The Incidence of Monoclonal Gammopathy of Renal Significance in a Population with Monoclonal Gammopathy and Renal Dysfunction: A Descriptive Analysis at a Single Institution
- 1. Department of Internal Medicince, Tufts Medical Center, Boston, MA
- 2. Division of Nephrology, Tufts Medical Center, Boston, MA
- 3. The John Conant Davis Myeloma and Amyloid Program, Division of Hematology-Oncology, Tufts Medical Center, Boston, MA
Abstract
Background: Monoclonal gammopathy of renal significance (MGRS) is a term coined to describe patients with monoclonal gammopathy and concomitant renal pathology, typically due to immunoglobulin deposition or a fragment thereof, without evidence of an overt hematologic malignancy. Early identification of MGRS and treatment with chemotherapy can prevent progression to kidney failure. There is limited data on the characteristics of populations with concomitant monoclonal gammopathy of unknown significance (MGUS) and chronic kidney disease (CKD), and on the prevalence of diagnosed MGRS in this particular population.
Methods: Through retrospective chart review, we identified 246 patients with ICD-9 or -10 codes denoting both MGUS and chronic kidney disease (CKD) between the years of 2000 and 2017. Patients with related overt malignancies such as multiple myeloma, Waldenstrom’s macroglobulinemia, and amyloidosis at onset were excluded, leaving 144 evaluable patients.
Results: The median eGFR was 48 mL/min/1.73 m² at the time of MGUS diagnosis, and the median M-protein was 0.54 g/dL in patients with a quantifiable gammopathy.
Conclusion: MGRS needs to be considered in patients with a monoclonal protein and chronic kidney disease. Renal biopsies are underutilized. Based on our findings, we propose a simple algorithm for workup of suspected MGRS.
What is already known about this subject: Monoclonal gammopathy of renal significance (MGRS) is a disorder of deposition of monoclonal protein in the kidney without overt multiple myeloma or lymphoproliferative disorder, and can result in irreversible kidney failure.
What this study adds: This study describes the characteristics of a population who harbors both a monoclonal protein and CKD, and identifies the prevalence of MGRS in this cohort. We postulate that MGRS is likely underreported due to a lack of kidney biopsies and propose a simple algorithm for the workup of MGRS.
What impact this may have on practice or policy: This study may result in increased awareness of MGRS as a disease as well as an increased need for the pursuit of a renal biopsy in this particular at-risk population.
Keywords
• Cancer
• Chronic kidney disease
• Monoclonal gammopathy
• Multiple myeloma
• Renal biopsy
Citation
Kumar AD, Inker LA, Drew DA, Comenzo RL, Varga C (2021) The Incidence of Monoclonal Gammopathy of Renal Significance in a Population with Monoclonal Gammopathy and Renal Dysfunction: A Descriptive Analysis at a Single Institution. JSM Renal Med 4(1): 1016.
INTRODUCTION
The term monoclonal gammopathy of renal significance (MGRS) was coined in 2012 to describe patients with a monoclonal gammopathy and deposition of the secreted immunonoglobulin or its light or heavy chain components in the kidney (1). The diagnosis of MGRS requires hematological workup in search of a monoclonal protein and histologic confirmation via kidney biopsy (2). However, there are reported cases of patients who lack a monoclonal protein on serum or urine electrophoresis/ immunofixation, thus, making a kidney biopsy even more critical in identifying this rare disorder (3) .
Patients with MGRS will progress to end stage renal disease (ESRD) without intervention. There is an associated risk of disease recurrence in patients who have undergone kidney transplant if the culprit plasma cell or B cell clone is not eradicated (3, 4). There has been increased attention given to distinguishing MGRS from monoclonal gammopathy of unknown significance due to the recognition that therapy targeting clonal plasma cells in MGRS can prevent further immunologlobulin deposition, reduce long-term kidney damage, and improve survival (4-6). Early intervention increases the chances of eventual renal recovery (4).
Although several articles exist describing MGRS, there are very few that have investigated the prevalence of MGRS and the characteristics of a patient population with co-existing MGUS and chronic kidney disease (CKD) at a tertiary care institution.
