Clinical Application of Three-Dimensional Printing and Tissue Engineering for Maxillofacial Reconstruction. A Review of Reported Cases
- 1. Department of Oral and Maxillofacial Surgery, Harvard School of Dental Medicine, USA
Abstract
The purpose of this review article was to summarize the published clinical cases, using three-dimensionally (3D) printing technology and/or tissueengineered (TE) approach to reconstruct segmental bone defects of the jaws. PubMed database was used to conduct the searches. Data collected included: demographics, location and defect size, diagnosis, material used to 3D-print the device and/or TE approach, 3D printing technology, outcome and followup. Fifteen publications met the criteria, containing 20 case reports. Treatment of segmental defects of the mandible (n=12) and of the maxilla (n=8) were reported. Diagnosis of the cases: tumors (n=15), alveolar clefts (n=4), and trauma (n=1). 3D-printed devices used: mesh (n=4), plate (n=1) and implant (n=4). One case described a 3D-printed Polycaprolactone (PCL) scaffold. In four cases a 3D-printed device (mesh n=1; plates n=3) together with a TE approach was used. Six patients were treated using a TE approach. Stereolithography (SLA; n=6), selective laser sintering (SLS; n=5) and selective laser melting (SLM; n=3) were 3D printing technologies used. The devices were mainly manufactured of titanium (Ti; n=16). The mean follow-up period was 16.25 months (3-60 months). In conclusion, it appears to date, only 1 clinical case of a 3D-printed scaffold for TE has been published.
Keywords
- Three-Dimensional Printing
- Titanium
- Tissue Engineering
- Jaws
- Reconstruction
Citation
Mueller ML, Thamm JR, Troulis MJ, Guastaldi FPS (2020) Clinical Application of Three-Dimensional Printing and Tissue Engineering for Maxillofacial Reconstruction. A Review of Reported Cases. JSM Oro Facial Surg 4(1): 1013.
INTRODUCTION
The standard for reconstruction of maxillofacial bone defects is the use of autogenous bone grafts harvested from the iliac crest, calvaria or vascularized free flaps. The drawbacks of these procedures are associated with donor site morbidity [1,2]. Reconstruction of large maxillofacial bone defects is clinically challenging due to the limited availability of transplantable autogenous bone grafts and the complex geometry of the bones.
As surgical techniques become less invasive, the burden to eliminate donor-site morbidity increases. Hence, the goal is to use autologous bone bioengineering with computer-aided design and computer-aided manufacturing (CAD/CAM) technology to produce 3D-printed scaffolds [1,3-6].
3D printing technology, also named additive manufacturing or rapid prototyping technology, was first described in 1986. This emerging technology based on a CAD/CAM process became of high interest for the reconstruction of maxillofacial bone defects and in the field of TE, due to its capacity of creating complex implants with a custom, patient-specific design and high precision [7].
Most commonly commercially pure titanium (cpTi) is chosen for 3D-printed maxillofacial devices, mostly due to its high properties for printing, and due to its excellent properties such as biocompatibility in vivo, corrosion resistance, and adequate mechanical strength [8,9]. Moreover, titanium-6aluminium4vanadium (Ti-6Al-4V) alloy has also been used as a material of choice to produce 3D-printed devices because of its superior mechanical strength compared to cpTi [10-12?]. Currently, it is mainly used for reconstruction of shape and form when it is combined with TE. With such approach, donor side morbidity will may be eliminated.
The overall goal is to create customized bone scaffolds, or coconstructs (bone, teeth, nerve, etc.) with precise form and shape to reconstruct missing structures. Defects can be analyzed by imaging techniques like computed tomography (CT) or magnetic resonance image (MRI). The data is processed with CAD software creating a digital prototype enabling 3D-printing of a patientspecific implant for bone reconstruction. First implementations of this technique have already been performed in preclinical and clinical studies, with positive approaches of personalized tissue-engineered devices and scaffold in the clinical setting of maxillofacial reconstruction [13-17].
During the last decade several preclinical studies have been published, using TE grafts with growth factors and cytokines, such as bone morphogenetic proteins (BMP) and stem cells, for the reconstruction of large bicortical defects [28-24].
The purpose of this review article was to summarize the published clinical cases, using 3D printing technology and/or TE approach to reconstruct segmental bone defects of the jaws.
