The Impact of DHA on Attentional Performance: A Series of Case Studies
- 1. Center for Reading, Pittsburg State University, USA
Abstract
Docosahexaenoic acid (DHA) is an omega-3 fatty acid vital for neurological development and function. Children with attention-deficit/hyperactivity disorder (ADHD) have been found to have low levels of blood plasma levels of DHA. The present series of case studies examined the potential of improving the attentional abilities of individuals with ADHD with the supplementation of DHA. The Test of Variables of Attention (TOVA), a psychometrically valid tool used to evaluate attentional abilities, was employed as an objective measure of attention to evaluate the effects of supplementation. The results indicated that DHA supplementation of 267mg b.i.d., 12 hours apart, was effective in improving the attentional abilities of individuals with ADHD.
Citation
Hurford DP, Fender AC (2026) The Impact of DHA on Attentional Performance: A Series of Case Studies. JSM Pediatr Neurol 6(1): 1022.
Keywords
• ADHD; Attentional Difficulties; Treatment; TOVA; DHA
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a clinical neurodevelopmental disorder involving difficulty with inattention, hyperactivity, and impulsivity, or a combination of these. It affects approximately 6% to 8% of youth around the world [1], and more than 10% of youth in the United States [2], including approximately 30% to 50% of individuals with specific learning disabilities such as dyslexia [3,4]. ADHD involves deficits in executive functioning, such as difficulty sustaining attention, planning, organizing, initiating tasks, completing tasks, managing time and/or materials, inhibiting responses, controlling emotions, being flexible, and metacognition. Over one-third of youth with ADHD have co-occurring oppositional defiant disorder [5], and ADHD can also co occur with other psychiatric and medical issues, such as behavior disorders [6], enuresis [7], and conduct disorder [8]. Relative to their peers, children with ADHD are more likely to experience emotional difficulties, such as anxiety [9], depression [10], and poor self-esteem [11]. ADHD has a significant impact on one’s life and the lives of those around them. Individuals with ADHD are more likely to engage in risky behaviors [12], which can result in vehicle accidents [13], use of illicit substances [14], and/or substance abuse, risk for pregnancy, and sexually-transmitted infections [15,16]. Individuals with ADHD also often experience difficulties with interpersonal skills and relationships [17]. They are less likely to apply to college [18], are more likely to experience difficulties in college when enrolled [19], and are less likely to graduate [20]. Adults with ADHD are more likely to be terminated from employment [18], divorce [21], and/or live with their parents [22]. ADHD is evident across academic, occupational, and interpersonal settings and can affect people throughout their youth and into adulthood [23]. Evaluation should include objective [24], and subjective assessments, as well as thorough clinical interviewing. Traditional strategies for ADHD management frequently include behavioral (e.g., environmental changes, accommodations, intensive skill instruction) and pharmaceutical intervention [25]. Medication has been used as a treatment for ADHD with relative success for some time [26]. Stimulant medications, such as methylphenidate and amphetamine formulations, as well as nonstimulant medications, such as norepinephrine reuptake inhibitors and alpha-agonists (clonidine and guanfacine), significantly improve ADHD symptoms [27] and, in combination with behavioral intervention, FDA approved medications are recommended as first-line treatment considerations for youth ages 6 to 18 years [28]. Unfortunately, many medications have side effects [29,30], such as suppressed appetite and adverse events [27]. As a result, many families are wary of using medications [31,32]. While families tend to initiate pharmaceutical intervention to support their child’s academic functioning, fewer parents demonstrate a preference for medication only (11%) than behavioral intervention only (28%), both interventions (25%), or neither (36%), and these goals and preferences are an important component in determining initiation of treatment [33]. In addition, pharmacological intervention is not recommended as a first-line treatment for children preschool-aged [34], or younger [28]. Further, medication is not recommended for youth with subclinical levels of inattention and/or impulsivity [28].