MATERIALS AND METHODS
We received approval from the Tufts Institutional Review Board to conduct a descriptive analysis. We subsequently searched two electronic health records (Soarian, used primarily for inpatient admissions and MOSAIQ, used for outpatient hematology/oncology clinics at our institution) to identify patients from the year 2000 to 2017 who had associated ICD-9 or -10 codes corresponding to both monoclonal gammopathy and chronic kidney disease. A total of 246 patients were identified meeting these criteria.
We eliminated patients who had a known diagnosis of multiple myeloma or Waldenstrom’s macroglobulinemia at study onset, but retained those who began as MGUS and underwent malignant transformation over the course of the study. We also eliminated patients who were incorrectly coded as having MGUS, leaving 148 patients. We then eliminated an additional 4 patients who did not meet the criteria of CKD by eGFR below 60 or presence of a marker of kidney damage (proteinuria/albuminuria, abnormalities of urine sediment, structural abnormalities, tubular disorders). This left a total of 144 cases on whom all subsequent data collection and analysis was performed (Supplemental Figure 1).
For each patient, we collected baseline characteristics (age, gender, race, smoking status, co-morbid conditions), details of how the patient came to the attention of a hematologist/ oncologist, details of the monoclonal gammopathy (bone marrow biopsy results, serum kappa/lambda light chains, serum and urine protein electrophoresis and immunofixation), and details of the underlying kidney disease (suspected etiology of the kidney disease, stage, eGFR, creatinine, urine protein/creatinine, urine albumin/creatinine, kidney biopsy results, treatment). We also evaluated clinician documentation to determine if MGRS was considered as a possible diagnosis. We then determined whether these characteristics differed between patients who underwent kidney biopsy and patients that did not undergo kidney biopsy
RESULTS
One hundred and forty-four patients fulfilled criteria of harboring both an MGUS and CKD from 2000 to 2017 at a tertiary care center. The majority were predominantly male (59.7%) and white (67.1%), had a median age of 61 years at study onset, and carried diagnoses for the following co-morbid conditions: hypertension (78.3%), coronary artery disease (30.6%), diabetes mellitus (35.0%). (Table 1).
|
Table 1: Baseline characteristics of total study population (n=144). |
|
IgM isotype |
15 (10.4%) |
||
|
Baseline characteristics |
IgA isotype |
16 (11.1%) |
|||
|
Sex (n, %) |
|
IgD isotype |
1 (0.7%) |
||
|
Male |
85 (59.0%) |
Light chain only (either kappa or lambda) |
8 (5.5%) |
||
|
Female |
59 (40.9%) |
||||
|
Heavy chain only |
1 (0.7%) |
||||
|
Race (n, %) |
|
||||
|
Biclonal |
23 (16.1%) |
||||
|
White |
96 (66.7%) |
||||
|
Other* |
20 (13.9%) |
||||
|
Black |
21 (14.6%) |
||||
|
eGFR (median, mL/min/1.73m2) |
48 (35-64) |
||||
|
Asian |
22 (15.2%) |
||||
|
Creatinine (median and IQR, mg/dL) |
1.4 (1.09-1.74) |
||||
|
Other |
5 (3.5%) |
||||
|
Albumin/creatinine (median, mg/g) |
90 (18.45-725.5) |
||||
|
Hypertension (n, %) |
114 (78.4%) |
||||
|
CKD stage (n, %) |
|||||
|
Coronary artery disease (n, %) |
44 (30.6%) |
||||
|
1 |
9 (6.3%) |
||||
|
Diabetes mellitus (n, %) |
50 (34.7%) |
||||
|
2 |
22 (15.3 %) |
||||
|
M-spike* (median and IQR**, g/dL) |
0.54 (0.3-0.8) |
3A |
27 (18.8%) |
||
|
κ in kappa gammopathies (median and IQR) |
20.3 (12.6-31.6) |
3B |
25 (17.4%) |
||
|
λ in lambda gammopathies (median and IQR) |
30.7 (20.65-66.9) |
4 |
9 (6.3%) |
||
|
Monoclonal |
N (%) |
5 |
6 (4.2%) |
||
|
IgG isotype |
60 (41.7%) |
Unknown |
46 (31.9%) |
||
The median M-spike was 0.54 g/dL (0.3-0.8 g/dL), with IgG kappa being the most common isotype.
