Selective Culture of Human Skeletal Myoblasts by Brefeldin A
- 1. Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, Japan
Abstract
Purification of skeletal muscle myoblasts from other cells such as fibroblasts is essential in basic research and cell-based regenerative medicine. In this report a simple non-serological method is proposed to purify desmin-positive myoblasts using Brefeldin A.
Ordinary passaged human skeletal muscle-derived cells were cultured for 3 days with five exocytic pathway inhibitors (Brefeldin A, Monensin, AACOCF3, Exo-1 and Golgicide A). The myoblasts identified by desmin exhibited relatively superior growth compared to the other cells, both in 7 - 15 ng/mL of Brefeldin A and in 850 – 2500 ng/mL of Golgicide A. In the case of 10 ng/mL Brefeldin A, the cell numbers increased 6.7-fold in desmin-positive cells and 2.4-fold in desmin-negative cells while they increased 11.7-fold and 15.6-fold in absence of Brefeldin A, respectively. In addition, 7-day cultures in 10 ng/mL Brefeldin A of four independent cell strains suggested that moderate starting purities resulted in high purities over 90%, while extremely low starting purities (< 1%) did not support stable purifications. Lastly, a strain purified through a 7-day culture with 10 ng/mL Brefeldin A was subjected to myotube induction culture. Fluorescent imaging using a confocal microscope revealed their ability to form multinucleated myotubes and myofibers with sarcomeric structures.
In conclusion, the purification of desmin-positive cells by culturing human skeletal musclederived cells with Brefeldin A provides a simple method to obtain purified myoblasts for use in basic research and cell-based regenerative medicine.
Keywords
• Myoblast
• Purification
• Muscle
• Regenerative medicine
• Cell therapy
Citation
Kikuchi T, Shimizu T (2016) Selective Culture of Human Skeletal Myoblasts by Brefeldin A. JSM Regen Med Bio Eng 4(1): 1020
ABBREVIATIONS
SkMC: Skeletal Muscle-derived Cell
INTRODUCTION
In developmental and regeneration studies of skeletal muscle, the preparation of purified myogenic cells is of critical importance for reliable, reproducible experiments. Myoblasts are the major myogenic cells derived from muscle satellite cells, which are the tissue-resident stem cells responsible for skeletal muscle regeneration [1]. However, when the cells are enzymatically dissociated from skeletal muscle specimens they are inevitably contaminated with non-myogenic fibroblasts, and unfortunately, these fibroblasts often grow faster than myoblasts in conventional expansion cultures [2]. Thus, in vitro expansion of myoblasts requires selective culture or a purification process. To solve this problem, several serological methods exploiting a surface antigen, such as CD56, as a myoblast marker have been proposed including clonal selection methods [3], magnetic affinity cell sorting [4-6] and fluorescence-activated cell sorting [7-9]. Although these methods provide sufficient selectivity for purifying myoblasts, they are not economical for large scale production of myoblasts due to their use of antibodies. On the other hand, pre-plating methods utilizing different attachment kinetics of the two types of cells are widely used as an alternative [2,10-14]. Co-culture systems with macrophages for selective acceleration of myoblast growth have also been reported [15]. These non-serological methods may be applicable to large scale production, even though their selectivity may be less than those of serological methods.
Recently, the usage of myoblasts has expanded within regenerative medicine and cultured meat [16] beyond just basic research. In these fields, simpler and more efficient expansion methods for myoblasts are required for the production of a large number of myoblasts with minimal or moderate contamination. Against that background, we were searching for another method to acquire purified massive myoblasts and determined that the quantitative or qualitative differences in the exocytic pathway between myoblasts and fibroblasts could be used to selectively culture myoblasts.