MATERIALS AND METHODS
Search strategy
For literature search, the electronic database of PubMed was used. The search runs were performed using the terms: “threedimensional printing”, “titanium mesh”, “tissue engineering”, “scaffold”, “maxillofacial reconstructive surgery”, “segmental defect”, “mandible”, and “mandibular defect”. Publications were screened and cross-referenced for any cases not available on PubMed. Reports included were limited to studies published in English language from January 2004 to December 2019.
Study selection
Narrowing the findings, the focus was only on case reports, reporting the use of 3D printing technology and/or TE approach in order to reconstruct segmental bone defects of the jaws. Cases reporting minor defects (<1 tooth size), and cases describing bone autografts without 3D printing were excluded. Inclusion and exclusion was determined by two authors, if any discrepancies occurred, a third person made the determination.
RESULTS
Overall, 921 manuscripts were identified. Exclusion criteria narrowed the findings to 62 papers. After excluding duplicate case reports, 38 preselected articles were reviewed. Finally, 16 original publications, reporting 20 cases, were found eligible after applying the inclusion and exclusion criteria [13-17,25-35] (Figure 1).
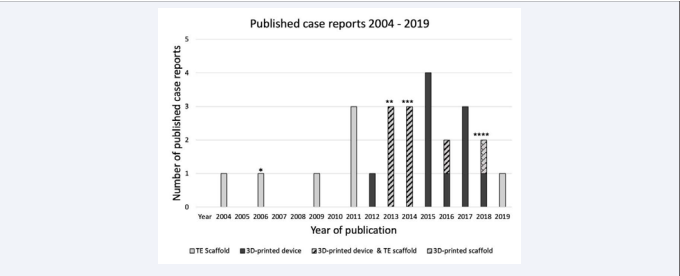
Figure 1 Amount of all published case reports (n=20), starting in 2004, on segmental defects in the maxilla and in the mandible, treated either with a 3D printing technology or TE approach, or the combination of both methods.
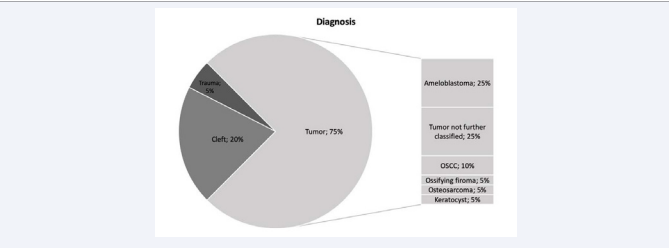
Of the 20 cases analyzed, 7 were females and 10 were males. The mean adult age was 52 years, ranging from 25 to 82 years (n=13). There were 4 pediatric cases and the mean age was 10 years, ranging from 8 to 13 years. Eight of the nineteen cases reported reconstructed defects of the maxilla and 12 of the mandible. Of the eight, 4 cases reported alveolar cleft reconstruction, while 4 cases were due to tumor ablation. In the mandible 11 cases were due to tumor and 1 case was trauma related. Defects size was reported in 4 cases (mean=8.1 cm, range=6-10 cm) (Figure 2).
Figure 2 Ratio of diagnosis leading to treatment. OSCC: Oral squamous cell carcinoma
3D Printing and/or Tissue Engineering approach
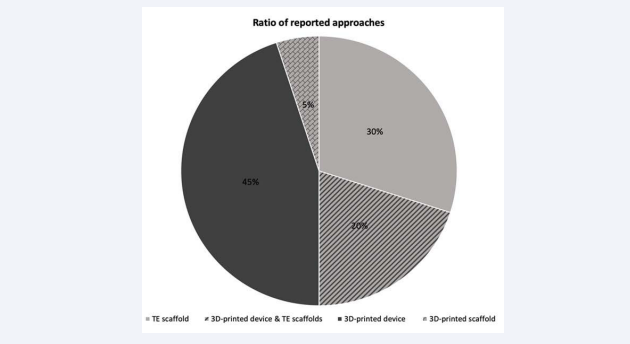
For standardization purpose, custom reconstruction plates/ implants and custom meshes were considered and refferd to be “devices” in the manuscript. Ten of the 20 reported cases were treated using 3D-printed devices. Six patients were treated using TE approach. In four cases the combination of a 3D-printed device together with the TE approach was performed (Figure 3).
