A 7-Year Assessment of Clinical Outcomes of Periosteum Derived Autologous Micrografts with PLGA in Maxillary Sinus Lift Procedures: An Observational Study
- 1. Department of Clinical Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Italy
Citation
Todaro C, Virgi N (2025) A 7-Year Assessment of Clinical Outcomes of Periosteum-Derived Autologous Micrografts with PLGA in Maxillary Sinus Lift Procedures: An Observational Study. JSM Regen Med Bio Eng 7(1): 1024
INTRODUCTION
Tooth loss is a condition that significantly affects quality of life, with impacts on aesthetics, nutrition, and general well-being. The primary causes are dental caries and periodontal disease [1]. Addressing this issue requires increasingly efficient, long-lasting, and prompt treatments. However, before placing a dental implant, it is essential to ensure adequate bone volume. If insufficient, bone regeneration must precede implant placement. Over the years, several techniques have been introduced, including pterygoid implants, bone grafting, and sinus floor augmentation [2].
The sinus lift technique, introduced by Boyne and James in 1980 using autologous bone grafts, was later re fined in 1986 by Tatum with the development of a lateral approach. Today, sinus augmentation can be categorized into two primary techniques.
The first, known as the Summer’s technique, involves the use of osteotomes to perform controlled fractures of the maxillary sinus floor, followed by graft material placement. This method creates space by elevating the sinus membrane and filling the cavity with graft material. It is less invasive and reduces surgical time but is suitable only when the residual bone height is not less than 4-6 mm.
The second approach, the lateral window technique, is preferred when the residual bone height is below 6 mm. This more invasive method involves creating a lateral bone window and elevating the Schneiderian membrane. The resulting cavity is then filled with natural or synthetic graft materials [2].
Each of these procedures requires grafting materials capable of supporting and promoting osseointegration, osteoconduction, osteogenesis, and osteoinduction. Available materials are commonly classified into five groups:
• Grafts of natural origin (autografts, allografts, xenografts)
• Synthetic substitutes (e.g., hydroxyapatite-based, polymeric, or metallic materials)
• Composite bone substitutes
• Substitutes containing viable osteogenic cells
• Substitutes enriched with growth factors
There is a growing demand for high-performance materials in dental applications [3-6]. Current trends suggest that hybrid materials-comprising scaffolds combined with biological components-represent the future of dental bone tissue engineering [7]. These materials often incorporate mesenchymal stem cells and/or growth factors [8].
In this observational follow-up study [3], the biological component consisted of autologous and homologous periosteum-derived micrografts obtained via Rigenera® technology. This class II medical device is already widely used across regenerative fields including dentistry, orthopedics, dermatology, wound healing, and cardiology [9,12]
In a previous study, Rodriguez et al., demonstrated that autologous periosteum-derived micrografts combined with PLGA and hydroxyapatite (HA), enhanced bone formation in sinus augmentation procedures. Radiographic and histological data supported the conclusion that micrografts improved outcomes compared to PLGA/HA alone [10].
This study therefore seeks to add to the evidence base by evaluating the efficacy and long-term stability (up to 7 years) of autologous micrografts combined with PLGA, assessed through radiographic and histological analyses.
PRESENTATION OF CASE
Patients
This retrospective observational study included patients requiring bone regeneration and sinus augmentation to facilitate dental implant placement. The treatment protocol involved the application of Rigenera® micro-grafting technology in combination with Fisiograft® (Ghimas, Casalecchio di Reno (BO), Italy). The study received ethical approval from the Ethics Committee of the University of Pavia.
A total of five patients (two males and three females), aged between 36 and 71 years, were enrolled. All patients were in good systemic health (classified as ASA I or II), with no systemic conditions or ongoing treatments that could interfere with osseointegration. One week before surgery, all patients underwent professional dental hygiene treatment and were instructed to maintain oral hygiene at home using 0.2% chlorhexidine mouthwash after tooth brushing twice daily. Informed consent was obtained from each participant after they were fully briefed on the nature of the study. Follow-up visits were scheduled periodically for up to 8 years postoperatively. During these visits, standard maintenance protocols were applied when necessary [13,15].
Autologous Micrografts
Autologous micrografts (AMGs) were prepared using the Rigeneracons medical device, which generates a suspension of tissue fragments approximately 80 µm in size [12]. These fragments consist of progenitor cells preserved within their native extracellular matrix, enriched with growth factors. The Rigeneracons device comprises a grid, a helix, and a reservoir. The helix presses the tissue sample onto the grid, which features 100 holes, each equipped with six micro-blades that mechanically disaggregate the tissue.
