanoparticles [3], activation of dendritic cells (DCs) via toll like receptors (TLR) after co-culture with nanoparticles poly(methyl vinyl ether-co-maleic anhydride) [4], and increased T cell costimulatory molecules upon activation of bone marrow derived DC by functionalized nanoparticles [5]. Among nanomaterials, carbon nanoparticles are of special interest because of the tremendous number of potential applications. Therefore, several studies have been carried out on the biocompatibility and biodistribution of nanodiamonds (NDs) in vivo in mice and rats. Vaijayanthimala et al [6] reported that fluorescent nanodiamonds (FNDs) are non-toxic (as indicated by histopathological analysis), and they added that FNDs have wide ranging biocompatibility and perfect chemical and photophysical stability in FND-injected rats over 5 months. In another study, Puzyr et al [7] reported no inflammatory response in detonation NDs-treated mice after 3 months. In addition, NDs were shown to be non-toxic at the cellular level [8]. DPV576, the product that was used in the current study, is a mixture of nanodiamond (ND) and nanoplatinum (NP). The mean particle diameter of ND is 120 nm with an oval shape, and that of NP is 20 nm with a round shape [9]. This range in shape and size may facilitate their uptake by a variety of cells. We have shown that aged mice exposed to ND- and NP-coated garments (DPV576-C) exhibit increased T lymphocyte response [10]. Our earlier research has also shown that DPV576 has the ability to activate human DCs and induce their maturation, as indicated by the increased expression of costimulatory molecules and cytokine production [9]. In the present study, we examined the effects of the ND/NP mixture dispersed in an aqueous solution, DPV576, on human T cell proliferation and cytokine IL-2 production.
MATERIALS AND METHODS
DPV576 liquid
A mixture of ND and NP solution known as DPV576 was used. Details regarding particle size, shape, and composition in the DPV576 solution have been previously reported [9]. DPV576 was supplied by Venex Co., Ltd. Kanagawa, Japan.
Antibodies
Fluorescein-conjugated monoclonal antibody against CD69, Phycoerythrin-conjugated anti CD25 (IL-2 receptor), and PerCp-conjugated anti CD3, anti CD4, and CD8 antibodies were purchased from B. D. Biosciences, San Jose, CA.
Cell culture medium and mitogens
Serum-free AIM V medium (Gibco, Grand Island, NY) was used in all experiments. Phytohemagglutinin L (PHA) was purchased from Sigma Chemical Co. St Louis, MO, and was dissolved in AIM V medium.
Subjects
Whole blood was obtained from 12 healthy volunteers (21 75 yrs old, median 84.5 yrs). The protocol was approved by the Institutional Review Board [IRB], Charles Drew University, Los Angeles, CA.
Cell proliferation assay
Whole blood was used to assess the proliferative response of lymphocytes to the polyclonal T cell mitogen. Proliferation was assessed by measuring [3H] thymidine uptake in mitogen stimulated lymphocytes as previously described [11]. Briefly, whole blood was diluted 1:4 with RPMI AIM V medium, following which 100 μl was cultured in triplicate in 96-well round bottom tissue culture plates (Costar, Corning, NY) at 37°C in humidified 5% CO2 in the presence or absence of polyclonal T cell mitogen PHA (10 µg/ml) with or without DPV576 liquid. The concentrations of DPV576 ranged between 1.25% and 5% v/v, and nanomaterial was added either at the beginning of the culture or at various times during the culture period. At 24 hrs prior to termination of culture, 1µCi of [3H]thymidine (specific activity, 6.9 Ci/mmol; Perkin Elmer Waltham, MA) was added to each well. The cultures were harvested onto a glass fiber filter by using an automated cell harvester (Skatron, McLean, VA), and [3H]thymidine incorporation was assessed by a liquid Scintillation counter. Variation among triplicates did not exceed ± 15%.
The proliferation of CD4+ and CD8+ T cells was also determined by flow cytometry using CFSE dye dilution method as previously described [12].
