Progress of Gut-Brain Axis in the Development of Autism Spectrum Disorder (ASD) in Children
- 1. College of Life Science, Zhejiang Chinese Medical University, China
- 2. Key Laboratory of TCM Encephalopathy of Zhejiang Province, Zhejiang Chinese Medical University, China
- 3. Department of Physiology, Zhejiang Chinese Medical University, China
- #. Equal contribution to the article
Abstract
Autism, also known as Autism Spectrum Disorder (ASD), is a serious neurodevelopmental disorder that has attracted considerable attention owing to its increasing incidence worldwide. In recent years, the role of the Gut-Brain Axis (GBA) in ASD development has attracted much attention. The Gut-Brain Axis (GBA) is a bidirectional communication system between the gut and the brain and is a key component of the gut microflora, which is closely related to ASD. Studies have shown that the composition of the gut microbiota in patients with ASD differs significantly from that in healthy individuals, such as changes in the abundance of specific bacterial genera, reduction in beneficial flora, and changes in the abundance of fungi, which are affected by a variety of factors such as diet, geography, and age. Changes in gut flora can trigger metabolite abnormalities, increased intestinal permeability, immune system abnormalities, and abnormalities in the HPA axis and neural pathways, which in turn affect the development of ASD. In addition, gut flora can be involved in the expression of ASD related behavioral phenotypes by affecting the levels of neurotransmitters such as 5-hydroxytryptamine, γ-aminobutyric acid, histamine, and dopamine. In this regard, interventions based on the gut-brain axis, such as probiotics, dietary therapies, and fecal microbial transplantation, have shown potential to improve ASD symptoms but still face challenges, such as insufficient sample size and lack of clinical validation. This paper aims to review the mechanisms of the gut brain axis in ASD and evaluate existing intervention strategies to provide new ideas and directions for the early diagnosis and personalized treatment of ASD.
Keywords
• ASD
• Gut - Brain Axis
• Gut Microbiota
• Intervention Measures
Citation
Kong W, Shen D, Yuan H, Qiu H, Zheng K, et al. (2025) Progress of Gut-Brain Axis in the Development of Autism Spectrum Disorder (ASD) in Children. JSM Pediatr Neurol 5(1): 1020.
INTRODUCTION
Autism, also known as Autism Spectrum Disorder (ASD), is a group of neurodevelopmental disorders characterized by social communication deficits, repetitive stereotyped behaviors, and narrow interests [1]. In recent years, its incidence has increased significantly owing to various complex environmental and genetic factors. As of 2022, the prevalence rate among children aged 3-17 in the United States was 3.42% [2]. In China, the prevalence also reaches 0.70% in children aged 6–12 years [3]. Globally, the prevalence of ASD is slightly less than 1% and is significantly higher in males than in females, with a male-to-female ratio of approximately 2:1 to 5:1 [4]. The high prevalence of ASD not only brings a heavy mental burden to the families of patients but also exerts great pressure on the social economy. In the United States, for example, the cost of ASD treatment was estimated to be $223 billion/year in 2020, and is expected to rise to $589 billion/year in 2030, $1.36 trillion/year in 2040, and even up to $5.54 trillion/year by 2060 [5]. ASD has become a global childhood disease. However, owing to its complex pathogenesis, there is a lack of effective treatment options in clinical practice, further aggravating the burden on patients’ families and society.In recent years, an increasing number of studies have focused on the gut-brain axis and have found that it plays a key role in the development of childhood autism. The gut-brain axis is a complex two-way communication system that connects the gut and brain, including the central nervous, endocrine, and neuroimmune systems, among other important systems. In this system, the brain can regulate the composition of gut microorganisms and their functions through the nervous system; conversely, secretions produced by gut microorganisms can influence the normal function and behavior of the brain with the help of the nervous, immune, and endocrine system Gastrointestinal symptoms such as constipation, abdominal pain, intestinal gas, and diarrhea are extremely common in patients with ASD [6]. Studies have shown that approximately 91% of people with ASD develop gastrointestinal disorders [7,8]. These findings strongly suggest that GI disorders in individuals with autism are likely mediated by the gut-brain axis. Therefore, an in depth understanding of the mechanisms of the gut-brain axis in childhood autism is crucial for the development of new therapeutic strategies for ASD.
RELATIONSHIP BETWEEN GUT MICROBIOME AND AUTISM
Gut microbial sequencing technologies
16S rRNA high-throughput sequencing: In studies investigating the differences between ASD and gut bacteria, 16S rRNA high-throughput sequencing has been widely applied. Liu et al. [9], used this technique to find significant differences at the genus level in children with ASD for Chlamydia, Barnesella, Microbacterium, Chlamydia, Rumatococcus, and Clostridium. In addition, Dan et al. [10], found decreased abundance of Prevotella, a bacterium that produces succinic acid and enhances the T-cell immune response, in patients with ASD using 16S rRNA analysis, and that its reduction may be associated with immune disorders in ASD [11]. In addition, Li et al. [12], used this technique to demonstrate that children with ASD have an elevated Fasciola gigantica/Bacillus pseudomallei (F/B) ratio, mainly due to a decrease in Bacteroides pseudomallei, which affects polysaccharide digestion. However, 16S rRNA was inferred based on operational taxonomic units (OTUs) with limited species level analysis, which may affect accuracy.
Birdshot macrogenome sequencing: In contrast, shotgun metagenomic sequencing can detect microbial species and infer metabolic pathways and functions [13]. Zhang et al [14]. used this method to find that ASD patients had an increase in B. cereus and Lactobacillus rhamnosus, a decrease in Bifidobacterium longum and B. cereus, and a decrease in B. cereus, and a decrease in B. cereus and B. cereus, and a decrease in B. cereus, a decrease in B. cereus and B. cereus. carboxylic acid cycle related metabolites suggestive of mitochondrial dysfunction. Wang et al. [15], also used this method to demonstrate that ASD patients had a decrease in Anaplasma oryzae commonum and an increase in E. delayeri and Clostridium botulinum, as well as an elevation of 11 metabolic pathways including abnormalities in benzoic acid degradation, tyrosine metabolism, and cortisol synthesis. These analyses are useful in understanding the influence of metabolic changes in the intestinal flora on the development of ASD.
Artificial Intelligence Technology: In addition to the above techniques, with the development of technology, artificial intelligence techniques have also been used for gut flora analysis. In the study of intestinal flora, with the help of artificial intelligence-assisted analysis, the changes in intestinal flora can be analyzed more quickly, easily, and economically, and it is even expected to realize an accurate diagnosis of ASD. For example, Wang et al. [16], used Random Forest (RF) modeling to identify Fusobacterium spp. CAG-248 and Prevotella spp. as potential ASD biomarkers. Similarly, Lucia et al. [17], used Recursive Integrated Feature Selection (REFS) to identify an increase in Clostridium spp, Octococcus spp., and Paramycetes spp. in patients with ASD from 16S rRNA data, and identified 26 amplicon sequence variants to distinguishing ASD from controls. It has become a trend to use a combination of computerized and other gene sequencing techniques to explore changes in the gut flora of ASD patients [18]. The use of artificial intelligence assistance on traditional sequencing methods combines the advantages of speed and accuracy. The results of a large number of studies mentioned above have demonstrated that changes in the abundance of bacterial taxa, as well as alterations in the biodiversity of gut bacteria are closely related to the development of autism. A large number of studies have been conducted using different means to show that there are significant differences in the diversity of gut flora in children with ASD compared to the healthy population (Table 1).