The median creatinine was 1.4 mg/dL (1.09-1.74 mg/dL) and eGFR was 48 mL/min/1.73m2 (35-64 mL/min/1.73m2 )
One hundred and thirteen patients (79.0%) were evaluated at the hematology/oncology clinic at our institution. Patients were referred most commonly through their primary care physician (38.1%) or nephrologist (22.1%). Eighty patients (55.6%) were actively being followed at the end of data collection, and 13 patients (9.1%) were confirmed to be deceased.
Fifty-two of the 144 patients (36.1%) had at least one bone marrow biopsy and 1 patient had an attempted but failed bone marrow biopsy. Kidney biopsy was performed in 18 of 144 (12.5%) patients. Two of the 18 cases underwent nephrectomies.
The patients who underwent kidney biopsy had higher median age at study onset: 62 years (54-69 years) Versus 57 had a higher median age (50.5 - 61 years), lower eGFR: 41 mL/ min/1.73m2 (22.25-60 mL/min/1.73m2 ) versus 49 (36-65 mL/ min/1.73m2 ), higher creatinine: 1.71 mg/dL (1.23-2.5 mg/dL) versus 1.38 mg/dL (1.04-1.70 mg/dL), and greater albumin/ creatinine ratio: 886 (93.5-2189) versus 75.5 (15.45-181.5). However, there was no discernable pattern or difference with regards to plasma cell marker parameters between the groups (Supplemental Figure 2).
In the total study population, kidney disease was mostly attributed to the following causes by the evaluating physician: hypertension (40%), repeated insults from acute kidney injury (14.6%), and diabetes (13.9%); MGRS was considered in 20 patients (13.9%). In the 18 patients who underwent (72.2%), vascular/hypertensive changes (61.1%), tubular disorders (44.4%); MGRS was confirmed in 3 patients (16.7%).
One patient was a 66 year old male with IgM kappa gammopathy with proteinuria who was found by kidney biopsy to have monoclonal immunoglobulin deposition disease (MIDD) and diabetic glomerulopathy. He was treated with rituximab for four weeks, but further treatment was not pursed due to deteriorating hematologic markers and kidney function. The second patient was a 74 year old male with lower extremity edema and nephrotic range proteinuria (5 grams/day). Kidney biopsy demonstrated MIDD, but interestingly his serum, urine, and bone marrow showed no evidence of a plasma cell clone. He was treated with chemotherapy (cyclophosphamide, bortezomib, and dexamethasone) at an outside facility and was lost to follow up. The third patient was a 74 year old male with IgG kappa MGUS, ESRD requiring hemodialysis, and MIDD on kidney biopsy. He did not receive chemotherapy initially due to active infection, and later was not thought to be a candidate due to cited vague benefit and high risk for complication.
Eight of the 144 patients (5.6%) progressed to smoldering or symptomatic multiple myeloma, and 1 (0.7%) progressed to Waldenstrom’s macroglobulinemia.
DISCUSSION
MGRS is a subset of MGUS whereby immunoglobulin deposition occurs in the kidney without overt myeloma or cast nephropathy. The mechanism of kidney damage is postulated to occur directly from renal deposition leading to tubular toxicity, or indirectly via either the precipitation of immunoglobulins/immune complexes or by activation of complement (4, 7). Kidney biopsy remains the only definitive diagnostic tool for MGRS. Notably, kidney biopsy is not without risk, and can be associated with hematuria (4.9%), hematoma (0.6%), or hypotension/shock (0.6%)(8).
We demonstrate that MGRS is considered only in a small subset of patients with MGUS and CKD, and kidney biopsy is pursued in an even smaller fraction of this population. This finding is consistent with current recommendations not to investigate MGUS if alternative causes of kidney disease exists, and is likely in part due to the fact that MGRS is a relatively new entity, with which many healthcare providers may not be familiar.
We predict that a significant number of cases of MGRS are being missed, and thus, recommend a lower threshold for pursuing kidney biopsy in patients with a monoclonal protein and a declining eGFR of unclear etiology or worsening proteinuria, as demonstrated by our proposed algorithm (Figure 1).