The system operates on a size-exclusion principle: only fragments of 80 µm or smaller pass through the grid and are collected in the reservoir below, while larger fragments remain on the surface. The disaggregation requires the addition of 1 ml of saline solution (0.9% NaCl), through a dedicated port [11,15].
Using a dental surgical motor and a specific adapter, the sample is disaggregated at 70 rpm with 15 N·cm torque for 3 minutes [10]. The resulting micrograft suspension is then aspirated with a syringe and used to soak the selected grafting material, in this case Fisiograft.
Scaffold Composition
Successful bone regeneration and osseointegration depend on multiple factors, including the selection of an appropriate scaffold material. An ideal material should possess mechanical properties-such as stiffness, elasticity, and porosity-closely resembling those of natural bone to minimize stress shielding and prevent further bone loss. Furthermore, the scaffold should replicate the bone’s physiological architecture to support both osteogenesis and angiogenesis. It must allow the formation of a vascular network to ensure the diffusion of nutrients, oxygen, growth factors, and minerals.
In this study, the scaffold used was PLGA (Fisiograft),a synthetic, biocompatible, and biodegradable polymer widely applied in tissue engineering and drug delivery. One of PLGA’s key advantages is its tunable degradation rate, determined by molecular weight and the lactic/ glycolic acid ratio. Fisiograft is an alloplastic material composed of a 50:50 PLA/PGA ratio, which degrades more rapidly than 70:30 or 85:15 compositions, and it was used here in granular form.
Numerous studies have confirmed its clinical efficacy in procedures such as sinus augmentation, socket preservation, and the treatment of peri-implant and periodontal defects, as well as bone fenestrations and dehiscence.
Surgery and Micrografting Procedure
Before local anesthesia (Articaine 4% with 1:200,000 epinephrine) was administered, all patients received antibiotic prophylaxis with 2 g of amoxicillin
The sinus lift procedures were performed using the lateral window technique. After flap elevation, piezosurgery (Mectron, Carasco GE, Italy) with an OT5 insert was used under submerged healing conditions. Periosteum derived micrografts were harvested from either the deep palatal mucosa or the alveolar periosteum using a scalpel or biopsy punch. The tissue was then disaggregated with the Rigeneracons device using 1 ml of saline solution. The micrograft solution was used to saturate the granulated PLGA (Fisiograft), which was then used to fill the sinus cavity. The surgical site was closed with single sutures.
Postoperative care included a 7-day course of antibiotics (amoxicillin/clavulanic acid 1 g every 12 hours) and 0.2% chlorhexidine rinses twice daily for four weeks. Sutures were removed two weeks after surgery
Radiographic Evaluation
All patients underwent cone-beam CT scans prior to the sinus lift and implant placement surgeries. Follow-up radiographic evaluations were performed using periapical endoral radiographs for up to 7 years after surgery.
Histological Analysis
Five months after sinus augmentation, dental implants were placed. At that time, bone biopsies (3 mm in diameter) were collected using a trephine bur for histological examination. Samples were fixed in 10% for-malin, decalcified using EDTA (Osteodec; Bio-Optica, Milan, Italy), and embedded in paraffin.
Sections 5 µm thick were prepared with a microtome (Leica Biosystem, Milan, Italy) and mounted on coated slides. Deparaffinization was performed using xylene, followed by graded ethanol washes and rehydration. Hematoxylin-eosin staining was then applied to assess new bone formation.
RESULTS
Clinical Results
In all cases, periodontal probing depths remained within physiological limits (≤ 5 mm), with no clinical signs of inflammation such as erythema or swelling, and no bleeding on probing was observed [16]. The implant survival and success rate was 100% across all patients. Figure 1 illustrates the radiographic progression in one of the patients treated in this observational study.
Figure 1 Radiographic follow-up over 7 years in a patient treated with autologous periosteum-derived micrografts and PLGA. (a) Preoperative radiograph. (b) Radiograph at 5 months post-implant placement following sinus augmentation. (c) Radiograph at 7 years postoperatively.
Radiographic follow-up over 7 years in a patient treated with autologous periosteum-derived micrografts and PLGA. (a) Preoperative radiograph. (b) Radiograph at 5 months post-implant placement following sinus augmentation. (c) Radiograph at 7 years postoperatively (Figure 2).
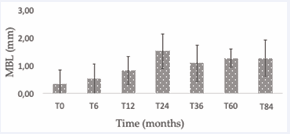
Figure 2 Graphically displays these data
Additionally, marginal bone loss (MBL) remained below 1.5 mm throughout the observation period. Table 1 summarizes the average MBL values and standard deviations across different timepoints.