Recently, research of non-pharmacological interventions has increased [35]. Supplements have been considered for the treatment of ADHD, such as omega-3 fatty acids [36,37], particularly docosahexaenoic acid (DHA; [38]). DHA is an essential omega-3 fatty acid found in fatty fish such as salmon, which is crucial for brain development and function, especially in children [39]. Studies have found that children with ADHD have low plasma levels of DHA [40-42]. This suggests that a viable mechanism for treatment might include supplementation with DHA. Although this may be the case, the evidence has been equivocal (see [43], [27], and [37]’s meta-analyses). If effective, DHA supplementation has multiple benefits: essentially no side effects for children or adults [44], and is safe with little possibility of overdose [45].
Although DHA can be endogenously metabolized from its precursor alpha-linolenic acid (ALA), the conversion of ALA to DHA is not efficient. Therefore, the acquisition of DHA comes from consumption. Dietary supplementation may be suggested, particularly during fetal neurological development [46]. DHA is essential for maintaining cell wall functioning which assists inter-neuronal communication [47,48], and repair [49]. DHA is required for fetal brain development [50], hence the reason that there are high concentrations of DHA in breastmilk (32% by weight; [51]).
The neuronal mechanisms that are specifically affected by DHA involve receptor sites by helping to regulate ion channels, releasing neurotransmitters, and influencing synaptic plasticity (e.g., [52,53]). DHA modulates the sodium/potassium pump as a function of its role in regulating membrane function and structure [54]. Metabolites of DHA (e.g. docosanoids) have been implicated in the protection of neurons from oxidation stress (e.g., [55,56]).
Insufficient levels of DHA in a person’s diet potentially negatively affects neuronal functioning including those described above [57], and generally leads to cognitive and behavioral deficits that can last throughout the affected person’s life [58]. The suggested mechanism for describing the relationship between deficient DHA levels and suboptimal neurological functioning include deficient cell membrane efficiency which leads to a host of difficulties related associated with poor functional neurotransmission, and lower levels of glucose absorption in the brain, among other issues [59,60]. Insufficient DHA is related to an increased probability of cognitive decline associated with psychiatric disorders [61-64].
.The present series of case studies investigated the effectiveness of DHA supplementation as a strategy to assist with reducing symptomology of ADHD. To evaluate the potential effectiveness of DHA supplementation, pre- and post-treatment performance in the participants’ attentional performance was examined with the Test of Variables of Attention, Ninth Edition (TOVA-9). The TOVA has been used in a variety of studies to evaluate the potential effectiveness of treatment (e.g., [65-69]). The TOVA is a continuous performance test (CPT), an objective method of measuring attention and impulse control. It is primarily used to aid in the evaluation and diagnosis of ADHD and to evaluate the effectiveness of treatment applications. During administration of the TOVA, the individual holds a microswitch in their preferred hand and responds by depressing the microswitch with their thumb as quickly as possible every time the identified target is presented without sacrificing accuracy. When the non-target is presented, the individual is asked to refrain from responding. The target or non-target occurs every two seconds for the duration of the 22.6-minute administration. The target during the first half of the administration occurs infrequently (1:3.5 target-to nontarget ratio), while in the second half, the target density is high (3.5:1 target-to-nontarget ratio). The TOVA-9 provides five scores: omission and commission errors, response time and response time variability, and an overall Attention Comparison Score (ACS). Response time variability, a hallmark of ADHD [70-72], accounts for 80% of the variability between individuals with and without ADHD.
METHOD
Participants
Children. Three White male children were involved in the case studies. The initial participant was 8 years 4 months old. After the initial case study, two additional male children were included, aged 10 years 2 months and 11 years 8 months.
Adult. The adult participant was 28 years 7 months old and also a White male.