T-cells expression of CD69 and CD25
The expression of activation markers on T cells was performed using FACS Calibur (Becton Dickinson, San Jose, CA). Diluted whole blood was cultured in the presence or absence of DPV576 and was stimulated with PHA. At 24 hrs after incubation, 100 µl of cell suspension were incubated with 10 µl of FITC anti CD69, PE anti-CD25, and PerCp anti-CD3, and the erythrocytes were lysed with lysing solution (BD Biosciences). Cells were then washed and suspended in phosphate-buffered saline containing 0.1% azide. Flow cytometry was performed using FACS Calibur equipped with argon ion laser emitting at 488nm (for FITC, PE, and PerCp excitation) and a spatially separate diode laser emitting at 631 nm (for APC excitation). Ten thousand cells were acquired and analyzed using CellQuest software. Forward and side scatters were used to gate and exclude cellular debris, and FL3 channels were used to gate CD3+ T cells. Thereafter, an electronic gate was placed on CD3+ T cells during analysis, and the associated expression of CD69 and CD25 was determined.
IL-2 production
IL-2 production was examined by a specific ELISA kit (BD Pharmingen) as per the manufacturer’s protocol as previously described [12]. Peripheral blood mononuclear cells (PBMCs) were treated with PHA alone and with PHA + DPV576 at 2.5%v/v.
Statistics
All experiments were repeated with samples from 12 individual donors. The probability that the mean values of two experimental groups were identical was tested by the two-tailed t-test for paired samples. The level of significance was set at p<0.05.
RESULTS AND DISCUSSION
T cell proliferation and T cell subsets (CD4+, CD8+)
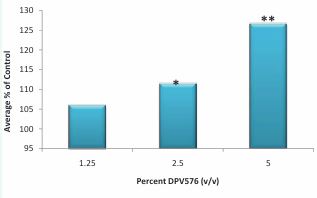
We examined the direct effect of ND/NP mixture dispersed in an aqueous solution (DPV576) on the induction of human T cell proliferation in an in vitro culture model. The data in Figure 1 show that DPV576 treatment resulted in an increase in T cell proliferation induced by PHA in a dose-dependent manner. The increase was detected at a low concentration of 1.25% v/v, became significant at a concentration of 2.5%v/v (p<0.05), and was maximized post-treatment with DPV576 at a concentration of 5%v/v (p<0.001) as compared to the control untreated cells. DPV576 alone has no effect on T cell proliferation (data not shown). Subsequently we determined whether or not DPV576 preferentially modulated the proliferation in T cell subsets: CD4+ T cells (helper T-cells) and CD8+ T cells (suppressor/ cytotoxic T-cells). For this purpose, CFSE-labeled PBMC were stimulated in vitro with PHA in the presence or absence of DPV576, and the proliferation of CD4+ and CD8+ T cells was visualized by FACS analysis. The results of two subjects are presented in Table 1, which shows that DPV576 enhanced proliferation in both CD4+ and CD8+ T cells. T lymphocytes develop in the thymus from a common lymphoid progenitor. They are typically classified into CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T helper cells that recognize peptides presented by MHC-I and MHC-II, respectively. Several studies have demonstrated that CTLs play an important role in immunity against cancer via direct cytolytic activity against tumor cells [13, 14]; they also play a major antiviral role [15]. In addition, accumulating evidence reveals that CD4+ T cells are important in the protection against cancer and viral infection [16-18].
Figure 1 Effect of DPV576 on T cells proliferation in vitro. Proliferation was assessed by measuring [3H] thymidine uptake in mitogen stimulated lymphocytes. Whole blood was diluted 1:4, following which 100 μl was cultured with polyclonal T cell mitogen PHA (10µg/ml) and DPV576 at concentrations of 1.25-5% v/v. 3H incorporation was assessed by a liquid scintillation counter. Variation among triplicates did not exceed ± 15%. *p<0.05, **p<0.001.
Table 1: Effect of DPV576 on the proliferation of CD4+ and CD8+ cells in culture.
