Prognostic Value of Plasma Diquat Concentration in Acute Oral Diquat Poisoning Patients: A Retrospective Study
- 1. Emergency Department, The Second Hospital of Hebei Medical University, 215 Heping West Road, Shijiazhuang, China
Abstract
Background: Diquat (DQ) poisoning is an important public health and social security agency. This study aimed to develop a prognostic model and evaluate the prognostic value of plasma DQ concentration in patients with acute oral DQ poisoning, focusing on how its impact changes over time after poisoning.
Methods: This was a retrospective study using time-dependent Cox regression analyses on 80 patients with acute oral DQ poisoning at our hospital between January 2019 and May 2022.
Results: Overall in-hospital case fatality rate was 36.25% (29/80). The Schoenfeld residual of plasma DQ concentration on admission is evidence of the violation of the proportional hazards assumption. Plasma DQ concentration on admission (HR = Exp (0.032-0.059×ln(t))) and PSS within 24 h of admission (HR: 4.470, 95%CI: 1.604 ~ 12.452, P = 0.004) were independent prognostic factors in time-dependent Cox regression model. The HR of plasma DQ concentration on admission gradually decreased with time. AUROC of plasma DQ concentration on admission was higher than ingestion amount at each time point.
Conclusions: Plasma DQ concentration on admission and PSS within 24 h of admission are independent prognostic factors for in-hospital case fatality rate of patients with acute oral DQ poisoning. The prognostic value of plasma DQ concentration decreased with time.
Keywords
• Diquat
• Poisoning
• Plasma DQ Concentration on Admission
• Prognosis
• Time-Dependent Cox Regression Analyses
• Multiple myeloma
• Renal biopsy
CITATION
Meng N, Tian Y, Sun Y, Dong Y, Lv B, et al. (2023) Prognostic Value of Plasma Diquat Concentration in Acute Oral Diquat Poisoning Patients: A Retrospective Study. JSM Renal Med 5(1): 1018.
INTRODUCTION
Diquat (1,1’-ethylene-2,2’-bipyridinium ion; DQ) belongs to bipyridinium herbicides. Although it has a similar herbicidal action to Paraquat (PQ), there are significant distinctions in its poisoning mechanisms and clinical effects. Cases of DQ poisoning have been steadily increasing since paraquat was banned in many countries [1,2]. One of the most common causes of DQ poisoning is an inadvertent or intentional intake of concentrated liquid products containing DQ. When a large amount of DQ is ingested, it can cause multiple organ dysfunction and perhaps death. DQ poisoning has a case fatality rate of up to 52.5% [3]. After PQ poisoning, treating DQ poisoning has become another difficult task for clinicians. Therefore, timely clinical outcome evaluation and risk assessment for critically ill DQ poisoning patients and essential for appropriate medical resource allocation, which has become an important public health and social security agency.
The disease is divided into mild poisoning, moderate-to- severe poisoning, and fulminant poisoning, and the survival probability of patients is roughly evaluated based on the ingested dose (DQ2+) and different clinical manifestations [3,4]. However, the real absorbed dose (DQ2+) varies widely due to subjective expression, varied gastrointestinal absorption function, and different intervention timing of gastric lavage and catharsis. Poison detection is a crucial component of acute poisoning clinical diagnosis. Plasma poison concentration has a great value in evaluating prognosis and guiding treatment. Hart et al created concentration-time curves to represent estimates of the survival probability of acute PQ poisoning [5]. Hampson et al found that when PQ plasma concentrations were above 3 mg/L, the patient had a bad prognosis, despite haemoperfusion [6]. The prognostic value of plasma DQ concentration in patients with acute DQ poisoning has never been studied. Therefore, the goal of this study was to identify prognostic factors of patients with acute oral DQ poisoning, as well as to assess the prognostic value of plasma DQ concentration on admission, which might contribute to clinical evaluation and treatment.
MATERIALS AND METHODS
Patients
We retrospectively identified patients who were treated in our hospital between January 2019 and May 2022. The patients were included in the study according to the following criteria: (1) a history of oral DQ ingestion, (2) 14 years of age or older, (3) DQ was detected in the plasma and/or urine samples taken on admission, (4) time interval from DQ ingestion to the Emergency Department (ED) ≤ 36 h. Patients were excluded if they had: (1) mixed toxicants poisoning, (2) no-oral exposure routes, (3) blood purification in local hospitals; (4) a history of serious lung disease or severely impaired hepatic or renal function.