Figure 3 Ratio of the reported treatment methods. Ten of the 20 cases reported the use of 3D-printing techniques or the combination of 3D-printing together with TE approach (4/20). Six of the 20 cases was treated only by TE approaches. Only one 3D-printed scaffold was reported.
In all reported cases describing 3D-printing techniques, 3D-imaging was obtained from conventional CT-imaging prior to manufacturing the patient-specific devices and the scaffold.
To construct the 3D-printed devices, a variety of printing techniques were used. Six of 14 cases were produced by stereolithography (SLA), followed by selective laser sintering (SLS), reported in 5/14 cases, whereas 3/14 used selective laser melting (SLM). The devices were mainly printed out of titanium (Ti). In only 1 case the scaffold was printed with biodegradable PCL.
Sixteen of the 20 cases were treated with a Ti device. Ti alloy (n=4) and Ti-6-Al-4V alloy (n=5) were the most frequently reported Ti composites used. With regard to the form of the Ti devices, Ti meshes (11/16), followed by Ti plates (4/16) and Ti implants (4/16) were used. In some cases multiple Ti devices were used simultaneously. Four cases reported the use of a Ti device only. Thirteen cases reported the use of Ti devices, directly produced via 3D-printing. In contrast, cases described the application of pre-bent Ti plates or meshes.
Three cases were treated only by a 3D-printed Ti device of the overall 14 cases where 3D-printing and 3D-printing & TE were used. In 5/14 a Ti device or a PCL scaffold, seeded with bone marrow stem cells (BMSCs) was reported. Seven of 14 cases described autogenous bone filled into a Ti mesh. Two cases reported the use of Bio-Oss together with autogenous bone. Four cases described the additional use of BMP-2. In 3/14 betatricalcium phosphate (β-TCP) granules were loaded onto the Ti mesh, after incubation in media containing BMP-2. In the TE approach group, 3 cases reported a pre-bent Ti mesh containing β-TCP, Bio-Oss or particulate cancellous bone and 3 cases used a hydroxyapatite (HA)/β-TCP plug, functioning as a framework. To fulfill the TE triad, in all six cases BMSCs, adipose stem cells (ASCs) or non further purified bone marrow aspirate were seeded onto the scaffold, followed by applying BMP-2 or BMP7 in most cases or platelet-derived growth factor (PDGF) in 3/5 cases. Three cases reported a prefabrication of the implanted device by its incubation inside the patient’s body.
Defining a successful outcome without complications, postoperative corrections or total revision, the majority (19/20) mentioned a “successful treatment”. Six cases stated a “successful outcome” with objective criteria such as bone mineral density, measured in Hounsfield Units (HU), millimeters of mouth opening, and stable occlusion. Cases in which postoperative second surgical interventions have been of necessity, or complications occurred, were considered as partially successful. In 1 case postoperative complications was mentioned, such as dehiscence, fistula occurrence and small necrosis. This was treated with an additional scapula bone graft placed over the gap. No cases required the removal of the reconstruction method used. There was a lack in reporting the outcome with objective obtained data.
The mean follow-up period for all patients (n=20) was 16.25 months (range=3 months to 5 years). Especially, children (8 to 13 years) had a mean follow-up of 4.3 months, while adults (25 to 82 years) had a mean follow-up of 19.25 months. Supplemental Table 1 shows all data collected from the 20 published cases using 3D printing technology and/or TE scaffolds to reconstruct segmental bone defects of the jaws.
Table 1: Data collected from the 20 published cases using 3D printing technology and/or TE scaffolds to reconstruct segmental bone defects of the jaws.