|
Treatment |
% proliferation (subject #1) |
% proliferation (subject #2) |
||
|
CD4+ |
CD8+ |
CD4+ |
CD8+ |
|
|
None |
5 |
6.5 |
4.6 |
7 |
|
PHA |
87.7 |
73 |
65 |
82 |
|
PHA + 2.5% DPV576 |
91 |
80 |
72 |
83 |
|
PHA + 5% DPV576 |
92 |
84 |
75 |
86 |
CFSE-labeled mononuclear cells (1 x 106 /mL) were incubated with PHA (10µg/ml) in the presence or absence of DPV576. Percentages of proliferation of CD4+ and CD8+ T cells were determined with fluorochrome lab. On day 3 post-incubation cells were stained with PerCp labeled with mAb. CD4+ or CD8+ proliferating cells were identified by gating on CD4+ cells and CD8+ cells and analyzed for CFSE fluorescence intensity. The clonal expansion of CD4+ and CD8+ T cells is an important early event in mounting an effective immune response against cancer and infections. Carbon black nanoparticles have been shown to suppress the proliferative response of splenic T cells derived from transgenic DO11.10 mice [19]. In contrast, the results presented here demonstrate that DPV576 significantly enhances human T cell proliferation. In addition, other nanoparticles such as metal oxide TiO2 and Al2 O3 have been shown to increase T cell proliferation [20].
Effect of DPV576 on IL-2 Receptor and IL-2 Production
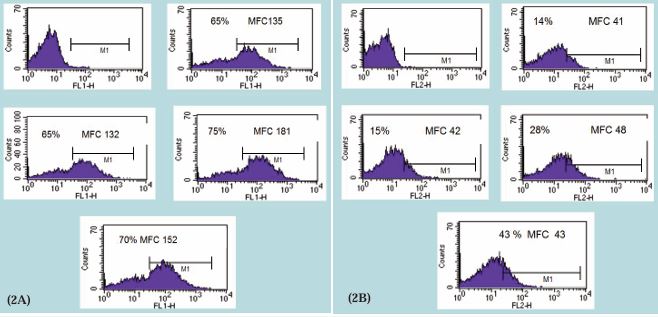
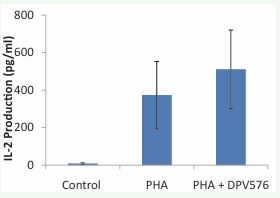
T cell expansion requires the expression of receptors for IL-2 (the growth factor for T cells) and the production of IL-2. Therefore, in the present study we examined IL-2 receptor expression and IL-2 production. For this purpose, whole blood was cultured with PHA in the presence or absence of DPV576 for 24 hrs, and the surface expression was determined by multicolor flow cytometry. Data in Figure 2A&B show that treatment with DPV576 resulted in an increase in the percentage of cells expressing CD69 and CD25 on the cell surface. The observed increase was dose dependent. Subsequently we determined the ability of DPV576 to influence the production of IL-2. The results illustrated in Figure 3 show that PBMCs alone without either PHA or DPV576 (2.5% v/v) induced a negligible amount of IL-2 production. However, treatment with PHA alone induced increases in IL-2 production that were further augmented in the presence of PHA + DPV576. T cell markers CD69 and CD25 are involved in lymphocyte proliferation, function as signal transmitting receptors in lymphocytes, and are rapidly up regulated in T cells upon activation. The activation marker CD (cluster of differentiation) appears on the surface of T cells during their activation. The expression of CD69+ appears early, and CD25+ appears late post receptor stimulation [21]. CD69+ is involved in activating signal transduction, leading to the synthesis of IL-2 and the receptor for IL-2 (IL-2R) [21]. Several studies reported decreased expression of the activation markers CD25 (a component of the receptor for IL-2) and CD69 in cancer patients [22, 23]. Therefore, the increased expression of these markers post exposure to DPV576 in T cells may suggest the use of this agent for the treatment of cancer.
Figure 2 Effect of DPV576 on the expression of early activation markers (A) CD69 and (B) CD25. Whole blood was cultured with PHA in the presence or absence of DPV576 for 24 hrs, and the surface expression was determined by multicolor flow cytometry, shown as the percentage of cells expressing activation marker and the density of CD69 and CD25 (mean fluorescent channel intensity [MFC]).
IL-2 plays an important role in the differentiation and the proliferation of T cells [24-26]. Several studies have shown that nanoparticles modulate IL-2 production. Cobalt nanoparticles were found to inhibit the release of IL-2 by human mononuclear cells [27]; similarly, the colloid of gold nanoparticles decreased the level of IL-2 by mouse splenocytes [28]. On the other hand, mice immunized with ovalbumin in the presence of [Gd@C(82)(OH)(22)](n) nanoparticles exhibited increased production of IL-2 [29], and the colloid of copper nanoparticles, caused an increase in IL-2 by mouse splenocytes [28]. Carbon nanoparticles have also been examined for their ability to induce IL-2 production. Fullerenes, molecules composed entirely of carbon, when administered to mice, resulted in increased proliferation of splenocytes and increased splenic production of IL-2 [30]. In the present study, results showed increased IL-2 production post treatment of human PBMCs with DPV576, suggesting that DPV576-induced T cell proliferation may be due in part to the increased production of IL-2 and its receptors.