The exocytic pathway is an intracellular mechanism to secrete processed proteins and lipids, which is centered upon the Golgi apparatus with complicated vesicle formations and fusions. When the mechanism becomes blocked, the transport of these molecules is stagnated resulting in an accumulation of secretary proteins in the endoplasmic reticulum [17]. If the accumulation is prolonged, an endoplasmic reticulum stress response (also referred as unfolded protein response or UPR) is provoked and the cells finally develop apoptosis [18-20]. Thus, different sensitivities to blockage of the exocytic pathway can induce selective apoptosis or arrest growth in moderate conditions. Considering the culture of skeletal muscle-derived cells, we speculated that fibroblasts were more sensitive to UPR than myoblasts because fibroblasts secrete collagens in physiological situations, such as wound healing process. With that expectation, we examined the difference in sensitivity of myoblasts and fibroblasts to several major inhibitors of the exocytic pathway, and found that supplementing Brefeldin A at specific concentrations most effectively purified desmin-positive myoblasts.
MATERIALS AND METHODS
Cell culture
Four independent strains (Strain A-D) of human skeletal muscle-derived cells (SkMCs) of passage two were purchased from Lonza Japan Ltd. (Tokyo, Japan; Cat. No. CC-2561). These cells were cultured and expanded through two additional passages (total four passages) in SkGM-2 BulletKit (Lonza, CC3244 and CC-3246), and stored at -150°C until use.
Screening of inhibitors
Five exocytic pathway inhibitors (Brefeldin A, Monensin, AACOCF3, Exo-1 and Golgicide A) were added to SkMC cultures at various concentrations to study the selective effect on myoblasts. The stored SkMCs (strain A-C) were thawed and seeded in 24- well cell culture plates (Corning, #353226) at 5000 cells/well in SkGM-2 medium and incubated at 37°C in a humidified 5% CO2 and 95% air atmosphere. After 24 hr-incubation, each inhibitor was added directly to the wells and cultured for an additional 72 hr (total 96 hr). For each experiment, a control plate was seeded in the same condition and fixed at 24 hr to calculate the growth ratios during the additional 72 hr-culture. All culture plates were analyzed by image-base cytometry, as described below.
Time-course of selective culture
To see the time-course of desmin-positive ratios in the purification cultures using Brefeldin A, the stored SkMCs (strains A-D) were initially thawed and seeded in 24-well cell culture plates. After 24hr incubation, Brefeldin A was added directly to the wells at 10ng/mL and cultured for 3, 5 and 7 days. At each time point, the cells were fixed and analyzed for the desminpositive ratio and total cell number by image-based cytometry as described below.
Image-based cytometry
To measure purity of the myoblasts, total cells and desminpositive cells were counted in the 24-well plate format by imagebased cytometry. SkMCs in 24-well cell culture plates were fixed by a 4% formaldehyde solution of phosphate buffered saline (PBS) for 15 min, washed by PBS once and stored at 4°C in PBS. At the end of each experiment, all the wells were treated by anti desmin antibody (PROGEN, Cat.No.10598) at 2 μL/mL in PBS supplemented with 0.1% Triton-X and 5% Blocking One Solution (Nacalai tesque, Japan) for 30 min at room temperature. Then, after washing with PBS, the wells were treated with Alexa Fluor 488 anti-mouse IgG antibody (Life Technologies, USA) at 2 μL/ mL, Alexa Fluor 568 Phalloidin (Life Technologies) at 2 μL/mL, and 4’, 6-diamidino-2-phenylindole (DAPI) at 2 μg/mL in PBS supplemented by 5% Blocking One Solution. After 1 hr, the wells were washed by PBS once and stored until analysis in PBS at 4°C. Within a few days, the fluorescent images of the stained cells were acquired by the ImageXpress Ultra Confocal High-Content Analysis System (Molecular Devices, USA). For strains A and B, nine images were acquired from each well. Then, the total cell numbers and desmin-positive cell numbers were counted by the accessory software Meta Xpress. For strains C and D, 36 images were acquired from each well to compensate for the limited number of myoblasts in these strains. The total cell numbers were counted by Meta Xpress and the desmin-positive cells were counted manually. The desmin-negative cell numbers were calculated by subtracting desmin-positive cell numbers from total cell numbers.