Ethics Statement
The study was approved by Hebei Medical University institutional review board (IRB: 2020-C043). Furthermore, the study follows the STROBE guidelines and was conducted according to the principles of the Declaration of Helsinki [7]. Exemption from obtaining written informed consent was granted because of the retrospective observational nature of our study.
Treatment
All patients were immediately given gastric lavage, adsorption with activated charcoal, and diarrhea induction with purgative and/or high enemas to prevent the absorption of DQ. Forced diuresis, hemoperfusion and/or hemofiltration were applied to promote the excretion of DQ. Blood Purification was given to patients within 1-2 h after admission. The old therapeutic regimen referred to patients receiving 2-hour of HP, with a 6 to 8-hour interval time. The frequency of HP was adjusted according to the plasma DQ concentration. The new therapeutic regimen adopts the model of “HP+CVVH+HP”. The interval time between two HP was 9 to 10 hours, during which a CVVH was applied. The frequency of HP and CVVH was adjusted according to the plasma DQ concentration. Antioxidants (Vitamin C and melatonin) and low-dose glucocorticoids were applied to scavenge free radicals and inflammatory mediators. Other clinical treatments included maintaining fluid and electrolyte balance, organ function support, and so on [3,4].
Data Collection
Demographic data, clinical data, laboratory data, treatment, and outcomes were collected by two clinicians based on the unified form. Specifically, we collected information including gender, age, exposure routes, ingestion amount (including reproduced ingestion amount and accurate ingestion amount), time interval from DQ ingestion to gastric lavage, time interval from DQ ingestion to ED, time interval from DQ ingestion to blood purification, plasma DQ concentration on admission, lungs injury within 24 h of admission, liver injury within 24 h of admission, kidney injury within 24 h of admission, Central Nervous System (CNS) injury within 36 h of admission, the Acute Physiology and Chronic Health Evaluation (APACHE) II score and Poisoning Severity Score (PSS) within 24 h of admission [8,9], treatment regimens, frequency of Hemoperfusion (HP), frequency Of Continuous Veno-Venous Hemofiltration (CVVH) and hospital days. The primary endpoint was the death of patients in hospital, and survival days were recorded. Patients were grouped into survivors and non-survivors based on whether in-hospital death.
For patients with uncertain ingestion amount, reproduced ingestion amount was performed by two specially trained staff members. Initially, they prepared the mineral water and made it clear to the patients that the purpose was to perfectly duplicate ingestion amount of DQ. The patients then simulated the original poisoning situation by holding the mineral water in their mouths and not swallowing it. Subsequently, they spat the water into a 100-milliliter cylinder. Two staff members measured the water and recorded the average volume as the reproduced ingestion amount. A 200 mL measuring cup was used if the ingestion amount was large. When a patient took a whole bottle (not sprinkled) of DQ and the container was labelled with precise graduation, the patient’s ingestion amount could be directly recorded (accurate to ml or g).
Definition
Prior the study, definitions were defined. The following are the diagnostic criteria for lung injury [10]: (1) History of ingestion of DQ that is known to induce lung injury, (2) The clinical manifestations have been reported to be induced by DQ,
- Other causes of the clinical manifestations could be ruled out,
- Partial Pressure Oxygen2 (PaO2) < 80 mmHg on room air. Case definition of liver injury is proposed if one of the following thresholds is met [11] (1) alanine aminotransferase (ALT) ≥ 5 × Upper Limits of Normal (ULN), (2) alkaline phosphatase (ALP)≥ 2 × ULN (especially with an elevation of Gamma-Glutamyl Transferase (GGT) or after ruling out primary bone pathology in cases of isolated elevation of ALP), (3) ALT ≥ 3 × ULN plus Total Bilirubin (TB) > 2 × ULN. Case definition of kidney Injury is proposed if one of the following thresholds is met [12,13] (1) serum creatinine increased ≥ 1.5 times baseline, (2) Urinary output< 0.5 ml/kg/h during a 6-hour block. Case definition of CNS injury is proposed if one of the following thresholds is met [3,14] (1) The clinical manifestations with headache, dizziness, consciousness disturbance (drowsiness, confusion of consciousness, delirium, lethargy, coma), focal or generalized epileptiform seizures, etc.,(2) The brain imaging can be manifested as cerebral edema, brain stem infarction, and bleeding.
Quantitative Analysis of DQ Levels in Plasma
The plasma samples were collected at admission. Plasma DQ concentration was measured by a high-performance liquid chromatographic-tandem mass spectrometry (HPLC-MS/MS, shimadzu, Japan; AB Sciex, USA). The separation of analytes was achieved on a Pc Hilic S5 column (5μm, 2.0 mm×150 mm) (Osaka Soda, Beijing) held at 35?. The mobile phase consisted of a mixture of solvent a (20mM ammonium formate in water containing 0.1% formic acid) and solvent B (acetonitrile) delivered with a gradient at a flow rate of 0.3 mL/min. The standard curve was linear over a concentration range of 10-1000 ng/mL in plasma.