Measures
Test of Variables of Attention (TOVA). Version 9.0 of the Test of Variables of Attention (TOVA-9) was used to assess changes in attentional abilities prior to and after supplementation with DHA. It has acceptable psychometric properties [73]. Specific instructions regarding the nature of the task and the need to balance speed and accuracy is followed by a three-minute practice session, then test administration occurs. Corrective feedback is provided to participants if needed during the three-minute practice session, but not thereafter. The participant fixates on a small dot in the middle of the screen, and responds when the target appears (Figure 1). Both the target and non target are presented for 100 ms. The inter-target interval is 2 s. The ACS is a standard score in which the test-taker’s performance is compared to a group of individuals who participated in the standardization sample who were independently diagnosed with ADHD. Performance below 0 indicates that that test-taker likely has ADHD. The ACS was the TOVA variable assessed in this study.
Procedure
TOVA-9. The participants were assessed on the TOVA 9 in a quiet, dimly lit evaluation lab. The second time of administration was completed after the participant had received daily doses of DHA supplementation for six to eight weeks.
DHA. Participant 1 initially received 267mg q.d. in the morning. Subsequent to the second time of testing (Time 2), Participant 1 began taking 267mg b.i.d. twelve hours apart. All other participants received 267mg of DHA b.i.d. for their entire regimen. The source of the DHA was GNC’s Triple Strength Fish Oil (267mg DHA, 734mg EPA, 65mg other omega-3s), which has an enteric coating and can be purchased without a prescription.
RESULTS AND DISCUSSION
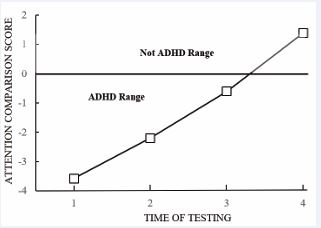
The present case studies were conducted to determine if daily supplementation of DHA would affect attentional performance. The first participant to receive the DHA supplementation improved his ACS from a –3.58 to a –2.2 (see Time 1 and 2 of Figure 2). The participant indicated that he felt that he could concentrate better, and others remarked that his attentional behavior had improved. A second administration of the DHA b.i.d. 12 hours after the first administration was added. As can be seen in Figure 2, Time 3, the participant’s performance on the TOVA further improved. Dosage was then stabilized at 267mg b.i.d. with no further changes. The participant’s Time 4 performance was in the “not ADHD” range (Figure 2). At this point, the participant continued his current regimen of DHA supplementation.
Figure 1 Stimuli used in the TOVA. Note. The white square on the left with the small black square at the top is the target. The square to the right is the nontarget. One or the other appears every 2 seconds during the administration of the TOVA.
Figure 2 Participant 1’s ACS by Time of Testing. Note. ACS = Attention Comparison Score from the TOVA. ACS are provided in z-score units. Scores below 0 are considered to be in the ADHD range.
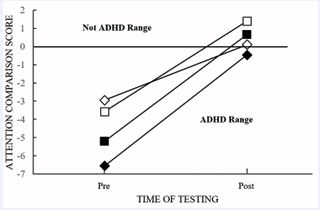
Due to the success of the DHA supplementation of the first participant, three additional participants were examined to determine if the results could be replicated. All participants received the same dosage of 267mg b.i.d. The first participant’s pre- and post-scores were included in Figure 3. Two of the participants who initially had significant challenges with attentional ability moved their performance into the “not ADHD” range, while the third was near the cutoff for the “not ADHD” range (Figure 3). A related t-test confirmed that the ACS were significantly better post-supplementation, t(3) = 6.8, p = .007, indicating ipsative and normative improvement, supporting the efficaciousness of DHA supplementation on an objective measure of attention.
Figure 3 Participants’ ACS by Time of Testing. Note. ACS = the black diamond shape indicates the performance of the adult white male.
CONCLUSIONS
The present study examined the potential remedial effects of daily DHA supplementation. All participants (all White males) performed significantly better after taking 267mg of DHA b.i.d. via supplementation. A more thorough examination of DHA is required that includes a more diverse population and randomized controlled trials. In addition to objective assessment via psychometrically adequate CPT, future studies should include a comparison of multiple interventions, blinded raters of functional behavioral improvement [36], and a placebo control. However, the current case studies suggest that DHA might have potential use in the treatment of ADHD that warrants further investigation.
