Deconstructing a Paradigm: Chemoradiation and the Resurgent Role of Surgery in Locally Advanced Cervical Cancer
- 1. Department of Surgical Oncology, National Cancer Institute, Cairo University, Eygpt
Abstract
Background: For over two decades, concurrent chemoradiation (CRT) has been the cornerstone of treatment for locally advanced cervical cancer (LACC). However, survival outcomes have stagnated, with distant metastasis now the primary mode of failure and CRT imposes a significant burden of permanent toxicity that impairs long-term quality of life.
Objective: This review critically appraises the evidence for CRT and evaluates the modern role of surgery in the management of LACC.
Methods: A narrative review was conducted, synthesizing evidence from pivotal randomized trials, contemporary retrospective cohorts, meta-analyses, and major societal guidelines.
Findings: While CRT remains a cornerstone, its universal application is being questioned due to toxicities and survival plateaus. For selected patients with early LACC (FIGO IB3/IIA2), modern radical hysterectomy offers a valid alternative, providing a superior long-term quality-of-life profile by avoiding radiation sequelae, but only if adjuvant radiotherapy can be avoided. Furthermore, the integration of immunotherapy is reshaping the therapeutic landscape for both modalities.
Conclusion: The management of LACC is evolving beyond a universal CRT paradigm towards a personalized approach. Primary surgery is a compelling option for a well-selected subset, aiming to optimize quality of life without compromising survival. The future lies in prospective trials that integrate modern surgery and novel systemic therapies to definitively guide patient-specific treatment choices and validate personalized paradigms.
Keywords
• Locally Advanced Cancer Cervix
• Chemoradiation
• Surgery
Citation
Khalifa H, Oreaba R (2025) Deconstructing a Paradigm: Chemoradiation and the Resurgent Role of Surgery in Locally Advanced Cervical Cancer. JSM Sexual Med 9(5): 1168.
INTRODUCTION
The longstanding paradigm of concurrent chemoradiation (CRT) as the universal standard for locally advanced cervical cancer (LACC) is facing a critical reassessment [1,2]. Despite its foundational role, long-term survival outcomes have plateaued, and CRT is associated with substantial and permanent morbidity that profoundly impacts quality of life (QoL) [3,4]. Driven by these limitations, surgical oncology has undergone a revolution. The development of the anatomically-based Querleu-Morrow classification and refined nerve-sparing techniques have modernized radical hysterectomy, reducing morbidity while maintaining oncologic efficacy [5]. Reflecting this progress, contemporary guidelines now recognize radical hysterectomy as a valid alternative to CRT for selected patients with early LACC (FIGO Stages IB3 and IIA2) [6]. This article aims to critically re-appraise the historical evidence for CRT, synthesize the modern evidence supporting primary surgery for selected patients, and discuss how these developments, combined with novel therapies, are driving a personalized paradigm shift in LACC management.
The Historical Pillar: Evidence for Chemoradiation
Prior to the 1990s, primary radiotherapy for LACC yielded suboptimal survival, prompting the investigation of cisplatin-based chemotherapy as a radiosensitizer to address both local and micrometastatic disease. Concurrent cisplatin-based chemoradiation (CRT) remains the standard of care for LACC, supported by level 1 evidence from pivotal trials published in 1999 (Table 1). These studies demonstrated a significant survival benefit over radiation alone, leading to a ~30-50% reduction in the risk of death [1,2,7]. Cisplatin’s efficacy stems from its ability to inhibit the repair of radiation-induced DNA damage. This compelling and consistent evidence prompted the NCI’s 1999 clinical announcement, establishing a paradigm that would define LACC management for over twenty years.
Table 1: Foundational Trials Establishing Chemoradiation as Standard of Care.
|
Trial / Analysis |
Population |
Intervention vs. Control |
Key Finding |
Conclusion |
|
GOG 85 |
Stages IIB-IVA |
Cisplatin/5-FU + RT vs. Hydroxyurea + RT |
Superior PFS/OS |
Established cisplatin-based combo as superior radiosensitizer |
|
RTOG 9001 |
Stages IB2-IVA |
CRT vs. Extended-Field RT |
5-yr OS: 73% vs. 58% |
CRT drastically improves survival |
|
GOG 120 |
Stages IIB-IVA |
Weekly Cisplatin + RT vs. other regimens |
Equivalent efficacy, better tolerability |
Established weekly cisplatin as the preferred regimen |
|
GOG 123 |
Bulky Stage IB |
CRT + Brachy vs. RT + Brachy |
51% reduction in mortality risk |
CRT superior to RT alone for bulky early disease |
5. A. Long-Term Toxicity and Quality of Life
While CRT is highly effective, it is associated with a significant burden of chronic, often permanent, side effects that profoundly impact long-term quality of life (Table 2). Modern techniques like IMRT can reduce but not eliminate these risks [4,8]. The sequelae are not merely clinical notes but life-altering conditions that affect daily functioning, body image, and mental health.
B. Stagnant Survival Outcomes and the Shift to Systemic Failure
Despite two decades of research, the regimen of weekly cisplatin with CRT remains the global standard, representing a therapeutic ceiling. Efforts to intensify concurrent chemotherapy or refine radiation delivery have failed to consistently improve survival [9,10]. Consequently, with modern brachytherapy achieving local control rates >90%, distant metastasis has become the dominant pattern of failure, accounting for 60-70% of recurrences [11]. This shift unmasks the principal weakness of the current paradigm: its inability to adequately control systemic disease, highlighting an urgent need for more effective systemic strategies.