Statistical Analysis
Baseline characteristics were described for categorical variables by n (%) and continuous variables using mean ± SD or median (Interquartile Range [IQR]) according to their distribution. Student t-tests, Non-parametric tests and Chi- square tests were used for analysis of the baseline characteristics. Univariate Cox proportional hazards regression was used to assess associations, measured as Hazard Ratios (HRs), between covariates and time. Variables that showed a P value less than 0.1 were included in multivariate Cox proportional hazards regression analyses. The proportional hazards assumption was checked using the Schoenfeld residuals method [15]. Linearity of continuous covariates was checked using the Martingale residuals method. In situations when the proportional hazards assumption of the Cox regression model does not hold, introducing a time- dependent variable (T_COV_) in Cox proportional hazards regression analyses provided a flexible method to evaluate non- proportionality. The natural logarithm of the time variable is used for the construction of the time-dependent covariates in time-dependent Cox regression model. The Hazard Ratio (HR) and 95% confidence interval (95% CI) were calculated. The prognostic value of plasma DQ concentration on admission was assessed by computing the area under a time-dependent Receiver Operating Characteristic Curve (ROC). The optimal cut-off value was represented highest Youden index (sensitivity + specificity - 1). Survival curves were generated by the Kaplan–Meier method and compared by the log-rank test. A P value less than 0.05 was considered statistically significant.
SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA) and R software (version 3.3.0, R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis. The R statistical packages “time ROC,” “survival,” and “survminer” was used to calculate the clinical characteristics table, Kaplan–Meier curves, and time-dependent ROC curves.
RESULTS
Baseline Characteristics
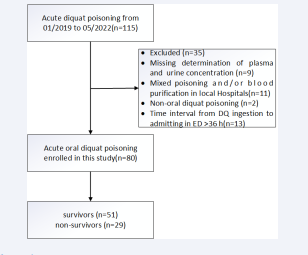
A total of 80 patients who met the criteria were included in the study (Figure 1).
Figure 1: Concentrated liquid products
78 patients took concentrated liquid products containing 200 g/L of DQ and 2 patients took concentrated liquid products containing 100 g/L of DQ. For recording, all patients reproduced ingestion amount was converted into concentrated liquid products containing 200 g/L of DQ. Baseline demographics and clinical characteristics of patients with acute oral DQ poisoning are shown in (Table 1).
Table 1: Baseline demographics and clinical characteristics of the diquat poisoning.
|
Variable |
Total (N = 80) |
Survivors (N = 51) |
Non-survivors (N = 29) |
P-value |
|
Age (years) |
26.67 ± 9.73 |
26.08 ± 15.0 |
27.72 ± 12.5 |
0.47 |
|
Male, n (%) |
37(46.3%) |
21 (41.2%) |
16 (55.2%) |
0.23 |
|
ingestion amount (ml) |
50 (80) |
20 (40) |
100 (137.5) |
< 0.01 |
|
Time to gastric lavage (h) |
2 (3) |
2 (4) |
1 (2) |
0.092 |
|
Time to our ED (h) |
6 (5) |
6 (5) |
6 (5) |
0.912 |
|
Time to blood purification (h) |
8.5 (5) |
9 (5) |
8.5 (5) |
0.912 |
|
PDQ (ug/ml) |
1.06 (9.57) |
0.35 (0.90) |
26.9 (46.65) |
< 0.01 |
|
Oragn injury |
||||
|
Lungs injury 24-h, n (%) |
17 (21.3%) |
3 (5.9%) |
14 (48.3%) |
< 0.01 |
|
Liver injury 24-h, n (%) |
6 (7.5%) |
1 (2.0%) |
5(17.2%) |
0.04 |
|
Kidney injury 24-h, n (%) |
31 (38.8%) |
5 (9.8%) |
26 (89.7%) |
< 0.01 |
|
CNS injury 36-h, n (%) |
28 (35.0%) |
4 (7.8%) |
24 (82.8%) |
< 0.01 |
|
APACHE-II 24-h |
6 (8) |
4(4) |
13(12) |
< 0.01 |
|
PSS 24-h |
1 (2) |
1 (1) |
4 (1) |
< 0.01 |
|
treatment regimens |
|
|
|
0.17 |
|
New regimens |
57 (71.3%) |
39 (76.5%) |
18(62.1%) |
|
|
Old regimens |
23 (28.8%) |
12 (23.5%) |
11 (37.9%) |
|
|
Survival Time (d) |
—— |
—— |
1.30 (1.00) |
—— |
Abbreviations: ED: Emergency Department; PDQ: Plasma DQ Concentration on Admission; CNS: Central Nervous System; APACHE-II: Acute Physiology and Chronic Health Evaluation; PSS: Poisoning Severity Score; HP: Hemoperfusion; CVVH: Continuous Veno-Venous Hemofiltration.