REFERENCES
- Salari N, Ghasemi H, Abdoli N, Rahmani A, Shiri MH, Hashemian AH, et al. The global prevalence of ADHD in children and adolescents: a systematic review and meta-analysis. Ital J Pediatr. 2023; 49: 48.
- Danielson ML, Claussen AH, Bitsko RH, Katz SM, Newsome K, Blumberg SJ, et al. ADHD Prevalence Among U.S. Children and Adolescents in 2022: Diagnosis, Severity, Co-Occurring Disorders, and Treatment. J Clin Child Adolesc Psychol. 2024; 53: 343-360.
- Daucourt MC, Erbeli F, Little CW, Haughbrook R, Hart SA. A Meta- Analytical Review of the Genetic and Environmental Correlations between Reading and Attention-Deficit Hyperactivity Disorder Symptoms and Reading and Math. Sci Stud Read. 2020; 24: 23-56.
- Willcutt EG, Pennington BF, DeFries JC. Twin study of the etiology of comorbidity between reading disability and attention-deficit/ hyperactivity disorder. Am J Med Genet. 2000; 96: 293-301.
- Harvey EA, Breaux RP, Lugo-Candelas CI. Early development of comorbidity between symptoms of attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD). J Abnorm Psychol. 2016; 125: 154-167.
- Austerman J. ADHD and behavioral disorders: Assessment, management, and an update from DSM-5. Cleve Clin J Med. 2015; 82: S2-S7.
- de Sena Oliveira AC, Athanasio BDS, Mrad FCC, Vasconcelos MMA, Albuquerque MR, Miranda DM, et al. Attention deficit and hyperactivity disorder and nocturnal enuresis co-occurrence in the pediatric population: a systematic review and meta-analysis. Pediatr Nephrol. 2021; 36: 3547-3559.
- Njardvik U, Wergeland GJ, Riise EN, Hannesdottir DK, Öst LG. Psychiatric comorbidity in children and adolescents with ADHD: A systematic review and meta-analysis. Clin Psychol Rev. 2025; 118: 102571.
- Sciberras E, Lycett K, Efron D, Mensah F, Gerner B, Hiscock H. Anxiety in children with attention-deficit/hyperactivity disorder. Pediatrics. 2014; 133: 801-808.
- Riglin L, Leppert B, Dardani C, Thapar AK, Rice F, O’Donovan MC, et al. ADHD and depression: investigating a causal explanation. Psychol Med. 2021; 51: 1890-1897.
- Pedersen AB, Edvardsen BV, Messina SM, Volden MR, Weyandt LL, Lundervold AJ. Self-Esteem in Adults With ADHD Using the Rosenberg Self-Esteem Scale: A Systematic Review. J Atten Disord. 2024; 28: 1124-1138.
- Shoham R, Sonuga-Barke E, Yaniv I, Pollak Y. ADHD Is Associated With a Widespread Pattern of Risky Behavior Across Activity Domains. J Atten Disord. 2021; 25: 989-1000.
- Brunkhorst-Kanaan N, Libutzki B, Reif A, Larsson H, McNeill RV, Kittel-Schneider S. ADHD and accidents over the life span - A systematic review. Neurosci Biobehav Rev. 2021; 125: 582-591.
- London AS, Antshel KM, Grove J, Gutin I, Monnat SM. Self-Reported ADHD Diagnosis and Illicit Drug Use and Prescription Medication Misuse Among U.S. Working-Age Adults. J Atten Disord. 2025; 29: 1355-1366.
- Chen MH, Hsu JW, Huang KL, Bai YM, Ko NY, Su TP, et al. Sexually Transmitted Infection Among Adolescents and Young Adults With Attention-Deficit/Hyperactivity Disorder: A Nationwide Longitudinal Study. J Am Acad Child Adolesc Psychiatry. 2018; 57: 48-53.