Figure 3 IL-2 production post-treatment with PHA and PHA + DPV576 (2.5%v/v) in vitro.
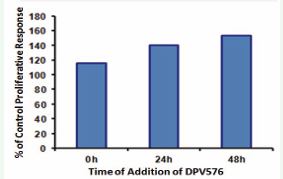
Figure 4 Temporal sensitivity to DPV576 induces cell proliferation. PHA was added at time zero, and DPV576 (2.5%) was added at the times shown on the abscissa. The data are normalized to the [3H] thymidine incorporation in PHA-activated lymphocytes cultured in the absence of DPV576.
Late Effects of DPV576 on the proliferative response
To assess the temporal sensitivity in the enhancement of proliferation, DPV576 (2.5%) was added to separate cultures at various times after PHA addition and was left in culture until [3H] thymidine incorporation was measured. Representative results in Figure 4 show that the stimulatory effect of DPV576 was observed when it was added at the beginning as well as after 48 hrs of culture initiation. These results indicate that DPV576 exerts its effects on early and late events involved in T cell clonal expansion. Our finding that DPV576 enhances T cell function when added after 48 hrs of cell activation suggests that this agent may be beneficial in boosting immune responses subsequent to the administration of a vaccine.
CONCLUSION
DPV576 treatment caused enhancement of human T lymphocyte proliferation in vitro via increasing the expression of IL-2 receptors and the production of IL-2. The defective expression of T cell growth factor and its receptors is well documented in cancer patients. Our results suggest that DPV576 may be of value in correcting these cellular defects. Moreover, this product may have the advantage of being safe and non-toxic.
ACKNOWLEDGEMENTS
This work was supported by Grant #C0030300 from Venex Company, Japan. Deyu Pan is supported in part by NIH-NIMHD grant U54MD007598 (formerly U54RR026138).
REFERENCES
- Baudoin JP, Jinschek JR, Boothroyd CB, Dunin-Borkowski RE, de JongeN. Chromatic aberration-corrected tilt series transmission electron microscopy of nanoparticles in a whole mount macrophage cell. Microsc Microanal. 2013; 19: 814-820.
- Yang M, Wada M, Zhang M, Kostarelos K, Yuge R, Iijima S, Masuda M . A high poly(ethylene glycol) density on graphene nanomaterials reduces the detachment of lipid-poly(ethylene glycol) and macrophage uptake. Acta Biomater. 2013; 9: 4744-4753.
- Yang EJ, Kim S, Kim JS, Choi IH. Inflammasome formation and “IL-1b release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012; 33: 6858-6867.
- Camacho A, Da Costa Martins R, Tamayo I, de Souza J, Lasarte JJ, Mansilla C, Esparza I . Poly (methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine. 2011; 29: 7130-7135.
- Carrillo-Conde B, Song EH, Chavez-Santoscoy A, Phanse Y, Ramer- Tait AE, Pohl NL, et al. Mannose-functionalized “pathogen-like” polyanhydride nanoparticles target C-type lectin receptors on dendritic cells. Mol Pharm. 2011; 8:1877-1886.
- Vaijayanthimala V, Cheng PY, Yeh SH, Liu KK, Hsiao CH, Chao JI, Et al. The long-term stability and biocompatibility of fluorescent nanodiamond as an in vivo contrast agent. Biomaterials. 2012; 33: 7794-7802.
- Puzyr AP, Baron AV, Purtov KV, Bortnikov EV, Skobelev NN, Mogilnaya OA, et al. Nanodiamonds with novel properties: a biological study. Diam Relat Mater. 2007; 16: 2124-2128.
- Schrand, AM, Hens, SA and Shenderova, OA. Nanodiamond particles: properties and perspectives for bioapplications. Crit Rev Sol State Mat Sci. 2009; 34: 18-74.