Old regimen: Received 2-hour of HP therapy, at a 6 to 8-hour interval time. New regimen: the model of “HP+CVVH+HP”. The interval time between two HP was 9 to 10 hours, during which a CVVH was applied. The frequency of HP and CVVH was adjusted according to the plasma DQ concentration.
Among the 80 patients, 29 (36.25%) patients died and 51 (63.75%) patients survived in the hospital. The non-survivors had a median (IQR) survival time of 1.3 (1.0) days and the longest survival time of 4.5 days after DQ poisoning. Between survivors and non-survivors, there were no significant differences in gender, age, time interval from DQ ingestion to gastric lavage, time interval from DQ ingestion to ED, time interval from DQ ingestion to blood purification, treatment regimens (all P > 0.05). But, survivors had significantly lower ingestion amount, plasma DQ concentration on admission, lungs injury within 24 h of admission, liver injury within 24 h of admission, kidney injury within 24 h of admission, and CNS injury within 36 h of admission (all P < 0.05). Furthermore, the comparisons of traditional scores between non-survivors and survivors also showed that non-survivors had significantlyhigher APACHE II score and PSS within 24 h of admission (all P< 0.05).
Proportional Hazards Assumption Verification
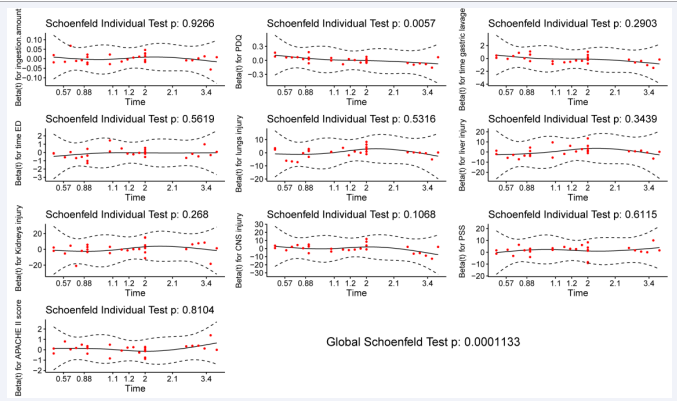
The proportional hazards assumption is verified using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. As shown in (Figure 2),
Figure 2: Schoenfeld individual test is not statistically significant (p > 0.05) for ingestion amount,
the Schoenfeld individual test is not statistically significant (p > 0.05) for ingestion amount, time interval from DQ ingestion to gastric lavage, time interval from DQ ingestion to ED, lungs injury within 24 h of admission, liver injury within 24 h of admission, kidney injury within 24 h of admission, and CNS injury within 36 h of admission, but statistically significant (p < 0.05) for plasma DQ concentration on admission. The Schoenfeld residual of plasma DQ concentration on admission is evidence of the violation of proportional hazards assumption. In addition, the global test shows a statistically significant (χ2 = 35.247 p = < 0.001) correlation between the Schoenfeld residuals and the variation of time, indicating that the Cox proportional hazards model is proven to be inappropriate in the multivariate analysis.
Univariate Cox Proportional Hazards Regression Analyses
According to the verification of proportional hazards assumption, plasma DQ concentration on admission is a time- dependent covariate. Therefore, the time-dependent Cox regression model is appropriate in dissecting the influences of these time-dependent covariates. The results from the univariate Cox regression analysis are presented in (Table 2).