Table 1: Differences in gut flora between ASD patients and healthy individuals using different techniques [19-30].
|
Methods |
Sample Gender (male, n (%)) |
Area |
Intestinal flora |
||
|
ASD |
TD |
|
Increase |
Decrease |
|
|
16S rRNA |
53 (82.8%) |
54 (84.3%) |
China |
Eubacterium limosum (mucus fungus) Alternaria (Alistipes) Candidate division TM7 isolate TM7c Streptococcus cristatus Streptococcus oligofermentans |
\ |
|
130 (90.9%) |
127 (88.8%) |
China |
\ |
Bacteroides Prevotella Phascolarctobacterium |
|
|
73 (73.8%) |
90 (60.8%) (60.8%) |
Australia |
Romboutsia timonensis |
\ |
|
|
197 (79.1%) (79.1%) |
52 (51.5%) |
Korea |
Macrocystis (Megamonas) Enterobacter cloacae (Pasteurella) |
\ |
|
|
59 (76.6%) |
39 (78%) |
China |
Lachnoclostridium Dorea |
Bacteroides Prevotella Faecalibacterium |
|
|
677 (70.7%) |
122 (75.7%) |
China |
Faecalibacterium genus (Faecalibacterium) Veillonella Parasutterella Blautia hydrotrophica |
\ |
|
|
Shotgun metagenomic sequencing |
15 (71.4%) |
22 (95.6%) |
USA |
Enterococcus faecium Megasphaera elsdenii Bacteroides fragilis |
\ |
|
36 (83.7%) |
17 (54.8%) |
China |
Eggerthella lenta (Eggerthella retarda) Clostridium botulinum |
Bacteroides vulgatus |
|
|
32 (82.1%) |
33 (82.5%) (82.5%) |
China |
Veillonella parvula Lactobacillus rhamnosus |
Bifidobacterium longum Prevotella (Prevotella) |
|
|
RT-PCR |
9 (90%) |
10 (100%) |
Slovakia |
Clostridia cluster I Desulfovibrio |
Bacteroidetes |
|
29 (87.8%) |
10 (62.5%) |
Poland |
\ |
Klebsiella genus (Klebsiella) Genus Bifidobacterium (Bifidobacterium) |
|
|
\ |
\ |
Australia |
Sutterella (Sutterella) Ruminococcus torques |
|
|
Differences in microbial composition
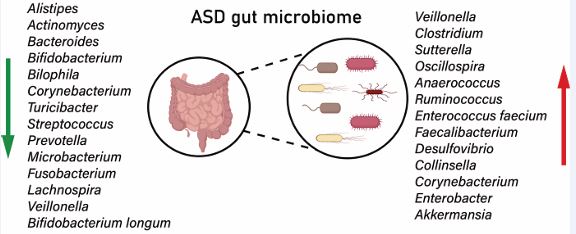
Gut microflora is an important component of the gut brain axis and is closely associated with human health. The human gut is inhabited by a large number and variety of microorganisms that form a complex interaction with the host, and their balance is essential for health. In recent years, studies have shown that ASD is closely related to gut dysbiosis, and that there are significant differences in the composition of the gut flora between children with ASD and healthy children.Among the diverse and changing gut bacteria in patients with ASD, changes in beneficial bacteria have been the focus of research.Wang et al. [31], found a decrease in Bifidobacterium bifidum and Bifidobacterium longum, and an increase in Clostridium perfringens and Ruminalococcus in patients with ASD using 16S rRNA technology, suggesting that a decrease in beneficial bacteria and an increase in potentially pathogenic bacteria may increase the risk of ASD. However, Dalia et al. [32], found elevated leves of lactobacilli in the intestines of patients with ASD, which may be associated with a decrease in short-chain fatty acids, a microbial metabolite associated with the intestinal tract. This result is in line with the study of Joby et al. [33], who demonstrated that the relative abundance of Lactobacillus in the gut of ASD patients was 32 times higher than that of healthy children, suggesting a strong association with the development of ASD. This discrepancy between the results of different studies may stem from the diversity of the study samples. These samples differed significantly in terms of geography, age, and dietary habits. Figure 1 illustrates the common bacterial taxa that have changed in the gut of ASD patients in several studies.
Figure 1: Bacterial taxa commonly altered in the gut of ASD patients. Regarding probiotics, there is a general reduction in the abundance of Bifidobacterium, Prevotella, and Faecalibacterium species. Opportunistic pathogens such as Clostridium, Sutterella, and Eggerthella lenta show a significant increase. The heterogeneity of microbiota changes is regulated by factors such as geography, age, and diet. For example, studies in Asian populations have observed a reduction in Bacteroidetes and an increase in the Firmicutes/Bacteroidetes (F/B) ratio, while in Western cohorts, there is an abnormal proliferation of Lactobacillus.
In addition to bacteria, the fungi in the gut flora of ASD patients have also changed. In terms of fungi, Rosaria et al. [34], found an elevated abundance of Candida in patients with ASD, whose toxins can cause neuroinflammation. Similarly, Francesco et al. [35], showed that Candida overgrowth can alter the gut flora structure and cause ASD. In contrary, Chamtouri et al. [36], found that the relative abundance of Candida did not significantly correlate with ASD [36], which may be due to the geographic setting in which the samples were selected, or the habits of the people who lived there. For other fungi, mainly Saccharomyces cerevisiae showed elevated levels in patients with ASD, which may be involved in pathogenesis through immune mechanisms [37]. Currently, there are relatively few studies on the relationship between fungi and ASD, and the specific mechanism of action is unclear, so subsequent studies should focus on the role of fungi in ASD. Owing to the differences in dietary conditions and living practices in different regions, different differences in intestinal microbial populations may occur, leading to a high degree of heterogeneity among different studies. For example, Lou et al. [38], found that alpha diversity in the ASD group showed a persistent decrease, whereas Wan et al [39]. observed an increase. Whereas Clostridium spp. showed an increase in Wafaa et al [40]. study, Shakuntla et al [41]. found that Clostridium spp. did not show any significant difference between ASD patients and healthy individuals. This shows that the geographical location of the children leads to differences in dietary habits, which in turn can cause heterogeneity in the intestinal flora. In addition to this, it has been pointed out that age also affects the composition of intestinal flora in ASD patients, with Enterobacteriaceae and Bifidobacterium spp. fluctuating significantly at different ages [38]. In addition, dietary diversity decreases the diversity of flora in patients with ASD. In addition, Single Nucleotide Variants (SNVs) in ASD patients are associated with a wide range of bacteria, which may affect disease progression. Therefore, future studies should identify gut microorganisms with commonalities as biomarkers for ASD. In summary, a large number of studies have shown that the composition and diversity of the gut flora of ASD patients are significantly different from those of the healthy individuals, which provides an important basis for early diagnosis and potential intervention in ASD.
Microbial-immune-neural axis
When the gut microflora is altered, the metabolites produced by the microorganisms are also altered, and the concomitant triggering of immune system abnormalities and HPA axis abnormalities will have an impact on the development of ASD.
Abnormal metabolites of intestinal flora: Metabolites of intestinal flora, such as Short-Chain Fatty Acids (SCFAs), including Acetic Acid (AA), Propionic Acid (PPA), and Butyric Acid (BTA), are important energy sources for intestinal epithelial cells. SCFAs are produced from dietary fiber by fermentation of intestinal microorganisms and can influence neural and brain development with the help of the blood circulatory system.SCFAs influence psychological functions by interacting with G protein-coupled receptors (GPCRs) and Histone Deacetylases (HDACs) and act on the brain through the immune pathway [42]. Studies have shown [43] that the levels of SCFAs in ASD patients are significantly higher than those in healthy individuals, mainly due to changes in the abundance of certain SCFAs producing bacteria in the gut, resulting in altered levels of SCFAs. Among SCFAs, PPA has received considerable attention. For example, He et al. [44], found high levels of PPA in children with ASD, and PPA levels were negatively correlated with Lactobacillus abundance, suggesting that Lactobacillus could be an effective substance for the treatment of ASD, and that PPA-producing bacteria (e.g., Clostridium spp., Mycobacterium spp.) could be used as biomarkers of ASD. Meanwhile, Frye et al. [45], found that elevated PPA leves leads to mitochondrial dysfunction, which in turn triggers ASD. Latifa et al. [46], also confirmed that elevated PPA leads to over-differentiation of neuroglia, which affects neurological function and triggers ASD. However, Kumar et al. [47], found that genistein (GNT) could counteract the neurotoxicity of PPA and improve ASD symptoms by regulating pro-inflammatory factors or autism-related proteins. In addition to SCFAs, other metabolites such as p-cresol (P-Cresol) also affect ASD development. Sheyla et al. [48]. found that p-cresol inhibited neural differentiation and synapse growth, suggesting that neuronal alterations in children with ASD may be associated with p-cresol. In addition, Brittany et al. [49], found that 4-ethylphenol sulfate (4EPS) levels were elevated in the blood of mice with ASD, and Bacteroides thickettsiae and Anaplasma ovale were the main 4EPS-producing organisms, and that 4EPS could promote the development of ASD. Thus, microbial metabolites can influence the production and development of ASD through various pathways.