Table 1: Average in millimeters of MBL and standard deviation (SD).
|
|
T0 |
T6 |
T12 |
T24 |
T36 |
T60 |
T84 |
|
Mean |
0,35 |
0,55 |
0,83 |
1,49 |
1,10 |
1,28 |
1,28 |
|
SD |
0,49 |
0,52 |
0,49 |
0,61 |
0,65 |
0,32 |
0,66 |
Histological Results
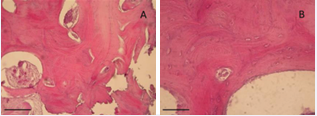
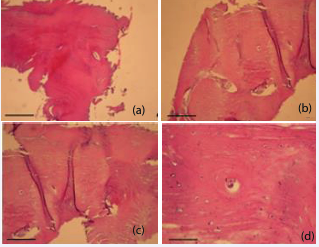
Histological analyses were performed at 3 and 7 months after sinus augmentation. Hematoxylin and eosin (H&E) staining (Figures 3 and 4), confirmed that the combination of micrografts with the PLGA scaffold supported bone formation.
At 3 months (Figure 3), lamellar bone structures were already visible at 10x and 20x magnification. The presence of calcified matrix and a developing Haversian system suggested early ossification.
Figure 3 Histological evaluation 3 months after surgery with Rigenera® technology and PLGA. (a) 10X magnification. (b) 20X magnification. Scale bar: 50 µm
At 7 months (Figure 4), histological sections demonstrated more mature bone features, including well-organized lamellae and osteocytic lacunae uniformly distributed and containing cells with osteo-blast-like morphology.
Figure 4 Histological evaluation 7 months after surgery with Rigenera® technology and PLGA. (a) 4X magnification. (b) 10X magnification. (c) 20X magnification. (d) 40X magnification. Scale bar: 50 µm.
DISCUSSION
This study presents the long-term (up to 7 years) clinical and histological outcomes of autologous micrografts (AMGs) used in sinus lift procedures for bone regeneration in humans. Throughout the entire follow-up period, no adverse events or complications attributable to the use of AMGs combined with PLGA were re-ported, indicating a favorable safety profile and long-term biocompatibility.
These findings expand upon previously published results from the same patient cohort with a six-month follow-up. Earlier preclinical investigations, including both in vitro experiments and in vivo murine models, have demonstrated the regenerative potential of AMGs in bone tissue engineering applications [17,18]. Additionally, a case report with a 3-year follow-up had already suggested the effectiveness of this protocol in a single patient [9]. The current study provides further support by documenting consistent outcomes across multiple patients over an extended observation period.
The evaluation of marginal bone loss (MBL) revealed values within the range considered physiologically acceptable by the existing literature. Adell et al., reported a mean MBL of 1.5 mm in the first year and a subsequent annual loss of 0.1 mm [19]. Similarly, Albrektsson et al., proposed a long-term physiological threshold of ≤ 0.2 mm/ year following the first year of functional loading [20]. In our study, the MBL measurements remained consistently below 1.5 mm over the full follow-up period, suggesting stable peri-implant bone conditions and successful osseointegration.
Thus, the protocol described, combining autologous micrografts with PLGA-can be considered a reliable and safe option for bone regeneration in sinus augmentation procedures. A major strength of this study lies in the long term follow-up and the integration of clinical, radiographic, and histological data.
Nevertheless, one limitation must be acknowledged: the absence of a control group treated with PLGA alone or alternative grafting materials. Future randomized controlled trials comparing different regenerative proto cols will be necessary to further validate these outcomes and strengthen the clinical evidence.
Despite this limitation, the consistency of results across all patients, together with the absence of complications and the maintenance of implant stability, strongly supports the use of autologous micrografts in clinical practice for bone regeneration procedures requiring long-term success.
CONCLUSIONS
The results of this long-term observational study demonstrate that the use of autologous periosteum derived micrografts in combination with PLGA for sinus lift augmentation is a safe and effective technique for bone regeneration in implant dentistry. Radiographic and histological data confirmed the stability of regenerated bone tissue and the maintenance of implant success over a follow-up period of up to seven years.
The absence of adverse events and the physiological levels of marginal bone loss observed throughout the follow-up further support the biocompatibility and reliability of this approach. These findings suggest that this regenerative strategy represents a predictable clinical protocol, offering long-lasting outcomes for patients requiring vertical bone augmentation prior to implant placement.
Future controlled clinical trials comparing this method with other grafting techniques will be essential to further validate its efficacy and define its indications in broader patient populations.