Table 2: Univariate cox regression analysis.
|
Variable |
cofe |
Se (cofe) |
Z |
P-value |
HR (95%CI) |
|
Age |
0.012 |
0.016 |
0.74 |
0.459 |
1.012 (0.980, 1.045) |
|
Gender |
0.395 |
0.374 |
1.057 |
0.291 |
1.488 (0.714,3.088) |
|
Ingestion amount (ml) |
0.011 |
0.002 |
6.519 |
< 0.01 |
1.011 (1.008, 1.014) |
|
Time to gastric lavage |
-0.078 |
0.062 |
-1.262 |
0.207 |
0.925 (0.818, 1.044) |
|
Time to our ED |
-0.023 |
0.036 |
-0.632 |
0.527 |
0.978 (0.911, 1.049) |
|
PDQ (ug/ml) |
0.068 |
0.01 |
7.131 |
< 0.01 |
1.071 (1.051, 1.091) |
|
T_COV_ PDQ |
-0.017 |
0.983 |
-1.119 |
0.263 |
0.983 (0.953, 1.013) |
|
Lungs injury 24-h |
1.883 |
0.383 |
4.915 |
< 0.01 |
6.577 (3.103, 13.94) |
|
Liver injury 24-h |
1.357 |
0.5 |
2.713 |
< 0.01 |
3.883 (1.457, 10.35) |
|
Kidney injury 24-h |
3.432 |
0.622 |
5.517 |
< 0.01 |
30.93 (9.139, 104.6) |
|
CNS injury 36-h |
2.984 |
0.503 |
5.937 |
< 0.01 |
19.76 (7.379, 52.91) |
|
APACHE-II 24-h |
0.117 |
0.015 |
7.798 |
< 0.01 |
1.124 (1.092, 1.158) |
|
PSS 24-h |
1.837 |
0.272 |
6.746 |
< 0.01 |
6.277 (3.681, 10.70) |
|
Treatment regimens |
-0.606 |
0.384 |
-1.579 |
0.114 |
0.546 (0.257, 1.158) |
Abbreviations: ED: Emergency Department; PDQ: Plasma DQ Concentration On Admission; CNS: Central Nervous System; APACHE-II: Acute Physiology and Chronic Health Evaluation; PSS: Poisoning Severity Score
Univariate Cox regression analysis revealed that ingestion amount, plasma DQ concentration on admission, lungs injury within 24 h of admission, liver injury within 24 h of admission, kidney injury within 24 h of admission, CNS injury within 36 h of admission, APACHE II score and PSS within 24 h of admission had statistical differences (all P < 0.01).
Multivariate Cox Proportional Hazards Regression Analyses
According to the univariate Cox regression analysis, ingestion amount, plasma DQ concentration on admission, lungs injury within 24 h of admission, liver injury within 24 h of admission, kidney injury within 24 h of admission, CNS injury within 36 h of admission, APACHE II score and PSS within 24 h of admission are included in the multivariate Cox regression analysis. Time interval from DQ ingestion to gastric lavage and time interval from DQ ingestion to ED are critical in assessing in-hospital deaths from acute DQ poisoning and are thus also included in the multivariate Cox regression analysis. There was no multicollinearity among the above indicators. This study satisfies the hypothesis of a linear relationship between the continuous variables and the outcome.
The time-dependent multivariate Cox proportional hazards regression analyses revealed that plasma DQ concentration on admission, T_COV_PDQ, and PSS within 24 h of admission are statistically significant(p < 0.05), as shown in (Table 3).
Table 3: Multivariate cox proportional hazards regression analyses.
|
Variable |
cofe |
se(cofe) |
Z |
P -value |
HR (95%CI) |
|
PDQ (ug/ml) |
0.032 |
0.016 |
2.049 |
0.04 |
1.033 (1.001, 1.066) |
|
T_COV_ PDQ |
-0.059 |
0.024 |
-2.52 |
0.0122 |
0.942 (0.900, 0.987) |
|
PSS 24-h |
1.497 |
0.523 |
2.864 |
0.004 |
4.470 (1.604, 12.452) |
Abbreviations: PDQ: Plasma DQ Concentration on Admission; PSS: Poisoning Severity Score.
Plasma DQ concentration on admission and T_COV_PDQ are both statistically significant, implying that the effect of plasma DQ concentration on admission varies with time. The time-varying effect of plasma DQ concentration on admission can be written as β(t) =0.032- 0.059×ln(t) and HR(t) = Exp(0.032-0.059×ln(t)). With 1.5 days (36 h) after poisoning, the HR of plasma DQ concentration on admission gradually decreased with time.
Comparison of the Impact of Plasma DQ Concentration on Admission and Ingestion Amount on Prognosis Using Time-Dependent ROC Analysis
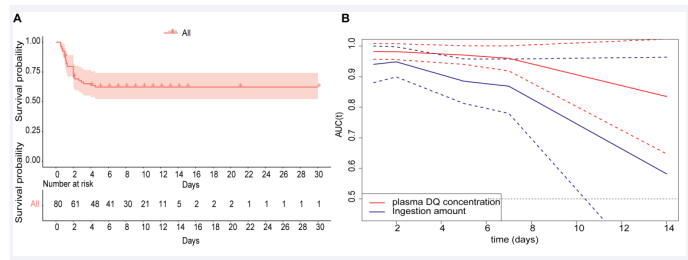
The cumulative survival probability in all cases were shown in (Figure 3A).