Abnormal intestinal permeability: Increased intestinal permeability is another important mechanism of the gut-brain axis in childhood autism. Changes in the gut flora and its metabolites affect the integrity of the gut barrier function, which in turn leads to altered gut permeability. The intestinal permeability of autistic patients is significantly higher than that of healthy children due to changes in the intestinal flora and abnormal metabolites. It is estimated that about 36.7% of ASD patients have abnormal intestinal permeability [50]. Piras et al. [51], suggested that the presence and severity of increased intestinal permeability in patients with ASD may be associated with altered urinary concentrations of specific metabolites. Luo et al [52] study, on the other hand, demonstrated that dysbiosis of the intestinal flora induces abnormal expression of intestinal mucosal proteins, inhibits mucin production, and damage the intestinal mucus layer, thus leading to intestinal permeability damage. In this regard, Fasano et al. [53], demonstrated that Vibrio cholerae-derived zonulin inhibits the production of intestinal tight junction proteins (TJs), leading to leaky gut phenomenon. However, in a study of intestinal barrier function in children with ASD, it was found that Zonulin levels in the serum of children with ASD were not significantly different from those of controls, but Occludin (a paracellular tight junction protein) was significantly lower in the serum of children with ASD [54]. Currently, Occludin is considered to be a marker of leaky gut phenomenon. In addition, due to increased intestinal permeability, increased blood levels of substances derived from the microorganism’s own structure, such as Lipopolysaccharide (LPS) can lead to the entry of interleukin-1β (IL-1β), IL-6 and other substances produced by immune responses, which activate the immune system, cause neuroinflammation and nerve damage, and thus exacerbate ASD symptoms. For example, Zhao et al. [55], demonstrated that LPS induced neuronal cell loss and microglia activation in mice, which in turn induced the activation of inflammatory cytokines in the bloodstream, leading to cognitive impairment in mice. Therefore, Alonazi et al. [56], proposed a therapy in which bee pollen in combination with probiotics could enhance the intestinal barrier function and control the expression of TJ proteins, thereby alleviating leaky gut and improving ASD symptoms. This shows that intestinal permeability is closely related to the occurrence of ASD, and it is necessary to further study the relationship between the two to further assist in the diagnosis and treatment of ASD.
Immune system abnormalities: As one of the largest immune organs in the human body, the gut plays an important role in the bidirectional connectivity of the gut-brain axis. Once altered, gut microorganisms prompt immune cells on the surface of gut mucosa exposed to pathogens to produce immune proteins, which in turn trigger an immune response [57]. This process is mediated by gut-associated lymphoid tissue (GALT) [58], which produces immunoglobulin (IgA) via lymphocytes. Kushak et al. [59], have long noted a trend of elevated IgA levels in ASD patients. Moreover, more studies have reported that different inflammatory signs were found in individuals with ASD. Plasma levels of pro-inflammatory factors (e.g., IL-1β, IL-6, and IL-8, etc.) were significantly higher than normal levels in patients with ASD [60,61]. In addition, Cuomo et al. [62], analyzed fecal DNA methylation in ASD patients and found that there was significant hypomethylation of the promoters of IL-1β and IL-6 in ASD patients, which led to an increase in the inflammatory response. The changes in the levels of pro-inflammatory factors were not only reflected in plasma and feces, but Than et al. [63], also detected elevated levels of four inflammatory cytokines (TNF-α, IL-4, IL-21, and BAFF) in the cerebrospinal fluid (CSF) of patients with ASD. In addition, Cerilli et al. [64], demonstrated in a recent study that ASD mice have elevated levels of molecules associated with inflammation and injury in the cerebellum and peripheral blood (PB) and extensive immune dysfunction in the bone marrow (BM) and spleen. These findings further suggest that neuroinflammation and immune system dysfunction may contribute to the production of autism-like behaviors, which in turn influence the severity of autism.
At the cellular level, microglia, a type of immune cell, dominate active immunity in the brains of ASD patients in an abnormally active manner compared to normal individuals, which can cause damage to the functional areas of the human brain. Duarte et al. [65], found that in the brains of mice with ASD, the density of microglia increased in a regionally specific manner to modulating inflammatory responses. In addition, Tetreault et al. [66], found that microglia in patients with ASD were not only altered in number, but also in their morphology, such as enlarged cell bodies and constricted and thickened protrusions. These changes suggest more intense microglia-mediated immune activity. To address this situation, Deng et al. [67], showed that the use of AG1478, an inhibitor of the neuromodulin 1 (NRG1) receptor (ErbB4), was effective in alleviating the abnormal activation of microglia and ameliorating ASD symptoms. Moreover, the offspring of mothers who acquire maternal immune activation (MIA) during pregnancy will exhibit ASD-related symptoms. In this regard, Salia et al. [68], transplanted the intestinal flora of MIA mice into the intestines of normal mice and found that the recipient mice had increased levels of the serum cytokines IL-4 and IL-7, which promoted the development of ASD. Zhang et al. [69], also demonstrated that significant microglia activation was seen in the prefrontal cortex of MIA offspring. They also found that these abnormalities may be caused by overexpression of sodium-potassium chloride cotransporter protein (NKCC1) in MIA progeny. Microinjection of the NKCC1 inhibitor bumetanide (BTN) into MIA offspring improved their autistic behavior. The above studies amply demonstrate that gut microbes can influence the development of ASD through the immune system. The manner in which gut microbes and their products enter the body to trigger an immune response and influence the development of ASD is closely related to the previously mentioned increased intestinal permeability.
The hypothalamic-pituitary-adrenal axis (HPA) is an important part of neuroendocrine regulation and is involved in the regulation of digestion, immunity, and emotion. The process of HPA regulation involves the release of corticotropin releasing hormone (CRH) from the hypothalamus, the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland under the stimulation of CRH, and the secretion of ACTH from the adrenal cortex under the stimulation of ACTH. The pituitary gland is stimulated by CRH to secrete adrenocorticotropic hormone (ACTH), which acts on the adrenal cortex to release cortisol into the bloodstream, which in turn affects various body systems. The effects of ACTH on the body’s systems can be seen in the following ways. Gut flora can influence the HPA axis through released neurotransmitters or SCFAs. For example, Huo et al. [70], demonstrated that changes in gut flora can disrupt the balance of the HPA axis, affecting the neuroendocrine system in the brain and leading to anxiety-like behavioral phenotypes. In the HPA axis, changes in the levels of cortisol, a key substance in the cascade response, are associated with ASD symptoms. In a triple-blind experiment, Dalile et al. [71], found that increasing the serum levels of SCFAs in healthy populations was associated with a significant decrease in cortisol in the face of acute psychosocial stress, accompanied by the development of ASD symptoms. In this regard, Wang et al. [72], showed that sodium butyrate, which can act as a histone deacetylase inhibitor, significantly increased histone acetylation at the corticotropin-releasing hormone receptor 2 (CRHR2) promoter in vitro, normalized cortisol and CRHR2 expression levels in vivo, and alleviated symptoms such as anxiety in patients with ASD. Thus, it is clear that changes in the HPA axis also contribute to ASD and that ASD symptoms can be alleviated by regulating the balance of the HPA axis. The neural pathways in the gut-brain axis mainly include the vagus nerve and enteric nervous system. The vagus nerve is one of the main neural pathways connecting the gut to the brain. The Enteric nerve system (ENS), on the other hand, is a neural network independent of the central nervous system that regulates motility, secretion, and immune function of the intestine. Gonzales et al. [73], found that in normal mice, after receiving transplantation of intestinal microbiota from patients with ASD, the expression of neuron connectivity proteins was reduced, which in turn induced ENS remodeling that leads to intestinal dysfunction. Meanwhile, Hosie et al. [74], hypothesized that mutations in genes encoding synaptic proteins, such as neural connexins, in individuals with ASD may lead to dysfunction of the ENS dysfunction and associated gastrointestinal symptoms in autism. In terms of the role of the vagus nerve, Sgritta et al. [75], transplanted Lactobacillus royale into the intestines of mice with ASD and found that the symptoms of the mice with ASD were significantly alleviated, however, after severing the vagus nerve the symptoms of ASD in the mice did not improve. This suggests that the therapeutic effect of Lactobacillus Royale on ASD depends on the vagus nerve. Similarly, Bravo et al. [76], found that mice enriched with Lactobacillus rhamnosus in the intestinal tract had attenuated social deficits due to the fact that Lactobacillus rhamnosus could increase the mass of GABA receptors on the vagus nerve, whereas ASD symptoms did not improve in mice after severing the vagus nerve. This further suggests that the vagus nerve is acting as an important pathway influencing the development of ASD. Thus, it is evident that the neural pathway, connecting the gut to the brain, is damaged to varying degrees during the development of ASD and mediates the signaling exchange between the gut microbes and the brain in a direct or indirect manner.
ROLE OF NEUROTRANSMITTERS AND SIGNALING REGULATION IN AUTISM
Neurotransmitters are chemicals that transmit information between neurons or between neurons and effectors, and mainly include 5-hydroxytryptamine (5-hydroxyptamine, 5-HT, also known as serotonin), γ-aminobutyric acid (γ-aminobutyric acid, GABA), histamine, and dopamine. DA) [77,78]. Gut flora is capable of producing neurotransmitters, so alterations in gut flora may lead to dysregulation of peripheral neurotransmitters, which in turn may lead to neurodevelopmental disorders [79].