DECLARATIONS
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. One of the authors works for the company that owns the Ri-genera Micrografting Technology.
Ethical Approval
Ethical Approval was provided by the authors institution.
Patient Consent
Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Research Registration
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Authors’ institution.
REFERENCES
- Saintrain MV, de Souza EH. Impact of tooth loss on the quality of life. Gerodontol. 2012; 29: e632-636.
- de Vicente JC, Hernández-Vallejo G, Braña-Abascal P, Peña I. Maxillary sinus augmentation with autologous bone harvested from the lateral maxillary wall combined with bovine-derived hydroxyapatite: clinical and histologic observations. Clin Oral Implants Res. 2010; 2: 430-438
- Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A; SCARE Group. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg. 2020; 84: 226-230
- Rodriguez Y, Baena R, Pastorino R, Gherlone EF, Perillo L, Lupi SM, et al. Histomorphometric Evaluation of Two Different Bone Substitutes in Sinus Augmentation Procedures: A Randomized Controlled Trial in
- Bollati D, Morra M, Cassinelli C, Lupi SM, Rodriguez Y, Baena R. In Vitro Cytokine Expression and In Vivo Healing and Inflammatory Response to a Collagen-Coated Synthetic Bone Filler. Biomed Res Int. 2016; 2016: 6427681.
- Baena RR, Lupi SM, Pastorino R, Maiorana C, Lucchese A, Rizzo S. Radiographic evaluation of regenerated bone following poly(lactic- co-glycolic) acid/hydroxyapatite and deproteinized bovine bone graft in sinus lifting. J Craniofac Surg. 2013; 24: 845-848.
- Ceccarelli G, Presta R, Benedetti L, Cusella De Angelis MG, Lupi SM, et al. Emerging Perspectives in Scaffold for Tissue Engineering in Oral Surgery. Stem Cells Int. 2017; 2017: 4585401
- Zhao R, Yang R, Cooper PR, Khurshid Z, Shavandi A, Ratnayake J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules. 2021; 26: 3007.
- Lupi SM, Rodriguez Y, Baena A, Todaro C, Ceccarelli G, Rodriguez Y, et al. Maxillary Sinus Lift Using Autologous Periosteal Micrografts: A New Regenerative Approach and a Case Report of a 3-Year Follow- Up. Case Rep Dent. 2018; 2018: 3023096
- Rodriguez Y, Baena R, D’Aquino R, Graziano A, Trovato L, Aloise AC, et al. Autologous Periosteum-Derived Micrografts and PLGA/HA Enhance the Bone Formation in Sinus Lift Augmentation. Front Cell Dev Biol. 2017; 5: 87.
- Aliberti F, Paolin E, Benedetti L, Cusella G, Ceccarelli G. 3D bioprinting and Rigenera® micrografting technology: A possible countermeasure for wound healing in spaceflight. Front Bioeng Biotechnol. 2022; 10: 937709
- Starita C, Arora CL, Trovato L. Tissue regeneration: an overview from stem cells to micrografts. J Int Med Res. 2020; 48: 300060520914794
- Lupi SM, Granati M, Butera A, Collesano V, Rodriguez Y, Baena R. Air-abrasive debridement with glycine powder versus manual debridement and chlorhexidine administration for the maintenance of peri-implant health status: a six-month randomized clinical trial. Int J Dent Hyg. 2017; 15: 287-294.
- Lupi SM, Redoglia L, Rodriguez Y, Baena A, Garbelli G, Rodriguez Y, et al. Detection of peri-implant inflammation by the use of a matrix metalloproteinase-8 chair-side test. Minerva Stomatol. 2019; 68: 168-176.
- Lupi SM, Pascadopoli M, Maiorani C, Preda C, Trapani B, Chiesa A, et al. Oral Hygiene Practice among Hospitalized Patients: An Assessment by Dental Hygiene Students. Healthcare (Basel). 2022; 10: 115.
- Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J Periodontol. 2018; 89: S304-S312
- Kawakami S, Shiota M, Kon K, Shimogishi M, Iijima H, Kasugai S. Autologous micrografts from the palatal mucosa for bone regeneration in calvarial defects in rats: a radiological and histological analysis. Int J Implant Dent. 2021; 25: 6.
- Araújo CRG, Astarita C, D’Aquino R, Pelegrine AA. Evaluation of Bone Regeneration in Rat Calvaria Using Bone Autologous Micrografts and Xenografts: Histological and Histomorphometric Analysis. Materials (Basel). 2020; 13: 4284
- Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981; 10: 387-416
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986; 1: 11-25