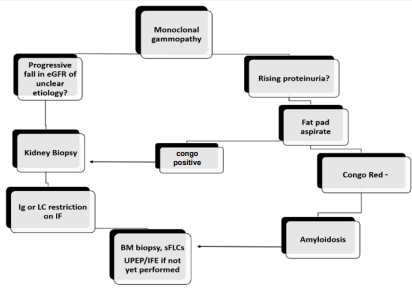
Figure 1 Algorithm for pursuing kidney biopsy. We recommend that patients with MGUS who have a progressive fall in eGFR or worsening proteinuria undergo the following steps leading to a kidney biopsy to evaluate for MGRS. eGFR: estimated glomerular filtration rate; Ig: immunoglobulin; LC: light chain; BM: bone marrow; sFLCs: serum free light chains; IF: immunofluorescence; UPEP/IFE: urine protein electrophoresis/immunofixation
Our algorithm differs from other proposed algorithms in that we include a fat pad aspirate early on in the investigation (9). A fat that is positive for congo red can avert the need for an invasive renal biopsy. If the fat is negative for amyloid deposit, then renal biopsy needs to be pursued. The benefit of a kidney biopsy and initiating anti-plasma cell therapy is less certain in patients presenting with ECOG 4, a lifespan of less than 6 months, or very advanced renal disease (eGFR <15), unless renal transplant is considered in the future. Patients who are at high risk for complications or those who would not tolerate MGRS treatment should also not undergo biopsy.
Owing to the retrospective nature of our study, we were limited by the fact that not all metrics were available for every patient. Furthermore, some patients had partial workup at other institutions. Our study may have also missed individuals with MGUS and CKD if they were not correctly coded as such. Additionally, given the small number of patients included in this brief report, no definitive or broad conclusions can be made. Conversely, what we can state with high certainty is that evaluation of a patient with suspected MGRS requires an interdisciplinary approach between hematology and nephrology (3).
Over the last year at our institution, we have used the above algorithm, which was designed in 2019, to establish an MGRS clinic, where patients are seen by providers from both hematology and nephrology to allow for joint decision making. We are continuing to collect data on this particular population and we hope to compare outcomes before and after the implementation of this algorithm. We predict that over time, as our understanding of MGRS grows, more patients will undergo kidney biopsy to identify this condition and more multidisciplinary MGRS clinics will form.
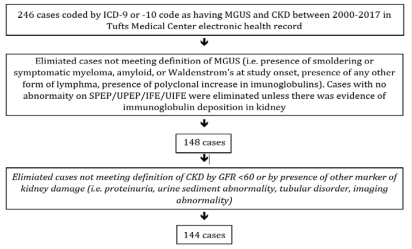
Supplementary Figure 1 Flow diagram demonstrating how cases initially identified by ICD codes were systematically excluded if they did not meet the definition of MGUS or CKD on further review.
Supplementary Table 1: Confirmed or possible cases of MGRS.
| Suspected MGRS cases | N = 20 |
| Biopsied n(%) | 5 (25%) |
| MGRS confirmed, treated | 2 (1.4%) |
| MGRS confirmed, untreated | 1 (0.7%) |
| Other abnormal kidney biopsy without formal diagnosis of MGRS | 2 (1.4%) |
| Not biopsied n (%) | 14 (9.7%) |
| Biopsy status unknown n (%) | 1 (0.7%) |
Supplementary Table 2: Key characteristics of patients based on kidney biopsy status.
| Patient Underwent Kidney Biopsy (n = 18) | Patient Did Not Undergo Kidney Biopsy (n = 116) | |||
| Median | Interquartile range | Median | Interquartile range | |
| Age at study onset (years) | 57 | 60.6-61 | 62 | 54-69 |
| M-spike (median, g/dL) | 0.41 | 0.4-0.75 | 0.6 | 0.3-0.8 |
| κ in kappa gammopathies (median) | 52.65 | 28.33-71.78 | 40.78 | 21.33-61.53 |
| λ in lambda gammopathies (median) | 51.0 | 34.9-69 | 30.3 | 22.75-70.75 |
| eGFR (median, mL/min/1.73m2) | 41 | 22.25-60 | 49 | 36-65 |
| Creatinine (median, mg/dL) | 1.71 | (1.25-2.5) | 1.38 | (1.04-1.7) |
| Albumin/creatinine (median, mg/g) | 886 | 93.5-2189) | 75.5 | (15.45-181.5) |








































































































