5-HT
5-HT, a neurotransmitter, is essential for the regulation of the nervous system. Tryptophan is the precursor substance of 5-HT, and the biosynthesis of tryptophan to produce indole and indole derivatives is completely dependent on tryptophanase (TPH1), which is only found in specific microorganisms. Changes in the species and number of colonies can interfere with tryptophan metabolism, thus indirectly influencing the amount of 5-HT synthesized. For example, Tian et al. [80], found that intestinal microbes affect TPH1 transcription and thus 5-HT synthesis, with significantly lower levels of 5-HT found in germ-free mice. As only a small fraction of tryptophan is converted to serotonin, changes in its levels may pose a significant risk to human health. More than 90% of 5-HT originates from enterochromaffin cells in the gastrointestinal tract, and the intestinal microbiota can act on enterochromaffin cells via short-chain fatty acids to promote colonic 5-HT production, which is an important determinant of intestinal 5-HT production and homeostasis [81]. A variety of gut microorganisms, such as E. coli spp. and Streptococcus spp. can assist in 5-HT production. ASD patients also exhibit higher levels of 5-HT in their bodies due to altered gut flora. For example, Tanaka et al. [82], demonstrated that high levels of 5-HT in mice lead to the development of ASD symptoms, and that social deficits in mice with ASD can be effectively ameliorated by knocking out the transporter through 5-HT (Sert). This suggests that the 5-HT signaling pathway and its downstream effects, such as neurogenesis and pro-inflammatory responses, are involved in the expression of behavioral phenotypes associated with ASD [83].
GABA
GABA As an inhibitory neurotransmitter capable of transmitting inhibitory signals, GABA is essential for the regulation of brain rhythms and spontaneous neuronal activity during neural development, including myelin formation and synaptic pruning.GABA is capable of disrupting the balance between excitation and inhibition in the brain, affecting neural communication from the brain to other parts of the body as well as the speed of the brain’s response to signals from other stimuli, resulting in the expression of, amongst other phenomena, hyper-responsiveness. Umesawa et al. [84], analyzed the correlation between GABA levels in four brain regions (primary visual cortex, left sensorimotor cortex, left Sensorimotor Area (SMA), and left ventral premotor cortex (vPMC)) and the symptoms of ASD, and found that the concentration of GABA in the left SMA of patients with ASD was reduced, and that the patients showed hyperresponsiveness. . This suggests that decreased GABA levels may be one of the potential causes of ASD symptoms. However, GABA levels are heterogeneous in ASD patients, Fung et al. [85], found in their study that GABA levels were negatively correlated with the Autism Spectrum Quotient (AQ) in male patients, whereas they were positively correlated with the AQ in female patients. In addition to this, Saleh et al. [86], confirmed that there are also differences in GABA levels between the left and right hemispheres of the brains of ASD patients, and they found that the right hemisphere of the brains of children with ASD showed high levels of GABA, while the left hemisphere did not have a significant difference. In addition, GABA receptor levels also affect ASD symptoms. Tabouy et al. [87], found that ingesting Lactobacillus Royale into ASD behaved mice resulted in an increase in GABA receptors and improved srepetitive and non-social behaviors in the mice. In addition, Hong et al. [88], found that γ-aminobutyric acid A receptor (GABA-A) α2 subunit protein levels were significantly reduced in the axon initiation segment of supragranular pyramidal cells in the prefrontal cortex of patients with ASD, which may be attributed to the reduced synthesis of GABA in the prefrontal cortex of patients with autism, which leads to the excitatory/inhibitory imbalance, and results in the disruption of GABA signaling, and patients show symptoms such as delayed responses. The defective excitatory/inhibitory balance in the prefrontal and hippocampus due to the loss of function of GABAergic interneurons is believed to underlie the symptoms of ASD, and research in this area will help us to better understand the pathogenesis of ASD and to propose effective therapeutic approaches.
Histamine
Histamine is a biogenic amine in the body and is best known for its role as a mediator of allergic reactions and as an important signaling molecule in the nervous system, intestinal tract, skin and immune system. Histamine is mainly produced and released by immune cells such as mast cells and basophils. Histamine can also be secreted by the intestinal microbiota, including Staphylococcus spp, Proteus spp, Enterococcus faecalis, and Klebsiella aerogenes [89,90]. Therefore changes in the gut flora of ASD patients can lead to changes in histamine levels in ASD patients as well. For example, Rashaid et al. [91], used enzyme-linked immunosorbent assay (ELISA) to determine histamine levels in the plasma of ASD patients, and found that the serum levels of histamine were higher in ASD patients, which were 5.3 times higher than those of the control group. Zhang et al. [92], analyzed a mouse model associated with autism, and found that there was a change in the abundance of the bacteria associated with histamine production, along with a change in the histamine metabolism-related neurotransmitter network activity was also altered, further elucidating the impact of histamine dysregulation on ASD. At the genomic level, a meta-analysis showed significant differences in the gene set of the histaminergic system in the brains of patients with ASD, which leads to histaminergic-mediated neuroinflammation exacerbating the symptoms of ASD [93]. Histamine acts primarily by binding to histamine receptors. In response to changes in histamine levels in individuals with ASD that lead to the development of associated symptoms, recent studies have shown promise in alleviating the symptoms of ASD, primarily by targeting histamine receptors in the brains of individuals with ASD (specifically H3R). For example, Venkatachalam et al. [94], found that an antagonist of histamine H3R, the novel multi-active compound ST-713, alleviated ASD symptoms in mice. Therefore, histamine receptor can be used as a potential target receptor for the treatment of ASD-related disorders, and can also enhance the transmission function of other neurotransmitters to influence the occurrence and development of ASD, including 5-HT, GABA, and DA [95]. In summary, the study of the correlation network composed of histamine and other neurotransmitters may also be an effective way to alleviate ASD.
DA
Dopamine is not only a precursor of norepinephrine and epinephrine, but also a neurotransmitter in its own right, produced in the substantia nigra (Substantia Nigra), ventral tegmental area (VTA), and hypothalamus (Hypothalamus) and released into the prefrontal cortex of the brain. It commonly modulates neuronal excitability and is therefore known as a reward neurotransmitter. Dohnalová et al. [96], found that bacteria in the gut of mice (Fusobacterium rectum and Fusobacterium regularis) activate the enteric nerves through the production of the small-molecule metabolite fatty acid amide (FAA), which, in turn, increases the level of dopamine in the brain’s ventral striatum. However, alterations in the gut flora of ASD patients lead to dysregulation of dopamine levels, and this dopaminergic-driven dysregulation of the reward pathway thereby contributes to the development of ASD. Domínguez et al. [97], demonstrated that mice with caspase-3 gene deletion in the dopaminergic system have a significant reduction in dopamine (DA) release at basal extracellular level, which is accompanied by impaired social interactions, ASD symptoms such as restricted interests and repetitive stereotyped behaviors. This is mainly due to dysfunction of the dopamine pathway. However, Schalbroeck et al. [98], showed different results, as they found no significant difference in striatal dopamine synthesis capacity between ASD patients and controls. This may be due to the different samples selected for the study as well as the assay methods. Dopamine D2-like receptors (D2, D3, and D4 receptors) modulate DA release as well as neurotransmitter release from neurons and improve dopamine pathway deficits [99]. Takeuchi et al. [100], used specific D2-like receptor agonists to effectively ameliorate the symptoms of ASD-associated psychiatric disorders. The above studies demonstrated that DA can be used as a marker for the diagnosis of ASD, and also dopamine receptors can be used as a potential therapeutic target to improve ASD symptoms.
GUT-BRAIN AXIS-BASED INTERVENTION FOR ASD
Due to the complexity of its pathogenesis, which involves environmental and genetic reasons, there is no effective cure for ASD. In the following section, we will introduce the improvement of ASD symptoms by intervening the gut-brain axis based on the fecal microbial transplantation technique, dietary therapy, and probiotic supplementation.