Characterization of purified myoblasts
To characterize the myoblasts purified by the addition of Brefeldin A, purified SkMCs were subjected to myotube formation induction culture. Initially, stored SkMCs of strain A were thawed and seeded to a flask (500 cm2 , Nunc) in SkGM-2 medium at 8×105 cells/flask. After 24 hr-culture, the medium was replaced with new SkGM-2 supplemented by 10 ng/mL of Brefeldin A. After the additional 7-day culture, the cells were harvested by Accutase (Innovative Cell Technologies, USA) and cry preserved. Again, the purified cells were thawed and seeded to a fibrin gel-coated dish in SkGM-2 medium. After 3 days, the medium was changed to NbActiv1 serum-free medium (BrainBits, USA) to induce myotube formation. After 7-day induction culture, the cells were fixed and subjected to fluorescent histochemistry. The cells were stained by DAPI and Alexa Fluor 568 Phalloidin overnight and washed with PBS, then observed by a confocal laser scanning microscope (LSM 510 META; Carl Zeiss Microscopy, UK). In addition, flow cytometric analysis for anti-desmin and TE-7 antibodies was conducted for the cryopreserved SkMCs before and after the purification to confirm the result of the image-based cytometry. In brief, the cells were stained by anti-desmin antibody and TE-7 antibody (Millipore, Cat.No.CBL271), then analyzed by a flow cytometer (Gallios™, Beckman Coulter).
RESULTS AND DISCUSSION
Brefeldin A and Golgicide A showed a myoblast selective effect
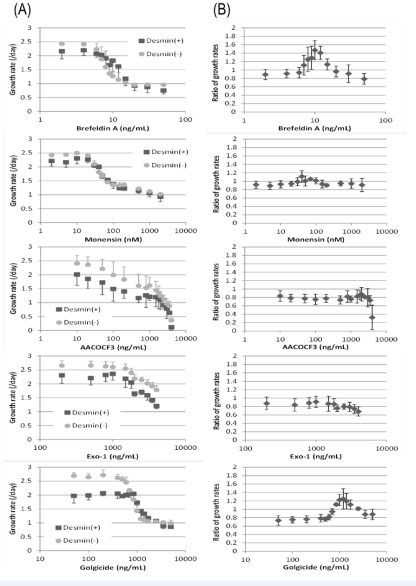
To test the myoblast selective effects of exocytic pathway inhibitors, SkMCs were cultured with five reagents at various concentrations, and then, analyzed for desmin positive ratio (Figure 1).
Figure 1 Effects of exocytic pathway inhibitors on growth rates of desmin-positive cells and desmin-negative cells from skeletal muscle-derived cells.Five exocytic inhibitors were added to the culture media at Day 1 and cultured until Day 4. (A) - The growth rates of desmin-positive cells and desmin-negative cells from Day 1 to Day 4. (B) - Ratios of both growth rates (growth rate of desmin-positive cells / growth rate of desmin-negative cells) calculated from the same data to emphasize the differences. Each plot indicates the average and the standard deviation of three independent cell strains.
Among the tested conditions, myoblasts identified by desmin exhibited relatively superior growth against the other cells, both in 7 - 15 ng/mL of Brefeldin A and in 850 - 2 500 ng/ mL of Golgicide A. In 40 - 100 nM of Monensin, desmin-positive and desmin-negative cells showed roughly equal growth rates. In the other conditions, desmin-positive cells showed slower growth than desmin-negative cells. In the optimal case for myoblast purification, where 10 ng/mL Brefeldin A was added, the cell numbers increased 6.7-fold in desmin-positive cells, and 2.4-fold in desmin-negative cells, whereas they were 11.7-fold and 15.6-fold, respectively in the absence of Brefeldin A at 72 hrs.