| Author/Year | Diagnosis | Location | 3D-printing/Tissue-engineering approach | Follow-up (months) | Outcome |
| Takano et al., 2019 | Oral Squamous Cell Carcinoma | Maxilla | Pre-bent Ti mesh + particulate cancellous bone + bone marrow | 10 | Successful |
| Ahn et al., 2018 | Cleft Palate | Maxilla | 3D-printed (SLS) PCL scaffold + BMSCs | 8 | Successful |
| Naujokat et al., 2018 (follow-up) Wiltfang et al., 2016 | Tumor | Mandible | 3D-printed (SLM) Ti-6Al-4V alloy mesh + BMP-2 + BioOss + autogenous bone + BMSCs | 18 | Partly successful |
| Qassemyar et al., 2017 | Ameloblastoma | Mandible | 3D-printed (SLM) Ti implant | 18 | Successful |
| Oral Squamous Cell Carcinoma | Mandible | 12 | |||
| Rachmiel et al., 2017 | Ameloblastoma | Mandible | 3D-printed (SLA) Ti implant + Bio-Oss + autogenous bone | 12 | Successful |
| Leiser et al., 2016 | Trauma | Mandible (bilateral) | 3D-printed (SLA) Ti implant + autogenous bone | 6 | Successful |
| Shan et al., 2015 | Ossifying Fibroma | Mandible | 3D-printed (SLA) Ti alloy mesh + fibula flap | 24 | Successful |
| Osteosarcoma | Mandible (bilateral) | 3D-printed (SLA) Ti alloy mesh + fibula flap | 60 | Successful | |
| Tumor | Maxilla | 3D-printed (SLA) Ti alloy mesh | 6 | Successful | |
| Tumor | Mandible | 3D-printed (SLA) Ti alloy mesh + autogenous bone | 6 | Successful | |
| Sándor et al., 2014 (follow-up) Sándor et al., 2013 (follow-up) Wolff et al., 2013 | Ameloblastoma | Mandible | Pre-bent Ti mesh + 3D-printed (SLS) Ti-6Al-4V alloy plate + β-TCP + BMP-2 + BMSCs | 29 | Successful |
| Ameloblastoma | Mandible | 51 | Successful | ||
| Ameloblastoma | Mandible | 27 | Successful | ||
| Ciocca et al., 2012 | Tumor | Mandible | 3D-printed Ti-6Al-4V alloy plate (SLS) + fibula flap | 12 | Successful |
| Behnia et al., 2011 | Cleft Palate | Maxilla | HA/TCP + PDGF + BMSCs | 3 | Successful |
| Cleft Palate | Maxilla | 3 | Successful | ||
| Cleft Palate | Maxilla | 3 | Successful | ||
| Mesimäki et al., 2009 | Keratocyst | Maxilla | Pre-bent Ti mesh + ASCs + β-TCP + BMP-2 | 4 | Successful |
| Warnke et al., 2006 (follow-up) Warnke et al., 2004 | Tumor | Mandible | Pre-bent Ti mesh + Bio-Oss + BMP-7 + BMSCs | 13 | Successful |
DISCUSSION
Considering the ideal treatment for the reconstruction of segmental bone defects of the jaws, autologous bone with limited donor site morbidity would be ideal. The treatment should be efficient, user friendly and cost effective. 3D-printing may allow the feabrication of repair of bone loss, due to its ability to produce patient-specific bone scaffolds in an affordable and efficient manner. Also, 3D-printed bone scaffolds could bypass the need of harvesting autogenous bone.
Several challenges are linked to the field of tissue regeneration by using custom 3D-printed scaffolds for clinical bone reconstruction. This is substantiated by the fact that up to date, only 14 clinical cases are reported in TE and 3D printing in the published literature.
The ideal treatment of tissue substance loss, is to be achieved by regenerative approaches which activate the body’s own mechanisms, by targeting natural cell mechanism and the human bodies intrinsic capacities of native tissue restoration [36]. In the field of TE, this approach consists out of a triad, containing a scaffold, seeded with cells and growth factors. This assumption can also be applied on the regeneration of bony defects. A 3D-printed scaffold should mimic the bony tissue, so that it is capable of osseointegration and attracts cells of the surrounding tissue by the function of growth factors and cell attractans. It should regenerate tissue close to the native healthy bone and being biocompatible and biodegradable. This approach is represented the best by the triad of TE.
Taking a closer look to the timepoint of publications, it turns out that in early stage clinical trials only TE approaches were reported [28,33,34]. In the recent years, the combination of TE approach and 3D printing technology represents the majority of cases [14,16]. In general, the total amount of published case reports increased over the years. A fact that may be explained by the encouraging findings of preclinical and translational research for the reconstruction of segmental bone defect and technological improvements made in the field of 3D printing technologies.
Thirteen cases were published, reporting 3D printing of Ti devices, mostly restricted to custom reconstruction plate or implants (n=8) and custom meshes (n=5). The one 3D-printed scaffold published as a case report, was for a cleft repair, the scaffold printed was polycaprolactone (PCL) combined with autologous BMSC’s [17]. Most case reports lack approaches of 3D-printed bioresorbable materials like biopolymers and bioceramics, although they are widely investigated in preclinical studies [24,37-39].