Fecal microbial transplantation
Fecal microbiota transplan-tation (FMT) is one of the interventions based on the gut-brain axis, which involves transplanting the fecal microbiota of a healthy individual into the gastrointestinal tract of a patient to achieve therapeutic goals.FMT improves the intestinal micro ecological environment, modulates the immune system and improves the brain’s function and behavior. Studies have confirmed [101] that the use of FMT is effective in improving the diversity of the intestinal flora, increasing bacterial taxa such as Bifidobacterium spp, Prevotella spp, Bifidobacterium spp, and Vibrio desulfuricans spp. and a significant reduction in the GI symptoms associated with ASD including significant improvement in symptoms of constipation, diarrhea, dyspepsia, and abdominal pain. However, Chen et al. [102], found that the relative abundance of Prevotella spp. was significantly reduced after transplantation by gut flora, but still improved ASD-related symptoms. This seemingly paradoxical phenomenon may be due to the different relationship between Prevotella spp. and specific strains of ASD. Not only is the structure of the bacteria in the gut of ASD patients effectively optimized by FMT, but plasma metabolite levels are also altered. For example, James et al. [103], found that analysis of plasma metabolites in children with ASD who underwent FMT revealed significant improvement in gastrointestinal and ASD-related symptoms in these children. At the same time, their metabolic profiles were significantly closer to those of the healthy group, in which, among three plasma metabolites that may represent the three general categories of metabolic abnormalities, sarcosine and 5’-inosine monophosphate showed significant improvement after treatment, whereas there was no significant change in tyramine O-sulphate, which suggests that it and its related metabolites may be a potential target for future therapy. For changes in serum chemokine levels, Chen et al. [102], observed that after FMT, including decreased levels of growth-regulated oncogene α (GRO-α) and macrophage inflammatory protein-1α (MIP-1α), monocyte chemotactic protein-3 (MCP-3), regulated activation of normal T-cell-expressed and secreted factors (RANTES), and eosinophil chemokine (Eotaxin) levels increased. In addition, metabolite changes are not only in plasma, Qureshi et al. [104], analyzed the fecal metabolites of children with ASD who underwent FMT and found that the difference in metabolites between the ASD and TD groups was reduced by 82-88% by 18 weeks, suggesting that FMT resulted in the metabolic profiles of the ASD group shifting in a direction to become more similar to those of the TD group. And according to a follow-up survey, after 2 years of FMT treatment, the diversity of gut flora in ASD patients remained stable and ASD symptoms continued to be relieved [105] , which suggests that FMT can be an effective method for long term treatment of ASD.
FMT
In addition to fecal microbial engraftment, changes in dietary structure and habits can affect the development and function of the central nervous system through the gut brain axis. Studies at [106] have shown that the ketogenic diet (KD) is effective in improving symptoms associated with ASD. KD refers to a high-fat, low-carbohydrate, and moderate-protein diet, in which approximately 90% of energy comes from fat, and 10% from carbohydrates and protein. KD has been found to be effective in improving ASD-related symptoms in both mouse and human trials [107,108]. The gut flora is a key mediator between diet and host physiology, and its species composition and function are influenced by diet, while the nutrients available to the host depend on microbial metabolism.KD can alter the abundance level of the intestinal flora, and Olson et al. [109]. found that KD significantly reduced the alpha diversity of the intestinal flora of mice, which implies that KD causes leads to the reduction of specific bacterial taxa. For the treatment of ASD, Newell et al. [107], found that KD ingestion produced an “antimicrobial”-like effect, significantly reducing the total abundance of host bacteria in the cecum and feces, and reversing the low ratio of Thick walled phyla to anaplasmatoid phyla in the cecum and feces, which is a common phenotype in autism spectrum disorders. Not only that, KD also improves nervous system energy metabolism through anti-inflammatory activity and anti-oxidative stress effects, while modulating neurotransmitter levels [110]. For example, Allan et al. [111], found that KD reduced plasma levels of pro inflammatory cytokines (IL-12p70, IL-1b) as well as brain derived neurotrophic factor (BDNF). They also observed changes in the gut microbiome and increased expression of butyric acid kinase in the gut, prompting more butyric acid to be produced and enter the bloodstream and brain. Although KD has a unique role in the treatment of neurological disorders, there are side effects, such as causing constipation, vomiting, and diarrhea [112], the most serious of which can inhibit physical development [113]. Fortunately, side effects from KD are uncommon. Although KD is not widely used in the treatment of ASD, its outstanding therapeutic effects suggest that it has the potential to become a new target and strategy for the treatment of ASD.In addition to KD, vitamin supplementation can also alleviate ASD symptoms. Vitamin A (VA) has an inducing effect on neuronal differentiation and neuroplasticity and also participates in the immune response, and a decrease in serum levels of VA causes a decrease in neurodevelopmental levels [114]. It was found [115] that retinoic acid, the active metabolite of VA, is involved in the proliferation and differentiation of CNS cells and regulates synaptic plasticity, and that reduced synthesis of retinol leads to the ASD phenotype. Yang et al. [116], showed that serum VA levels in children with ASD were significantly lower than those of normal children, especially boys. Liu et al. [117], demonstrated that supplementation with VA could significantly attenuate sodium valproate (VPA) induced ASD-related behaviors and restored the up regulation of NONRATT021475.2 (lncRNA) and Desert Hedgehog factor (Dhh) in the hippocampus of ASD rats. Not only can VA alleviate ASD symptoms, Javadfar et al [118], found that VD supplementation to individuals with ASD resulted in a significant decrease in serum 5-HT and IL-6 levels and an improvement in ASD symptoms. Long-term VD supplementation increased the degree of symptomatic improvement in children with ASD, with a particular improvement in language skills [119]. In conclusion, dietary therapy is of interest to patients and researchers as a relatively cost-effective treatment, but its therapeutic mechanisms as well as the side effects it produces are not yet fully understood, so further clinical studies are needed.
Probiotic supplementation
Probiotics are a group of active microorganisms that colonize the body and alter the composition of the flora of a particular part of the host in a way that is beneficial to the host. Probiotics promote homeostasis in the intestinal environment by competing for nutrients from pathogenic bacteria or by producing factors that promote an anti inflammatory immune cell phenotype. Studies have demonstrated that probiotic therapy can be effective in improving microbial abundance in patients. For example, administration of probiotic strain products resulted in a significant increase in the number of Bifidobacterium spp. and Lactobacillus spp. colonized in the gut of ASD patients, and a reduction in anxiety as well as an increase in deep sleep was observed in children with ASD [120]. Not only that, but probiotics have also been shown to improve intestinal inflammation. In a randomized trial, probiotics (Lactobacillus plantarum S128) in combination with oxytocin effectively improved the social behavior of patients with ASD, with a significant decrease in serum levels of the inflammatory markers S100B and IL-1β [121]. In addition, probiotic supplementation (including Lactobacillus, Bifidobacterium, and Streptococcus) significantly reduced fecal levels of TNF-α, which is associated with autism severity, in children with autism [122]. However, probiotics still face many challenges in the treatment of ASD. On the one hand, the selection of probiotic strains, the determination of dosage, and the optimal time of administration have not yet been standardized. On the other hand, although many studies have shown that probiotics are beneficial to ASD patients, the exact mechanism of action has not been fully clarified. Researchers have hypothesized that probiotics may play a role in regulating the balance of intestinal flora, improving the intestinal barrier function, regulating the immune system, and influencing the metabolism of neurotransmitters through a variety of pathways, but the interrelationships between the pathways and the specific molecular mechanisms need to be investigated in depth.
CONCLUSION
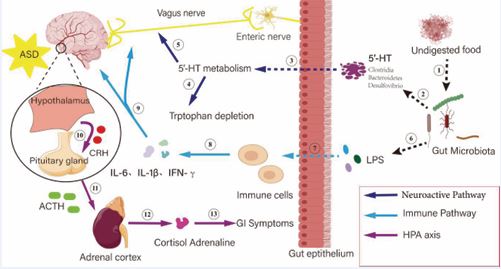
The gut-brain axis, as a bidirectional regulatory network connecting the intestine and the brain, plays a key role in the development of ASD in children, and the dysregulation of intestinal flora, abnormal metabolites, immune activation, and neurotransmitter disorders in patients with ASD suggest that dysfunction of the gut brain axis may be one of the core pathologic mechanisms. Imbalance of intestinal flora transmits signals to the brain through the vagus nerve and enteric nervous system, affecting the metabolism of neurotransmitters (e.g., 5-hydroxytryptophan, etc.) and tryptophan depletion; meanwhile, the increase of intestinal permeability leads to the entry of undigested food residues and bacterial endotoxins (e.g., LPS) into the circulatory system, which activates the immune cells and releases pro-inflammatory factors (IL-6, IL-1β, and IFN-γ), causing systemic inflammation and neuroinflammation. In addition, the metabolites of intestinal flora (e.g., short-chain fatty acids) regulate cortisol secretion through the HPA axis (hypothalamus-pituitary-adrenal axis), which exacerbates the stress response and abnormalities of central nervous system (Figure 2).