- Soldati L, Deiber MP, Schockaert P, Köhl J, Bolmont M, Hasler R, et al. Sexually Transmitted Diseases and Attention-Deficit/Hyperactivity Disorder: A Systematic Literature Review. J Psychiatr Pract. 2024; 30: 259-265.
- Kathju A. ADHD and its impact on interpersonal relationships. In New Developments in Diagnosing, Assessing, and Treating ADHD. IGI Global. 2021: 179-195.
- Kuriyan AB, Pelham WE Jr, Molina BS, Waschbusch DA, Gnagy EM, Sibley MH, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013; 41: 27-41.
- Gormley MJ, DuPaul GJ, Weyandt LL, Anastopoulos AD. First-Year GPA and Academic Service Use Among College Students With and Without ADHD. J Atten Disord. 2019; 23: 1766-1779.
- DuPaul GJ, Gormley MJ, Anastopoulos AD, Weyandt LL, Labban J, Sass AJ, et al. Academic Trajectories of College Students with and without ADHD: Predictors of Four-Year Outcomes. J Clin Child Adolesc Psychol. 2021; 50: 828-843.
- Bouchard G, Saint-Aubin J. Attention deficits and divorce. The Canadian Journal of Psychiatry. 2014; 59: 480-486.
- Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. Guilford Press. 2010.
- Jiménez-Muñoz L, Lopez-Fernandez O, Peñuelas-Calvo I, Delgado- Gómez D, Miguélez-Fernández C, López-González S, et al. Persistence of ADHD into adulthood and associated factors: A prospective study. Psiquiatría Biológica. 2025; 32: 100529.
- Soto EF, Kofler MJ, Singh LJ, Wells EL, Irwin LN, Groves NB, et al. Executive functioning rating scales: Ecologically valid or construct invalid? Neuropsychology. 2020; 34: 605-619.
- Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Macías Saint-Gerons D, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS One. 2017; 12: e0180355.
- Faraone SV, Newcorn JH, Cipriani A, Brandeis D, Kaiser A, Hohmann S, et al. Placebo and nocebo responses in randomised, controlled trials of medications for ADHD: a systematic review and meta-analysis. Mol Psychiatry. 2022; 27: 212-219.
- Peterson BS, Trampush J, Maglione M, Bolshakova M, Rozelle M, Miles J, et al. Treatments for ADHD in Children and Adolescents: A Systematic Review. Pediatrics. 2024; 153: e2024065787.
- Wolraich ML, Hagan JF Jr, Allan C, Chan E, Davison D, Earls M, et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019; 144: e20192528.
- Bannett Y, Gunturkun F, Pillai M, Herrmann JE, Luo I, Huffman LC, et al. Applying Large Language Models to Assess Quality of Care: Monitoring ADHD Medication Side Effects. Pediatrics. 2025; 155: e2024067223.
- Schein J, Cloutier M, Gauthier-Loiselle M, Bungay R, Guerin A, ChildressA. Symptoms associated with ADHD/treatment-related adverse side effects and their impact on quality of life and work productivity in adults with ADHD. Curr Med Res Opin. 2023; 39: 149-159.
- Coletti DJ, Pappadopulos E, Katsiotas NJ, Berest A, Jensen PS, Kafantaris V. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012; 22: 226-237.
- McKenna K, Wanni Arachchige Dona S, Gold L, Dew A, Le HND. Barriers and Enablers of Service Access and Utilization for Children and Adolescents With Attention Deficit Hyperactivity Disorder: A Systematic Review. J Atten Disord. 2024; 28: 259-278.
- Fiks AG, Mayne S, Debartolo E, Power TJ, Guevara JP. Parental preferences and goals regarding ADHD treatment. Pediatrics. 2013; 132: 692-702.
- Mechler K, Banaschewski T, Hohmann S, Häge A. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022; 230: 107940.
- Nazarova VA, Sokolov AV, Chubarev VN, Tarasov VV, Schiöth HB. Treatment of ADHD: Drugs, psychological therapies, devices, complementary and alternative methods as well as the trends in clinical trials. Front Pharmacol. 2022; 13: 1066988.