Figure 3: These results revealed that AUROC of plasma DQ concentration on admission and ingestion amount decreased with days after poisoning.
The 1-,2-,4- and 4.5-days cumulative survival probability in all cases were 87.5%, 70.5%, 63.7% and 62.3%, respectively. The median survival time was 19.33 days (95%CI: 16.256-22.410). No events occurred beyond 5 days in this study. We conducted a time-dependent ROC analysis for assessing the impact of plasma DQ concentration on admission and ingestion amount on the prediction of DQ poisoning patient survival or death. In this study, according to the time characteristics of the death event, we computed time-dependent AUCs (95%CI) to evaluate their predictive accuracy at 1,2,5,7 and 14 days, respectively (Table 4).
Table 4: Prognostic value of plasma DQ concentration on admission and ingestion amount on prognosis.
|
Indices |
plasma DQ concentration on admission |
Ingestion amount |
||||
|
AUC |
Lower 95% |
Upper 95% |
AUC |
Lower 95% |
Upper 95% |
|
|
1d |
0.983 |
0.957 |
1.008 |
0.94 |
0.88 |
1 |
|
2d |
0.982 |
0.956 |
1.007 |
0.948 |
0.899 |
0.998 |
|
5d |
0.97* |
0.94 |
1.001 |
0.885 |
0.812 |
0.958 |
|
7d |
0.96 |
0.919 |
1 |
0.869 |
0.78 |
0.958 |
|
14d |
0.835 |
0.648 |
1.023 |
0.582 |
0.201 |
0.964 |
Continuously changing AUCs and confidence intervals as days after poisoning were drawn using the plot AUC curve function, as shown in (Figure 3B). These results revealed that AUROC of plasma DQ concentration on admission and ingestion amount decreased with days after poisoning. In contrast, the AUROC of plasma DQ concentration on admission was higher than ingestion amount at each time point. There were statistically significant (p < 0.05) at 5 days after poisoning. The optimal cut-off point was 1.05 ug/ml (AUROC = 0.971, sensitivity= 100%, specificity = 62.69%) of plasma DQ concentration on admission at 5 days. But the prognostic value of both decreased significantly at 7 days after poisoning.
DISCUSSION
DQ, as a nonselective bipyridinium herbicide, belongs to moderately hazardous (class II) technical grade active ingredients in pesticides according to the WHO recommended classification of pesticides by hazard and guidelines to classification (2019) [16]. In the present study, the hospital case fatality rate of acute DQ poisoning was 36.25% (29/80). The longest survival time of 4.5 days after DQ poisoning, with 23 of the 29 patients dying within 2 days. DQ is more effective than other herbicides at generating Reactive Oxygen Species (ROS). Through reduction-oxidation (redox) cycling processes, it can produce reactive oxygen and reactive nitrogen, resulting in oxidative stress and multiple organ damage [3,17]. DQ poisoning mostly affects the kidneys, CNS, and lungs. A rise in serum creatinine and anuria are the most common sign of kidney injury. Varying degrees of consciousness disturbance and (or) epileptiform seizures could be noticed within 36 h of admission, particularly in non-survivors [18-20]. DQ can also damage the lungs, leading to upper respiratory pain, pulmonary edema, and respiratory depression. However, unlike PQ, pulmonary fibrosis has not been observed [3,21]. In the present study, blood purification was utilized to promote the excretion of absorbed poisons, but there were no significant differences in different treatment regimens, which consist with previous studies [22-24].
The Schoenfeld residuals were used to verify the proportional hazards assumption. The result showed that plasma DQ concentration on admission is a time-dependent covariate. As a result, we present a time-dependent Cox regression model for DQ poisoning prognosis estimate. Considering that the variation of time is generally correspondent to the skewed distribution, the natural logarithm of the time variable was used for the construction of the time-dependent covariates in time-dependent Cox regression model to reduce the influences of extreme values [25]. According to the multivariate Cox analysis, plasma DQ concentration on admission and PSS within 24 h of admission were independent prognostic factors for in-hospital death in acute oral DQ poisoning patients. Plasma DQ concentration on admission and T_COV_PDQ were both statistically significant, indirectly suggesting its departure from the proportional hazards assumption. We found that the HR of plasma DQ concentration decreases with varying time (≤ 1.5 days) due to the negative values of regression coefficients (coef) of T_COV_PDQ. This result is probably relative to the toxicokinetic characteristics of DQ in patients [3]. PSS is a simple, less time-consuming, and effective evaluation scale for predicting the severity and mortality of poisoning in emergency [26]. In the present study, PSS was treated as a continuous variable. For each unit increase in PSS, the hazard of in-hospital case fatality rate increased by 3.47 on the original scale.