Figure 2 The core mechanism of the gut-brain axis in autism spectrum disorders (ASD). ? Undigested food causes intestinal flora disorders; ? intestinal flora disorders cause 5-HT metabolism abnormalities; ? intestinal flora synthesize 5-HT; ? 5-HT metabolism triggers tryptophan depletion; ? signals of intestinal metabolism abnormality are uploaded to the brain through the vagus nerve; ? intestinal flora releases LPS to stimulate the intestinal epithelium; ? LPS causes an increase of intestinal permeability and activation of immune cells; ? immune cells secrete pro-inflammatory factors; ? Induce neuroinflammation and activate the HPA axis; ? Hypothalamus secretes CRH and prompts the pituitary gland to release ACTH; ? ACTH drives the adrenal glands; ? adrenal glands secrete cortisol; ? adrenaline triggers gastrointestinal symptoms.
and ultimately creates a vicious cycle of “intestines→immunity→brain→endocrine→intestines”. These multiple interactions of the nervous, immune and endocrine systems together lead to pathological changes such as synaptic dysfunction and microglia activation, which are ultimately manifested in the social disorders and stereotyped behaviors of ASD. Repairing gut-brain axis functions (e.g., regulating flora, targeting metabolic pathways) may become a new direction for intervention in ASD. Although preliminary studies have been conducted to reveal the potential role of the gut-brain axis in ASD, existing interventions still face many challenges at the clinical application level. Although probiotics, dietary therapy and fecal microbial transplantation have shown some therapeutic effects, these interventions are still in the experimental stage, with small sample sizes, insufficient clinical trials, and poor reproducibility of efficacy needing to be addressed. Therefore, future research needs to focus on strengthening the mechanism of the gut-brain axis in ASD, especially how the gut microbiota precisely regulates the interaction between the nervous system and the immune system. In order to achieve effective treatment for ASD, not only is it necessary to deepen the understanding of the mechanisms of the gut-brain axis, but also to focus on the development of individualized treatment protocols. The gut microbiota and clinical manifestations of each ASD patient vary, and thus treatment strategies need to be customized to the individual. With the continuous development of gut microbiota regulation technology, the gut-brain axis, as an emerging area of ASD treatment, may provide patients with more precise and long-term effects in the future, thus improving the quality of life of ASD patients.
ACKNOWLEDGMENT
WAK and LJG designed the research study. DS, WAK, HLY, KYZ, SC and LFH searching and collating literature. DS and WAK drawing figures. DS, WAK wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
FUNDING
This research was supported by the Pre-research Project of National Natural Science Foundation of China (771200F00121) and TCM Clinical Research Program of Zhejiang Province (2025-54)
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- Hyman SL, Levy SE, Myers SM; COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORALPEDIATRICS. Identification, Evaluation, and Management of childrenwith Autism Spectrum Disorder. Pediatrics. 2020; 145: e20193447.
- Yan X, Li Y, Li Q, Li Q, Xu G, Lu J, et al. Prevalence of autism spectrum disorder among children and adolescents in the United States from 2021 to 2022. J Autism Dev Disord. 2024; 22.
- Zhou H, Xu X, Yan W, Zou X, Wu L, Luo X, et al. Prevalence of Autism Spectrum Disorder in china: A nationwide multi-center population- based study among children Aged 6 to 12 Years. Neurosci Bull. 2020; 36: 961-971.
- Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism Spectrum Disorder. Nat Rev Dis Primers. 2020; 6: 5.
- Blaxill M, Rogers T, Nevison C. Autism Tsunami: The impact of rising prevalence on the societal cost of autism in the United States. J Autism Dev Disord. 2022; 52: 2627-2643.
- Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, et al. Nutritional and metabolic status of children with autism vs. Neurotypical children, and the association with autism severity. Nutr Metab (Lond). 2011; 8: 34.
- Frye RE, Rossignol DA. Identification and treatment of pathophysiological comorbidities of Autism Spectrum Disorder to achieve optimal outcomes. Clin Med Insights Pediatr. 2016; 10: 43- 56.
- Srikantha P, Mohajeri MH. The possible role of the microbiota-gut- brain-axis in autism spectrum disorder. Int J Mol Sci. 2019; 20: 2115.
- Liu ZC, Wu D, Qu AN, Wang LL. Study on intestinal microbiota diversity and functional prediction analysis in children with Autism Spectrum Disorder. [Diversity and functional prediction of gut microbiota in children with autism spectrum disorder]. Zhongguo Dang Dai Er Ke Za Zhi. 2022; 24: 1356-1364.
- Dan Z, Mao X, Liu Q, Guo M, Zhuang Y, Liu Z, et al. Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes. 2020; 11: 1246-1267.
- Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008; 9: 1261- 9126.
- Li H, Guo W, Li S, Sun B, Li N, Xie D, et al. Alteration of the gut microbiota profile in children with Autism Spectrum Disorder in China. Front Microbiol. 2024; 14: 1326870.
- Ranjan R, Rani A, Metwally A, McGee HS, Perkins DL. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun. 2016; 469: 967-977.
- Zhang M, Chu Y, Meng Q, Ding R, Shi X, Wang Z, et al. A quasi-paired cohort strategy reveals the impaired detoxifying function of microbes in the gut of autistic children. Sci Adv. 2020; 6: eaba3760.
- Wang M, Wan J, Rong H, He F, Wang H, Zhou J, et al. Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with Autism Spectrum Disorder. mSystems. 2019; 4: e00321-18.
- Wang W, Fu P. Gut Microbiota Analysis and In Silico biomarker detection of children with autism spectrum disorder across cohorts. Microorganisms. 2023; 11: 291.
- Peralta-Marzal LN, Rojas-Velazquez D, Rigters D, Prince N, GarssenJ, Kraneveld AD, et al. A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Sci Rep. 2024; 14: 814.
- Wang W, Fu P. Gut Microbiota Analysis and In Silico biomarker detection of children with Autism Spectrum Disorder across cohorts. Microorganisms. 2023; 11: 291.
- Dan Z, Mao X, Liu Q, Guo M, Zhuang Y, Liu Z, et al. Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes. 2020;11: 1246-1267.
- Wan Y, Zuo T, Xu Z, Zhang F, Zhan H, Chan D, et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut. 2022; 71: 910-918.
- Yap CX, Henders AK, Alvares GA, Wood DLA, Krause L, Tyson GW, et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell. 2021; 184: 5916-5931.
- Jung Y, Lee T, Oh HS, Hyun Y, Song S, et al. Gut microbial and clinical characteristics of individuals with autism spectrum disorder differ depending on the ecological structure of the gut microbiome. Psychiatry Res. 2024; 335: 115775.
- Ding X, Xu Y, Zhang X, Zhang L, Duan G, Song C, et al. Gut microbiota changes in patients with autism spectrum disorders. J Psychiatr Res. 2020; 129: 149-159.
- Li H, Guo W, Li S, Sun B, Li N, Xie D, et al. Alteration of the gut microbiota profile in children with autism spectrum disorder in China. Front Microbiol. 2024; 14: 1326870.
- Kang DW, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, et al. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe. 2018; 49: 121-131.
- Wang M, Wan J, Rong H, He F, Wang H, Zhou J, et al. Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with Autism Spectrum Disorder. mSystems. 2019; 4: e00321-18.
- Zhang M, Chu Y, Meng Q, Ding R, Shi X, Wang Z, et al. A quasi-paired cohort strategy reveals the impaired detoxifying function of microbes in the gut of autistic children. Sci Adv. 2020; 6: eaba3760.
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015; 138: 179-187.
- Jendraszak M, Ga??cka M, Kotwicka M, Regdos A, Pazgrat-Patan M, Andrusiewicz M. Commercial microbiota test revealed differences in the composition of intestinal microorganisms between children with autism spectrum disorders and neurotypical peers. Sci Rep. 2021; 11: 24274.
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013; 4: 42.
- Wang Y, Li N, Yang JJ, Zhao DM, Chen B, Zhang GQ, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota- gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res. 2020; 157: 104784.
- Abuljadayel D, Alotibi A, Algothmi K, Basingab F, Alhazmi S, Almuhammadi A, et al. Gut microbiota of children with autism spectrum disorder and healthy siblings: A comparative study. Exp Ther Med. 2024; 28: 430.
- Pulikkan J, Maji A, Dhakan DB, Saxena R, Mohan B, Anto MM, et al.Gut Microbial Dysbiosis in Indian children with Autism Spectrum Disorders. Microb Ecol. 2018; 76: 1102-1114.
- Iovene MR, Bombace F, Maresca R, Sapone A, Iardino P, Picardi A, et al. Intestinal dysbiosis and yeast isolation in stool of subjects with Autism Spectrum Disorders. Mycopathologia. 2017; 182: 349-363.
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017; 5: 24.