Titanium is the gold standard for being base material of plates and screws. While Ti is known for its load-bearing stability and beeing efficiently manufacturable by 3D-printing, there are also disadvantages, such as its non-biodegradability. Titanium serves as a carrier but not as a scaffold. Moreover, the rate of post-op complications like prosthesis infection, which is well observed from replacements of the hip are frequently reported [40]. These obstacles may be overcome with the use of substitute materials for defect reconstruction using 3D-printed bioresorbable materials like biopolymers, bioceramics, and composite biomaterials [41]. Still, it appears to date, that only one clinical case of a 3D-printed biodegradeable scaffold in the treatment of a segmental bone defect of the jaw has been published. In contrast, findings of this study suggest that 3D printing is currently predominantly used for custom titanium plate/implant and mesh fabrication.
Warnke et al. [34], and Wiltfang et al. [16], reported the same approach using a 3D-printed Ti device, but also the patient’s body as a bioreactor for prefabrication of the TE scaffold prior implantation. Differentiation of stem cells, early vascularization and ossification of the scaffold were aims to achieve by this approach. Combining 3D printing together with TE, seems to be a promising approach. In contrast the prefabrication of a scaffold in the patient’s body does not align with the idea of reducing the patient’s donor site morbidity of traditional vascularized bone grafts, being considered one of the main reasons for striving for advances in the emerging field of TE and especially 3D printing in maxillofacial surgery. A major aim is to decrease donor side morbidity.
The high numbers of SLA and SLS technology (11/14) reported for manufacturing mostly Ti devices is not surprising and can be explained by each of the technology’s advantages. SLA printing is still one of the most popular 3D printing technique, because of its huge versatility of materials that can be selected, considering the desired application of the 3D-printed device [42]. SLS technology can be used to shape complex geometries, especially detailed interior features of scaffolds [42]. Having a low average cost and still the ability of producing scaffolds with an enormous mechanical strength, SLS is a very popular and commonly used technique in manufacturing affordable devices at a high productivity [42]. Furthermore, those technologies allow the fabrication of biodegradable patient specific scaffold geometries.
Also, combing 3D printing with TE approach by directly printing cells and growth factors onto the device, are underrepresented in human case studies and could overcome the need of human bioreactors for the scaffold’s fabrication. Especially printing PCL scaffolds by extrusion technique allows to produce cell seeded scaffolds at a high density for whole bone regeneration [43] and could be a promising next step to bring 3D printing in the clinical setting for producing patient-specific scaffolds.
The production of custom 3D-printed scaffolds using medical imaging combined with computer modeling and design may be considered as a promising alternative for the reconstruction of major maxillofacial osseous defects. Over the last decade 3D printing technology is becoming affordable for surgeons and patients, devices and scaffolds can be manufactured cost and time efficient, leading to personalized implants that fit one unique individual matching the concept of individualized medicine. In addition, human research should be based on highquality, well-designed clinical studies using custom 3D-printed scaffolds to obtain scientific evidence against conventional grafting strategies.
Tissue engineering is a well established field of research in the preclinical setting and 3D printing has become a promising method to facilitate processing of complex-shaped bone grafts. We believe that greater emphasis should be placed on increasing the number of 3D-printed bioresorbable TE scaffolds. Application of 3D printing should not only be limited to conventioal Tidevices. In the field of maxillofacial surgery, clinical trials need to investigate, if non-biodegradable Ti-bone-substitutes are a favorable method in the elder, under the risk of implant infection and osteoporosis, to restore segmental bone loss.
These case reports are an important step to encourage OMFS surgeons, TE research groups, and biomedical engineers to debate existing challenges and act at the frontier of knowledge. ?This will enable the use of innovative and less invasive solutions for evidence based clinical practice using these technologies and approaches in a safe and effective way to benefit patients.
ACKNOWLEDGEMENT
This manuscript was presented at the 101st Annual Meeting of the American Association of Oral and Maxillofacial Surgeons, Boston, MA, September 16-19, 2019. This study was funded in part from grants: MGH-Department of Oral and Maxillofacial Surgery Education Research Fund (Boston, MA), Jean Foundation (NH), Fondation Bertarelli (Gstaadt, Switzerland) and MGHWalter C. Guralnick Fund (Haseotes-Bentas Foundation, Boston, MA).