At the beginning, we had thought that fibroblasts might be more sensitive than myoblasts to these inhibitors, because UPR should be more severe in fibroblasts due to their role in the production of collagens. However, the reagents other than Brefeldin A and Golgicide A showed no or very weak selective effects. The five reagents tested here have different effects on the exocytic pathway. Brefeldin A impedes the function of ADP-ribosylation factor proteins (Arf proteins) by inhibiting their guanine nucleotide exchange factors (ArfGEFs) [21-23]. Golgicide A also inhibits ArfGEFs, but is more specific to GBF1. It does not interact with BIG1 or BIG2 [24]. On the other hand, Monensin is an ionophore that increases Na+ and decreases K+ ions in the cytoplasm preventing the fusion of Golgi vesicles and the plasma membrane [25-27]. AACOCF3 (arachidonyl trifluoromethyl ketone) is a phospholipase-A2 inhibitor that causes the dispersion of Golgi stacks and trans-Golgi networks [28]. Exo-1 also interferes with the activity of Arf proteins, although the mechanism is thought to be an acceleration of GTP hydrolysis in ARF1-GTP complexes without inhibiting ArfGEFs [29]. On the basis of these presumptions, it can be speculated that the inhibition of ArfGEFs is a key factor, but more investigation is required to elucidate the precise mechanism.
Efficiency of myoblast purification by Brefeldin A was dependent on the starting purities
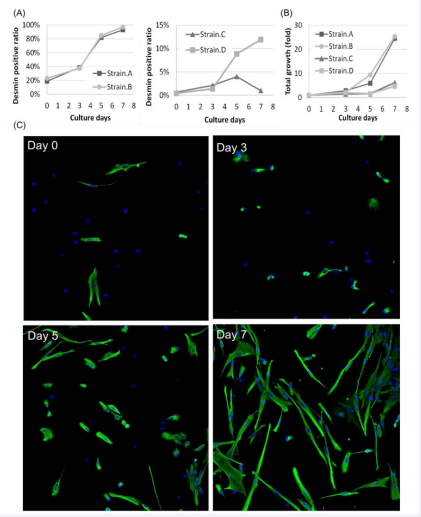
In 7-day culture with 10 ng/mL of Brefeldin A, SkMC strains having moderate starting desmin-positive ratios (Strain A: 19%, Stain B: 23%) were purified over 90% (Strain A: 93%, Strain B: 97%) while strains having extremely low starting desminpositive ratios (Strain C: 0.65%, Strain D: 0.41%) did not present a stable increase in purity (Strain C: 0.98%, Strain D: 12%) (Figure 2).
Figure 2 Time-course of myoblast purification by Brefeldin A. Four independent strains of skeletal muscle-derived cells were cultured for 7 days with 10 ng/mL of Brefeldin A. Green fluorescence indicates desmin, and blue fluorescence indicates nuclei. (A) - Desmin-positive ratio. (B) - Total cell growth. (C) - Immunofluorescent images of strain B.
However, even in the latter strains, the desmin-positive ratios all increased until at least day 5. Therefore, the loss or slowdown of the selective effects in these strains might be caused by a small cell fraction in SkMCs being both Brefeldin A-resistant and desmin-negative, and these cells had become the majority in the latter part of the purification cultures.
As implied by the above result, this purification method must be improved to be applicable to strains from various donors. It has been reported that the starting purities of myoblasts in SkMCs, and the temporal purity change during expansion culture varied from donor to donor [30,31]. According to these reports, a certain portion of SkMC strains exhibited a decrease in the desmin-positive ratio along with the expansion cultures. Therefore, it might be better if we used fewer passaged SkMCs than the four passage SkMCs we used as the starting material in this study.
On the other hand, SkMC strains A and B achieved higher purities that were over 90%. In respect to the appropriate myoblast purities, a comparison of muscle-derived cells with 10%, 50% and 75% desmin-positive ratios has been reported [32]. In this report, applying a tissue-engineering technique using fibrin gels, muscle-derived cells with 50% and 75% desmin-positive ratios presented a larger number of fusions and myotubes than that of 10%; and only 75% desmin-positive cells showed sarcomeric assembly. Therefore, our myoblast purification method could provide sufficient purities for similar studies.
Myoblasts purified by Brefeldin A supplementation could form myofibers
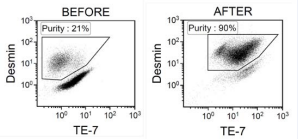
To identify the properties of purified myoblasts, flow cytometric analysis and a myotube induction culture was conducted. In the cytometric analysis, the purification of SkMC
strain A was confirmed and the measured purities were consistent with the results of image-based cytometry (Figure 3)
Figure 3 Flow cytometric analysis of skeletal muscle-derived cells before and after purification. Skeletal muscle-derived cells of strain A were cultured for 7 days with 10ng/mL Brefeldin A. The areas enclosed by polygons are assumed to be myoblasts.