- Chamtouri M, Merghni A, Miranda-Cadena K, Sakly N, Gaddour N, de Los Reyes-Gavilán CG, et al. Characterization of yeast isolated from the gut microbiota of Tunisian children with Autism Spectrum Disorder. J Fungi (Basel). 2024; 10: 730.
- Zou R, Wang Y, Duan M, Guo M, Zhang Q, Zheng H. Dysbiosis of Gut Fungal Microbiota in Children with Autism Spectrum Disorders. J Autism Dev Disord. 2021; 51: 267-275.
- Lou M, Cao A, Jin C, Mi K, Xiong X, Zeng Z, et al. Deviated and early unsustainable stunted development of gut microbiota in children with Autism Spectrum Disorder. Gut. 2022; 71: 1588-1599.
- Wan Y, Zuo T, Xu Z, Zhang F, Zhan H, Chan D, et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with Autism Spectrum Disorder. Gut. 2022; 71: 910-918.
- Kandeel WA, Meguid NA, Bjørklund G, Eid EM, Farid M, Mohamed SK, et al. Impact of clostridium bacteria in children with Autism Spectrum Disorder and Their Anthropometric Measurements. J Mol Neurosci. 2020; 70: 897-907.
- Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012; 5: 419-427.
- van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018; 596: 4923-4944.
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012; 57: 2096-2102.
- Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, et al. Altered gut microbiota and short chain fatty acids in Chinese children with Autism Spectrum Disorder. Sci Rep. 2019; 9: 287.
- Frye RE, Melnyk S, Macfabe DF. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in Autism Spectrum Disorder. Transl Psychiatry. 2013; 3: e220.
- Abdelli LS, Samsam A, Naser SA. Propionic acid induces gliosis and Neuro-inflammation through modulation of PTEN/AKT pathway in Autism Spectrum Disorder. sci Rep. 2019; 9: 8824.
- Kumar M, Mehan S, Kumar A, Sharma T, Khan Z, Tiwari A, et al. Therapeutic efficacy of Genistein in activation of neuronal AC/ cAMP/CREB/PKA and mitochondrial ETC-Complex pathways in experimental model of autism: Evidence from CSF, blood plasma and brain analysis. Brain Res. 2025; 1846: 149251.
- Guzmán-Salas S, Weber A, Malci A, Lin X, Herrera-Molina R, Cerpa W, et al. The metabolite p-cresol impairs dendritic development, synaptogenesis, and synapse function in hippocampal neurons: Implications for Autism Spectrum Disorder. J Neurochem. 2022; 161: 335-349.
- Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, HaneyJ, et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 2022; 602: 647-653.
- de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino Pet al. Alterations of the intestinal barrier in patients with Autism Spectrum Disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010; 51: 418-424.
- Piras C, Mussap M, Noto A, De Giacomo A, Cristofori F, Spada M, et al. Alterations of the intestinal permeability are reflected by changes in the Urine Metabolome of Young Autistic Children: Preliminary Results. Metabolites. 2022; 12: 104.
- Luo X, Yue B, Yu Z, Ren Y, Zhang J, Ren J, et al. Obacunone protects against ulcerative colitis in mice by modulating gut microbiota, Attenuating TLR4/NF-κB Signaling cascades, and improving disrupted epithelial barriers. Front Microbiol. 2020; 11: 497.
- Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000; 355:1518-1519.
- Nalbant K, Erden S, Yazar A, K?l?nç ?. Investigation of the relation between epithelial barrier function and autism symptom severity in children with Autism Spectrum Disorder. J Mol Neurosci. 2022; 72: 741-747.
- Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019; 9: 5790.
- Alonazi M, Ben Bacha A, Alharbi MG, Khayyat AIA, Al-Ayadhi L, El- Ansary A. Bee Pollen and probiotics’ potential to protect and treat intestinal permeability in propionic acid-induced rodent model of autism. Metabolites. 2023; 13: 548.
- Alharthi A, Alhazmi S, Alburae N, Bahieldin A. The Human gut microbiome as a potential factor in Autism Spectrum Disorder. Int J Mol Sci. 2022; 23: 1363.
- Alharthi A, Alhazmi S, Alburae N, Bahieldin A. The Human gut microbiome as a potential factor in Autism Spectrum Disorder. Int J Mol Sci. 2022; 23: 1363.
- Kushak RI, Buie TM, Murray KF, Newburg DS, Chen C, Nestoridi E, et al. Evaluation of intestinal function in children with autism and gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2016; 62: 687-691.
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011; 25: 40-45.
- Saad K, Abdallah AM, Abdel-Rahman AA, Al-Atram AA, Abdel-Raheem YF, Gad EF, Abo-Elela MGM, et al. Polymorphism of interleukin-1β and interleukin-1 receptor antagonist genes in children with Autism Spectrum Disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2020; 103: 109999.
- GU Yuhang, LIANG Dewu, QIN Xiaoliu, et al. Relationship between IL- 1β rs1143634 gene polymorphism, serum IL-1β levels and Autism Spectrum Disorders. Laboratory Medicine and Clinics,2024; 21: 2680-2684.
- Avolio E, Olivito I, Rosina E, Romano L, Angelone T, De Bartolo A, et al.Modifications of Behavior and Inflammation in Mice Following Transplant with Fecal Microbiota from Children with Autism. Neuroscience. 2022; 498: 174-189.
- Cerilli E, Dall’O GM, Chelini G, Catena B, Weinberger B, Bozzi Y, etal. Immune system dysfunction and inflammation in aging Shank3bmutant mice, a model of autism spectrum disorder. Front Immunol. 2024; 15: 1447385.
- Duarte-Campos JF, Vázquez-Moreno CN, Martínez-Marcial M, Chavarría A, Ramírez-Carreto RJ, Velasco Velázquez MA, et al. Changes in neuroinflammatory markers and microglial density in the hippocampus and prefrontal cortex of the C58/J mouse model of autism. Eur J Neurosci. 2024; 59: 154-173.
- Tetreault NA, Hakeem AY, Jiang S, Williams BA, Allman E, Wold BJ, et al. Microglia in the cerebral cortex in autism. J Autism Dev Disord. 2012; 42: 2569-2584.
- Deng Y, Ma L, Du Z, Ma H, Xia Y, Ping L, et al. The Notch1/Hes1 pathway regulates Neuregulin 1/ErbB4 and participates in microglial activation in rats with VPA-induced autism. Prog Neuropsychopharmacol Biol Psychiatry. 2024; 131: 110947.
- Salia S, Burke FF, Hinks ME, Randell AM, Matheson MA, Walling SG, et al. Gut microbiota transfer from the preclinical maternal immune activation model of autism is sufficient to induce sex-specific alterations in immune response and behavioural outcomes. Brain Behav Immun. 2025; 123: 813-823.
- Zhang HL, Hu S, Qu ST, Lv MD, Wang JJ, Liu XT, et al. Inhibition of NKCC1 Ameliorates Anxiety and Autistic Behaviors Induced by Maternal Immune Activation in Mice. Curr Issues Mol Biol. 2024; 46: 1851-1864.
- Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, et al. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front Cell Infect Microbiol. 2017; 7: 489.
- Dalile B, Vervliet B, Bergonzelli G, Verbeke K, Van Oudenhove L. Colon- delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial. Neuropsychopharmacology. 2020; 45: 2257-2266.
- Wang X, Sun Z, Yang T, Lin F, Ye S, Yan J, et al. Sodium butyrate facilitates CRHR2 expression to alleviate HPA axis hyperactivity in autism-like rats induced by prenatal lipopolysaccharides through histone deacetylase inhibition. mSystems. 2023; 8: e0041523.
- Gonzales J, Marchix J, Aymeric L, Le Berre-Scoul C, Zoppi J, Bordron P, et al. Fecal Supernatant from Adult with Autism Spectrum Disorder Alters Digestive Functions, Intestinal Epithelial Barrier, and Enteric Nervous System. Microorganisms. 2021; 9: 1723.
- Hosie S, Ellis M, Swaminathan M, Ramalhosa F, Seger GO, Balasuriya GK, et al. Gastrointestinal dysfunction in patients and mice expressing the autism-associated R451C mutation in neuroligin-3. Autism Res. 2019; 12: 1043-1056.
- Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019; 101: 246-259.e6.
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011; 108: 16050-16055.
- Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021; 13: 2099.
- Wang Y, Li N, Yang JJ, Zhao DM, Chen B, Zhang GQ, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota- gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res. 2020; 157: 104784.
- Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015; 29: 1395-1403.
- Tian P, Zou R, Wang L, Chen Y, Qian X, Zhao J, et al. Multi-Probiotics ameliorate Major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J Adv Res. 2023; 45: 117-125.
- Guo M, Zhu J, Yang T, Lai X, Liu X, Liu J, et al. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): A pilot study. Brain Res Bull. 2018; 137: 35-40.