Ingestion amount is often used as one of the indicators for determining disease grade and prognosis [3,4,17]. However, ingestion amount is often greatly affected by the subjective wishes of patients and doctors. To obtain relatively accurate ingestion amounts, this study was performed by two specially trained staff members to assist patients to reproduce the ingestion amount of DQ. The ingestion amount of DQ was 50 (80) ml. In addition, the ingestion amounts of non-survivors were significantly greater than survivors (P < 0.001). However, it was not an independent prognostic factor in multivariate Cox regression analysis. According to time-dependent ROC analysis, the AUROC of plasma DQ concentration on admission was larger than ingestion amount at each time point and was statistically significant (p < 0.05) at 5 days after poisoning. These results indicate that plasma DQ concentration on admission was superior to ingestion amount for the prediction of DQ poisoning patient survival or death. In this study, the longest survival time of 4.5 days after DQ poisoning, and no events occurred beyond 5 days. This may be relative to the fact that the most of non-survivors were fulminant poisoning.
By using a cut-off value (1.05 ug/ml, AUROC = 0.971, sensitivity= 100%, specificity = 62.69%) of plasma DQ concentration on admission at 5 days after poisoning, the cumulative survival rate of the high concentration group (≥ 1.05 ug/ml) was only 17.1%. Therefore, for patients with plasma DQ concentration above 1.05 ug/ml taken within 36 hours after ingestion, the prognosis is poor. And neither HP nor HP combined with CVVH could improve target organ damage. However, for patients with plasma DQ concentrations below 1.05 ug/ml taken within 36 hours after ingestion, HP and (or) CVVH should be administered actively administered early to increase toxicant excretion, reduce target organ damage, and improve patient prognosis.
Our study has some limitations. Firstly, the present study is a single-center retrospective study. The sample size is rather small for patients with acute oral DQ poisoning for the statistical analysis. A multi-center clinical study is required. Secondly, the most of non-survivors were fulminant poisoning and died within 2 days after poisoning, potentially leading to bias. Finally, due to ethical considerations, all patients included in this study were treated with blood purification. Although this study does not prove that blood purification can affect the prognosis of patients, its efficacy still needs to be further explored.
CONCLUSION
Plasma DQ concentration on admission and PSS within 24 h of admission are independent prognostic factors for in-hospital case fatality rate of patients with acute oral DQ poisoning. The prognostic value of plasma DQ concentration decreased with time.
REFERENCES
- Zyoud SH. Investigating global trends in paraquat intoxication research from 1962 to 2015 using bibliometric analysis. Am J Ind Med. 2018; 61(6): 462-470. doi: 10.1002/ajim.22835. Epub 2018 Mar 14. PMID: 29537078.
- Bang YJ, Kim J, Lee WJ. Paraquat use among farmers in Korea after the ban. Arch Environ Occup Health. 2017; 72(4): 231-234. doi: 10.1080/19338244.2016.1192982. Epub 2016 May 24. PMID: 27219666.
- Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol. 2018; 37(11): 1131-1160. doi: 10.1177/0960327118765330. Epub 2018 Mar 23. PMID: 29569487.
- Expert consensus group on the diagnosis and treatment of acute diquat poisoning. Expert consensus on the diagnosis and treatment of acute diquat poisoning. Chin J Emerg Med. 2020; 29(10): 1282- 1289. DOI: 10.3760/cma.j.issn.1671-0282.2020.10.002.
- Hart TB, Nevitt A, Whitehead A. A new statistical approach to the prognostic significance of plasma paraquat concentrations. Lancet. 1984; 2(8413): 1222-1223. doi: 10.1016/s0140-6736(84)92784-3. PMID: 6150271.
- Hampson EC, Pond SM. Failure of haemoperfusion and haemodialysis to prevent death in paraquat poisoning. A retrospective review of 42 patients. Med Toxicol Adverse Drug Exp. 1988; 3(1): 64-71. doi: 10.1007/BF03259932. PMID: 3285127.
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014; 81(3): 14-18.
- Persson HE, Sjöberg GK, Haines JA, Pronczuk de Garbino J. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998; 36(3): 205-13. doi: 10.3109/15563659809028940. PMID: 9656975.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13(10): 818-29. PMID: 3928249.