- Ghoneum M, Ghoneum A, Gimzewski J. Nanodiamond and nanoplatinum liquid, DPV576, activates human monocyte-derived dendritic cells in vitro. Anticancer Res. 2010; 30: 4075-4079.
- Ghoneum M, Ghoneum A, Tolentino L, Gimzewski J . Modulation of aged murine T lymphocytes in vivo by DPV576-C, a nanodiamond- and nanoplatinum-coated material. In Vivo. 2010; 24: 141-146.
- Ghoneum M. Anti-HIV activity in vitro of MGN-3, an activated arabinoxylane from rice bran. Biochem Biophys Res Commun. 1998; 243: 25-29.
- Ghoneum M, Agrawal S . Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int J Immunopathol Pharmacol. 2011; 24: 941-948.
- Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S . In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007; 204: 345-356.
- Gricks CS, Gribben JG . Cytotoxic T cell responses against immunoglobulin in malignant and normal B cells: implications for tumor immunity and autoimmunity. Curr Pharm Des. 2003; 9: 1889- 1903.
- Snyder JT, Alexander-Miller MA, Berzofskyl JA, Belyakov IM . Molecular mechanisms and biological significance of CTL avidity. Curr HIV Res. 2003; 1: 287-294.
- Wang J, Wang FW . Impact of age on clinical presentation, treatment, and cancer-specific survival of patients with small-cell carcinoma of the prostate. Clin Interv Aging. 2013; 8: 871-877.
- Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V . Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006; 12: 465-472.
- Sant AJ, McMichael A . Revealing the role of CD4(+) T cells in viral immunity. J Exp Med. 2012; 209: 1391-1395.
- Lefebvre DE, Pearce B, Fine JH, Chomyshyn E, Ross N, Halappanavar S, Tayabali AF . In vitro enhancement of mouse T helper 2 cell sensitization to ovalbumin allergen by carbon black nanoparticles. Toxicol Sci. 2014; 138: 322-332.
- Lozano-Fernández T, Ballester-Antxordoki L, Pérez-Temprano N, Rojas E, Sanz D, Iglesias-Gaspar M, et al. Potential impact of metal oxide nanoparticles on the immune system: The role of integrins, L-selectin and the chemokine receptor CXCR4. Nanomedicine. 2014; 10 :1301-1310.
- Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods. 2004; 293: 127-142.
- Harris J, Sengar D, Stewart T, Hyslop D . The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer. 1976; 37: 1058-1069.
- Whiteside TL. Signaling defects in T lymphocytes of patients withmalignancy. Cancer Immunol Immunother. 1999; 48: 346-352.
- Scrivener S, Kaminski ER, Demaine A, Prentice AG. Analysis of the expression of critical activation/interaction markers on peripheral blood T cells in B-cell chronic lymphocytic leukaemia: evidence of immune dysregulation. Br J Haematol. 2001; 112: 959-964.
- Murakami H, Ogawara H, Hiroshi H . Th1/Th2 cells in patients with multiple myeloma. Hematology. 2004; 9: 41-45.
- Johnston H, Pojana G, Zuin S, Jacobsen NR, Møller P, Loft S, Semmler- Behnke M . Engineered nanomaterial risk. Lessons learnt from completed nanotoxicology studies: potential solutions to current and future challenges. Crit Rev Toxicol. 2013; 43: 1-20.
- Bich-Thuy LT, Dukovich M, Peffer NJ, Fauci AS, Kehrl JH, Greene WC. Direct activation of human resting T cells by IL 2: the role of an IL 2 receptor distinct from the Tac protein. J Immunol. 1987; 139: 1550- 1556.
- Ma?aczewska J . The in vitro effect of commercially available noble metal nanocolloids on the splenocyte proliferative response and cytokine production in mice. Pol J Vet Sci. 2014; 17: 37-45.
- Yang D, Zhao Y, Guo H, Li Y, Tewary P, Xing G, Hou W . [Gd@C(82)(OH)(22)](n) nanoparticles induce dendritic cell maturation and activateTh1 immune responses. ACS Nano. 2010; 4: 1178-1186.
- Ding N, Kunugita N, Ichinose T, Song Y, Yokoyama M, Arashidani K, Yoshida Y . Intratracheal administration of fullerene nanoparticles activates splenic CD11b+ cells. See comment in PubMed Commons below J Hazard Mater. 2011; 194: 324-330.