- Tanaka M, Sato A, Kasai S, Hagino Y, Kotajima-Murakami H, Kashii H, et al. Brain hyperserotonemia causes autism-relevant social deficits in mice. Mol Autism. 2018; 9: 60.
- Guangyi Y ,Hongyan G ,Chun H .Targeting 5-HT as a Potential Treatment for Social Deficits in Autism.Neuroscience bulletin,2022; 38: 1263-1266 .
- Umesawa Y, Atsumi T, Chakrabarty M, Fukatsu R, Ide M. GABA Concentration in the Left Ventral Premotor Cortex Associates With Sensory Hyper-Responsiveness in Autism Spectrum Disorders Without Intellectual Disability. Front Neurosci. 2020; 14: 482.
- Fung LK, Flores RE, Gu M, Sun KL, James D, Schuck RK, et al. Thalamicand prefrontal GABA concentrations but not GABAA receptor densities are altered in high-functioning adults with autism spectrum disorder. Mol Psychiatry. 2021; 26: 1634-1646.
- Saleh MG, Bloy L, Blaskey L, Roberts TPL. GABA and glutamate measurements in temporal cortex of autistic children. Autism Res. 2024; 17: 2558-2571.
- Tabouy L, Getselter D, Ziv O, Karpuj M, Tabouy T, Lukic I, et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav Immun. 2018: 310-319.
- Hong T, Falcone C, Dufour B, Amina S, Castro RP, Regalado J, et al. GABAARα2 is Decreased in the Axon Initial Segment of Pyramidal Cells in Specific Areas of the Prefrontal Cortex in Autism. Neuroscience. 2020; 437: 76-86.
- Sánchez-Pérez S, Comas-Basté O, Duelo A, Veciana-Nogués MT, Berlanga M, Latorre-Moratalla ML, et al. Intestinal Dysbiosis in Patients with Histamine Intolerance. Nutrients. 2022; 14: 1774.
- De Palma G, Shimbori C, Reed DE, Yu Y, Rabbia V, Lu J, et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci Transl Med. 2022; 14: eabj1895.
- Rashaid AHB, Alqhazo MT, Nusair SD, Adams JB, Bashtawi MA, Al- Fawares O. Profiling plasma levels of thiamine and histamine in Jordanian children with autism spectrum disorder (ASD): potential biomarkers for evaluation of ASD therapies and diet. Nutr Neurosci. 2023; 26: 842-849.
- Fu Z, Yang X, Jiang Y, Mao X, Liu H, Yang Y, et al. Microbiota profiling reveals alteration of gut microbial neurotransmitters in a mouse model of autism-associated 16p11.2 microduplication. Front Microbiol. 2024; 15: 1331130.
- Wright C, Shin JH, Rajpurohit A, Deep-Soboslay A, Collado-Torres L, Brandon NJ, et al. Altered expression of histamine signaling genes in autism spectrum disorder. Transl Psychiatry. 2017; 7: e1126.
- Venkatachalam K, Eissa N, Awad MA, Jayaprakash P, Zhong S, Stölting F, et al. The histamine H3R and dopamine D2R/D3R antagonist ST- 713 ameliorates autism-like behavioral features in BTBR T+tf/J mice by multiple actions. Biomed Pharmacother. 2021; 138: 111517.
- Flik G, Folgering JH, Cremers TI, Westerink BH, Dremencov E. Interaction Between Brain Histamine and Serotonin, Norepinephrine, and Dopamine Systems: In Vivo Microdialysis and Electrophysiology Study. J Mol Neurosci. 2015; 56: 320-328.
- Dohnalová L, Lundgren P, Carty JRE, Goldstein N, Wenski SL, Nanudorn P, et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature. 2022; 612: 739-747.
- García-Domínguez I ,Suárez-Pereira I ,Santiago M , et al. Selective deletion of Caspase-3 gene in the dopaminergic system exhibits autistic-like behavior. Progress in Neuropsychopharmacology & Biological Psychiatry. 2021; 104: 110030.
- Rik SP, H FVV, Fee LOGD , et al. Striatal dopamine synthesis capacity in autism spectrum disorder and its relation with social defeat: an [18F]-FDOPA PET/ CT study[J].Translational Psychiatry.2021;11: 47.
- Watson DJ, Marsden CA, Millan MJ, Fone KC. Blockade of dopamine D? but not D? receptors reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int J Neuropsychopharmacol. 2012; 15: 471-484.
- Takeuchi S, Hida H, Uchida M, Naruse R, Yoshimi A, Kitagaki S, et al. Blonanserin ameliorates social deficit through dopamine-D3 receptor antagonism in mice administered phencyclidine as an animal model of schizophrenia. Neurochem Int. 2019; 128: 127-134.
- Dae-Wook K, BJA, CAG, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study.Microbiome. 2017; 5: 10.
- Chen K, Fu Y, Wang Y, Liao L, Xu H, Zhang A, et al. Therapeutic Effects of the In Vitro Cultured Human Gut Microbiota as Transplants on Altering Gut Microbiota and Improving Symptoms Associated with Autism Spectrum Disorder. Microb Ecol. 2020; 80: 475-486.
- Adams B J, Vargason T, Kang D, et al. Multivariate Analysis of Plasma Metabolites in Children with Autism Spectrum Disorder and Gastrointestinal Symptoms Before and After Microbiota Transfer Therapy. Processes. 2019; 7: 806.
- Fatir Q, James A, Kathryn H, et al. Multivariate Analysis of Fecal Metabolites from Children with Autism Spectrum Disorder and Gastrointestinal Symptoms before and after Microbiota Transfer Therapy.[J].Journal of personalized medicine.2020; 10: 152.
- Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019; 9: 5821.
- J.GCS, Silvie T. Ketogenic diets and Ketone suplementation: a strategy for therapeutic intervention. Frontiers in Nutrition. 2022.
- Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. 2016; 7: 37.
- Lee RWY, Corley MJ, Pang A, Arakaki G, Abbott L, Nishimoto M, et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol Behav. 2018; 188: 205-211.
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell. 2018; 173: 1728-1741.e13.
- Jiang Z, Yin X, Wang M, Chen T, Wang Y, Gao Z, et al. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022; 13: 1146-1165.
- Allan P N ,Yamamoto Y B ,Kunihiro P B , et al. Ketogenic Diet Induced Shifts in the Gut Microbiome Associated with Changes to Inflammatory Cytokines and Brain -Related miRNAs in Children with Autism Spectrum Disorder.Nutrients.2024; 16.
- Bertuccioli A, Cardinali M, Di Pierro F, Zonzini GB, Matera MR. Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota. Int J Mol Sci. 2022; 23: 8829.
- Li Q, Liang J, Fu N, Han Y, Qin J. A Ketogenic Diet and the Treatment of Autism Spectrum Disorder. Front Pediatr. 2021; 9: 650624.
- Hou N, Ren L, Gong M, Bi Y, Gu Y, Dong Z, et al. Vitamin A deficiency impairs spatial learning and memory: the mechanism of abnormal CBP-dependent histone acetylation regulated by retinoic acid receptor alpha. Mol Neurobiol. 2015; 51: 633-647.
- Xu X, Li C, Gao X, Xia K, Guo H, Li Y, et al. Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res. 2018; 28: 48-68.
- Yang T, Chen L, Dai Y, Jia F, Hao Y, Li L, et al. Vitamin A Status Is More Commonly Associated With Symptoms and Neurodevelopment in Boys With Autism Spectrum Disorders-A Multicenter Study in China. Front Nutr. 2022; 9: 851980.
- Liu Z, Wang J, Xu Q, Wu Z, You L, Hong Q, et al. Vitamin A supplementation ameliorates prenatal valproic acid-induced autism-like behaviors in rats. Neurotoxicology. 2022; 91: 155-165.
- Javadfar Z, Abdollahzad H, Moludi J, Rezaeian S, Amirian H, Foroughi AA, et al. Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: A randomized clinical trial. Nutrition. 2020; 79- 80: 110986.
- Lv N, Shang Q, Ma Caiyun, et al. Clinical study of vitamins A and D in the treatment of children with autism spectrum disorders. Clinical Research, 2021; 29: 55-57.
- Meguid NA, Mawgoud YIA, Bjørklund G, Mehanne NS, Anwar M, Effat BAE, et al. Molecular Characterization of Probiotics and Their Influence on Children with Autism Spectrum Disorder. Mol Neurobiol. 2022; 59: 6896-6902.
- Kong XJ, Liu J, Liu K, Koh M, Sherman H, Liu S, et al. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients. 2021; 13: 1552.
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015; 138: 179-187.