- Händel MN, Rohde JF, Rimestad ML, Bandak E, Birkefoss K, Tendal B, et al. Efficacy and Safety of Polyunsaturated Fatty Acids Supplementation in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients. 2021; 13: 1226.
- Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013; 170: 275-289.
- Hassanzadeh Mobini M, Boileau AJ. Omega-3 Polyunsaturated Fatty Acid Supplementation in Children with Attention-Deficit Hyperactivity Disorder (ADHD). Cureus. 2025; 17: e93175.
- Basak S, Duttaroy AK. Maternal PUFAs, Placental Epigenetics, and Their Relevance to Fetal Growth and Brain Development. Reprod Sci. 2023; 30: 408-427.
- Germano M, Meleleo D, Montorfano G, Adorni L, Negroni M, Berra B, et al. Plasma, red blood cells phospholipids and clinical evaluation after long chain omega-3 supplementation in children with attention deficit hyperactivity disorder (ADHD). Nutr Neurosci. 2007; 10: 1-9.
- Johnson M, Månsson JE, Ostlund S, Fransson G, Areskoug B, Hjalmarsson K, et al. Fatty acids in ADHD: plasma profiles in a placebo-controlled study of Omega 3/6 fatty acids in children and adolescents. Atten Defic Hyperact Disord. 2012; 4: 199-204.
- Miklavcic JJ, Ivity E, MacDonald IM, Urichuk L, Mazurak VC, Rinaldi C, et al. AA and DHA are decreased in paediatric AD/HD and inattention is ameliorated by increased plasma DHA. Human Nutrition & Metabolism. 2023; 31: 200183.
- Gillies D, Leach MJ, Perez Algorta G. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2023; 4: CD007986.
- Lien EL. Toxicology and safety of DHA. Prostaglandins Leukot Essent Fatty Acids. 2009; 81: 125-132.
- Lewis KD, Huang W, Zheng X, Jiang Y, Feldman RS, Falk MC. Toxicological evaluation of arachidonic acid (ARA)-rich oil and docosahexaenoic acid (DHA)-rich oil. Food Chem Toxicol. 2016; 96: 133-144.
- Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013; 97: 808-815.
- Muth AK, Park SQ. The impact of dietary macronutrient intake on cognitive function and the brain. Clin Nutr. 2021; 40: 3999-4010.
- Li X, Zhou S, Lin X. Molecular View on the Impact of DHA Molecules on the Physical Properties of a Model Cell Membrane. J Chem Inf Model. 2022; 62: 2421-2431.
- Lin L, Zheng S, Lai J, Ye D, Huang Q, Wu Z, et al. Omega-3 Polyunsaturated Fatty Acids Protect Neurological Function After Traumatic Brain Injury by Suppressing Microglial Transformation to the Proinflammatory Phenotype and Activating Exosomal NGF/TrkA Signaling. Mol Neurobiol. 2023; 60: 5592-5606.
- Greenberg JA, Bell SJ, Ausdal WV. Omega-3 Fatty Acid supplementationduring pregnancy. Rev Obstet Gynecol. 2008; 1: 162-169.
- Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007; 85: 1457- 1464.
- Cordero-Morales JF, Vásquez V. How lipids contribute to ion channel function, a fat perspective on direct and indirect interactions. Curr Opin Struct Biol. 2018; 51: 92-98.
- He Z, Xiong W, Yang Y, Zhang Y, Li B, Wang F, et al. Lipidomics Analysis Reveals the Effects of Docosahexaenoic Acid from Different Sources on Prefrontal-Cortex Synaptic Plasticity. Nutrients. 2025; 17: 457.
- Hishikawa D, Valentine WJ, Iizuka-Hishikawa Y, Shindou H, ShimizuT. Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS Lett. 2017; 591: 2730-2744.
- Bazan NG. Synaptic signaling by lipids in the life and death of neurons. Molecular neurobiology. 2005; 31: 219-230.