- Kubo K, Azuma A, Kanazawa M, Kameda H, Kusumoto M, Genma A, et al. Consensus statement for the diagnosis and treatment of drug- induced lung injuries. Respir Investig. 2013; 51(4): 260-277. doi: 10.1016/j.resinv.2013.09.001. Epub 2013 Oct 28. PMID: 24238235.
- Devarbhavi H, Aithal G, Treeprasertsuk S, Takikawa H, Mao Y, Shasthry SM, et al. Drug-induced liver injury: Asia Pacific Association of Study of Liver consensus guidelines. Hepatol Int. 2021; 15(2): 258- 282. doi: 10.1007/s12072-021-10144-3. Epub 2021 Feb 27. PMID: 33641080.
- Perazella MA, Rosner MH. Drug-Induced Acute Kidney Injury. Clin J Am Soc Nephrol. 2022; 17(8): 1220-1233. doi: 10.2215/CJN.11290821. Epub 2022 Mar 10. PMID: 35273009; PMCID: PMC9435983.
- Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012; 27(12): 4263-4272. doi: 10.1093/ndt/gfs375. Epub 2012 Oct 8. PMID: 23045432; PMCID: PMC3520085.
- Dobbs MR. Toxic encephalopathy. Semin Neurol. 2011; 31(2): 184- 193. doi: 10.1055/s-0031-1277989. Epub 2011 May 17. PMID: 21590623.
- Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis- Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018; 6(7): 121. doi: 10.21037/ atm.2018.02.12. PMID: 29955581; PMCID: PMC6015946.
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019. 2020.
- Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J Toxicol Clin Toxicol. 2000; 38(2): 123-128. doi: 10.1081/clt-100100926. PMID: 10778908.
- Schmidt DM, Neale J, Olson KR. Clinical course of a fatal ingestion of diquat. J Toxicol Clin Toxicol. 1999; 37(7): 881-884. doi: 10.1081/ clt-100102471. PMID: 10630275.
- Vanholder R, Colardyn F, De Reuck J, Praet M, Lameire N, Ringoir S. Diquat intoxication: report of two cases and review of the literature. Am J Med. 1981; 70(6): 1267-1271. doi: 10.1016/0002- 9343(81)90836-6. PMID: 7015857.
- Yu G, Jian T, Cui S, Shi L, Kan B, Jian X. Acute diquat poisoning resulting in toxic encephalopathy: a report of three cases. Clin Toxicol (Phila). 2022; 60(5): 647-650. doi: 10.1080/15563650.2021.2013495. Epub 2022 Jan 4. PMID: 34982016.
- Yang W, Ma X, Zhu Y, Meng X, Tian R, Yang Z. Paraquat but not diquat induces TGF-β expression and thus activates calcium-NFAT axis for epithelial-mesenchymal transition. Toxicol Res (Camb). 2021; 10(4): 733-741. doi: 10.1093/toxres/tfab055. PMID: 34484664; PMCID: PMC8403590.
- Hantson P, Wallemacq P, Mahieu P. A case of fatal diquat poisoning: toxicokinetic data and autopsy findings. J Toxicol Clin Toxicol. 2000; 38(2): 149-152. doi: 10.1081/clt-100100930. PMID: 10778912.
- Powell D, Pond SM, Allen TB, Portale AA. Hemoperfusion in a child who ingested diquat and died from pontine infarction and hemorrhage. J Toxicol Clin Toxicol. 1983; 20(5): 405-420. doi: 10.3109/15563658308990609. PMID: 6668627.
- Meng N, SunYQ, Dong YL. Toxicokinetic and the therapeutic effect of hemoperfusion in patients with acute diquat poisoning. Chin J Emerg Med, 2020, 29(11): 1403-1410. DOI: 10.3760/cma.j.is sn.1671-0282.2020.11.005.
- Zhang C, Li X, Li F, Li G, Niu G, Chen H, et al. Accurate prediction and further dissection of neonicotinoid elimination in the water treatment by CTS@AgBC using multihead attention-based convolutional neural network combined with the time-dependent Cox regression model. J Hazard Mater. 2022; 423(Pt A): 127029. doi: 10.1016/j. jhazmat.2021.127029. Epub 2021 Aug 25. PMID: 34479086.
- Churi S, Ramesh M, Bhakta K, Chris J. Prospective assessment of patterns, severity and clinical outcome of Indian poisoning incidents. Chem Pharm Bull (Tokyo). 2012; 60(7): 859-864. doi: 10.1248/cpb. c12-00171. PMID: 22790818.