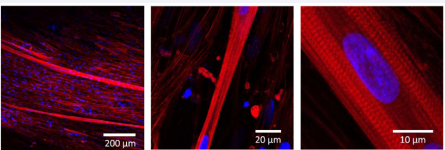
In this analysis, TE-7 antibody was used to identify fibroblasts. Unlike previous reports, the difference between myoblasts and fibroblasts in the fluorescent intensities of TE-7 seemed small and the purified myoblasts presented a wide range of intensities for TE-7. Finally, the purified myoblasts of strain A were subjected to a myotube induction culture. At 24 hr after the medium change for the induction medium (Day 1), a number of multinucleated cells emerged under a phase-contrast microscope. In the following culture, some of the multinucleated cells gradually became fiber-like shape with aligned nuclei, while many cells had detached from the fibrin gel forming cell aggregates. The fiberlike cells reached several millimeters in length until Day 7. Then, the cells were fixed and stained. Laser-scanning confocal imaging revealed that these long fiber-like multinucleated cells had sarcomeric structures, proving myofiber formation (Figure 4).
Figure 4 Myotube formation from purified myoblasts. Skeletal muscle-derived cells of strain A were purified through 7-day culture with 10ng/ mL Brefeldin A and subjected to myotube formation culture. Red fluorescence indicates F-actin (Phalloidin) and blue fluorescence indicates nuclei (DAPI).
Culture with Brefeldin A can be a cost-effective method for large-scale production of purified myoblasts
The aim of this study was to propose a novel purification method for myoblasts from skeletal muscle-derived cells. In general, digests of skeletal muscles consist of many types of cells including myofibers, muscle satellite cells, myoblasts, interstitial fibroblasts, endothelial cells and nerve cells. Among these cells, conventional serum-supplemented culture in plastic dishes mainly cultivates myoblasts and fibroblasts. These two fractions can be easily identified by immunohistochemistry using desmin and TE-7 antibodies [33]. Myoblast surface markers, such as CD56 and α7 integrin, are also well known [3,34], and these surface markers are useful for serological separation of myoblasts, because they yield sufficient purities for most experimental purposes. However, in the case of large scale production or in the cases where minimal contamination of fibroblasts is acceptable, easier and more economical methods are required. Myoblasts are not only used for basic research on myogenesis, but also in regenerative medicine for skeletal and cardiac muscle [35-38], in addition to food production such as cultured meat [16]. For example, in the manufacturing of myoblast sheets for treatment of diseased hearts, several hundred million cells are required [38,39]. At present, the purity of myoblasts has been controlled by the optimized culture method without purification steps. When this type of treatment expands to a large number of patients, it is expected that there will be some patients whose myoblast purity falls below the product specification. If the required number of cells is purified by magnetic affinity cell sorting, it could cost several hundred dollars for antibodies; however, using Brefeldin A will cost less than one dollar for the reagent. Further, with respect to biological safety, antibodies inevitably include animal- or human-derived components that may harbor infectious microorganisms or viruses, while Brefeldin A can be chemically synthesized. In comparison with already proposed non-serological methods, such as pre-plating methods, we believe that our method has advantage in selectivity, yield and labor-intensity for some applications [2,10-15].
CONCLUSION
The purification of desmin-positive cells in human skeletal muscle-derived cells by cultivation with Brefeldin A can provide a simple method to acquire moderately purified myogenic cells for studies of skeletal muscle and other applications of myoblasts in regenerative medicine.
ACKNOWLEDGEMENTS
This study was supported with funding from CellSeed Inc. (Tokyo, Japan). We would like to thank A. Nisbet for his useful comments and editing, and all who have supported this work.
Conflict of Interest
Tatsuya Shimizu is a shareholder of CellSeed Inc., and Tetsutaro Kikuchi was an employee of Cell Seed Inc. from December 2005 to March 2015.