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004; 101: 8491-8496.
- Cabal-Herrera A, Kigen B, Kapanga E, Samia A, Nabwera H, SamiaP. The impact of undernutrition and overnutrition on early brain development. Semin Pediatr Neurol. 2025; 55: 101212.
- Evbuomwan SA, Omotosho OE, Mgbojikwe I. Roles and mechanisms of docosahexaenoic acid (DHA) in neurodevelopment, neuronal functions, learning and memory. World News of Natural Sciences. 2022; 40: 104-119.
- Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012; 590: 2485-2499.
- Sinclair AJ. Docosahexaenoic acid and the brain- what is its role? Asia Pac J Clin Nutr. 2019; 28: 675-688.
- Chu CS, Hung CF, Ponnusamy VK, Chen KC, Chen NC. Higher Serum DHA and Slower Cognitive Decline in Patients with Alzheimer’s Disease: Two-Year Follow-Up. Nutrients. 2022; 14: 1159.
- Deshmukh GV, Niaz H, Bai R, Kim DH, Kim JW, Asghar J, et al. The Role of Omega-3 Fatty Acid Supplementation in Slowing Cognitive Decline Among Elderly Patients With Alzheimer’s Disease: A Systematic Review of Randomized Controlled Trials. Cureus. 2024; 16: e73390.
- Hennebelle M, Plourde M, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P, Cunnane SC. Ageing and apoE change DHA homeostasis: relevance to age-related cognitive decline. Proc Nutr Soc. 2014; 73: 80-86.
- Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LB, Ciappolino V, Agostoni C. DHA Effects in Brain Development and Function. Nutrients. 2016; 8: 6.
- Bangun SR, Putra BS, Atmojo WT, Sevriana ES, Hanifa YNM, Pangestuti RCA. Play therapy efficacy in ADHD-symptom reduction as measured by the Test of Variables of Attention (TOVA). J Child Adolesc Ment Health. 2023; 35: 118-128.
- Rodrigo Jiménez D, Foguet-Boreu Q, Juvanteny EP, Izquierdo Munuera E. Effectiveness of a psychoeducational group intervention developed by primary care nurses on symptom control of pediatric patients with ADHD. ADHD parent study. Health Psychol Behav Med. 2022; 10: 1176-1189.
- Khateri A, Afshar M. The efficacy and implementation of AKL-T01digital therapeutic in management of attention-deficit/hyperactivity disorder (ADHD) in children: A rapid review of recent evidence: AKL-T01: Efficacy and implementation in ADHD. Chronic Diseases Journal. 2025: 180-189.
- Kirk HE, Richmond S, Gaunson T, Bennett M, Herschtal A, Bellgrove M, et al. A 5-week Digital Intervention to Reduce Attention Problems in Children with ADHD: A Double-Blind Randomized Controlled Trial. J Atten Disord. 2024; 28: 1454-1466.
- Ritfeld GJ, Kable JA, Holton JE, Coles CD. Effectiveness of Psychotropic Medications in Children with Prenatal Alcohol and Drug Exposures: A Case Series and Model of Care. Child Psychiatry Hum Dev. 2024; 55: 744-753.
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, et al. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psychol Rev. 2013; 33: 795-811.
- Moses M, Tiego J, Demontis D, Bragi Walters G, Stefansson H, Stefansson K, et al. Working memory and reaction time variability mediate the relationship between polygenic risk and ADHD traits in a general population sample. Mol Psychiatry. 2022; 27: 5028-5037.
- Wiker T, Norbom LB, Beck D, Agartz I, Andreassen OA, Alnæs D, et al. Reaction Time Variability in Children Is Specifically Associated With Attention Problems and Regional White Matter Microstructure. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023; 8: 832-840.
- Shaw S. Review of the Test of Variables of Attention (9th Edition). In Carlson JF, Geisinger KF, Jonson JL (eds.), The twenty-first mental measurements yearbook. Buros Center for Testing. 2021: 803-806.