Table 2: The Burden of Long-Term Toxicity from CRT*
|
Organ System |
Common Toxicities |
Clinical Impact & Prevalence |
|
Gastrointestinal |
Chronic proctitis: diarrhea, urgency, incontinence [4] |
Major impact on social life and diet; >40% of survivors report chronic symptoms [Kirchheiner, 2017]. |
|
Vaginal & Sexual |
Stenosis, fibrosis, dryness, dyspareunia [8] |
Devastating impact on sexual health and intimacy; a primary concern for younger patients. |
|
Gonadal |
Ovarian failure in premenopausal women [6] |
Irreversible surgical menopause, sacrificing fertility and endocrine health. |
|
Other |
Lymphedema, pelvic fractures, secondary malignancies |
Chronic disability, pain, and increased long-term health risks. |
C. The “One-Size-Fits-All” Problem: A Blunt Instrument
The universal application of CRT across the clinically heterogeneous spectrum of LACC (Stages IB3 to IVA) is a fundamental limitation of the current paradigm. This approach fails to distinguish between patients with vastly different risks, leading to both overtreatment and undertreatment (Table 3) [6,12].
Table 3: The "One-Size-Fits-All" Problem Illustrated*
|
Aspect |
Patient with Early LACC (e.g., Stage IB3) |
Patient with Advanced LACC (e.g., Stage IIIB) |
|
Primary Risk |
Local |
Distant Metastasis |
|
CRT Approach |
Universal Application |
Universal Application |
|
Result |
Overtreatment: Exposed to definitive long-term toxicity for a risk that could be managed with a single local modality (surgery). |
Undertreatment: Inadequate systemic control for a disease with high risk of microscopic dissemination. |
|
Personalized Alternative |
Primary surgery to avoid radiation toxicity. |
CRT intensified with effective systemic therapy (e.g., immunotherapy). |
maintaining oncologic efficacy, making contemporary radical hysterectomy a more viable primary treatment. It is also critical to note that the surgical approach impacts outcomes. The landmark LACC trial demonstrated that minimally invasive radical hysterectomy was associated with worse disease-free and overall survival compared to open surgery for early-stage cervical cancer. This has firmly established open surgery as the recommended approach for radical hysterectomy in the curative setting, underscoring that technical excellence extends beyond classification to the method of access itself.
B. Primary Surgery for Stages IB3/IIA2: A Hypothesis in Need of Validation
Reflecting this progress, contemporary guidelines now endorse radical hysterectomy as a standard option for selected patients with early LACC [6]. Several retrospective cohort studies and meta-analyses have reported comparable survival outcomes between primary surgery and CRT for this population [14, 15]. However, this evidence is derived from non-randomized data subject to significant selection bias and must be interpreted with caution. The paramount goal of a surgery-first strategy is to avoid radiotherapy altogether. This is only achievable in patients with a high likelihood of having resectable disease without high-risk pathological features (positive nodes, parametrial involvement, positive margins), which would necessitate adjuvant CRT. The critical importance of selecting patients who will not need adjuvant therapy is starkly illustrated by the results of the EORTC 55994 trial.
Table 4: Evolution of Radical Hysterectomy Classification
|
Feature |
Piver-Rutledge-Smith (1974) |
Querleu-Morrow (2008) |
|
Basis |
Extent of tissue removal ("how much") |
Anatomical landmarks and structures ("what") |
|
Nerve Preservation |
Not considered |
Central to the classification (e.g., Type C1 vs. C2) |
|
Reproducibility |
Low, varies by surgeon |
High, allows for standardized training and reporting |
|
Primary Goal |
Maximize cancer control |
Balance oncologic efficacy with functional preservation |
C. Neoadjuvant Chemotherapy followed by Surgery (NACT-S): A Failed Alternative
The EORTC 55994 trial, the major Phase III RCT comparing NACT-S to definitive CRT, found that NACT-S was not non-inferior and was associated with significantly higher morbidity (Table 5) [16]. This poor outcome was largely driven by the ~50% of patients in the surgical arm who required adjuvant radiotherapy, resulting in toxic trimodality therapy. Therefore, NACT-S is not a standard alternative to CRT outside of specific scenarios like resource-limited settings or clinical trials.
Table 5: Key Prospective RCT: EORTC 55994
|
Trial (Year) |
Comparison |
Key Finding |
Implication for Practice |
|
EORTC 55994 (2018) |
NACT-S vs. CRT |
Trend towards worse OS (HR 1.29). Significantly higher morbidity. |
CRT remains standard. NACT-S is not non-inferior. The high rate of trimodality therapy is detrimental. |
7. The Critical Comparison: Weighing the Evidence
A. Oncologic Outcomes: Strategies, Not Just Modalities
The EORTC 55994 trial fundamentally reframes the comparison from “surgery vs. CRT” to a comparison of treatment strategies [16]. Its finding of a trend towards worse overall survival with NACT-S (HR 1.29) and higher morbidity was a direct consequence of poor patient selection, where ~50% of patients required adjuvant CRT, resulting in toxic trimodality therapy. In contrast, the Indian ICMR trial reported no significant survival difference, though concerns regarding radiotherapy quality complicate its interpretation. The patterns of failure also differ; while modern CRT fails predominantly at distant sites, surgery may fail more often locally, though isolated local recurrences may be amenable to salvage radiotherapy.
B. Quality of Life and Morbidity: A Clear Hierarchy
The “true” comparative QoL is a critical trade-off between different morbidity profiles over time. The evidence reveals a consistent hierarchy (Table 6):
Table 6: Comparative Morbidity and Quality of Life Profiles
|
QoL Domain |
Primary CRT |
Primary Surgery (No Adjuvant RT) |
Trimodality Therapy |
|
Bowel Function |
Significant long-term issues (proctitis, diarrhea) [4] |
Generally normal long-term function |
Severe, chronic issues |
|
Bladder Function |
Chronic cystitis, reduced capacity |
Near-normal with nerve-sparing |
High risk of chronic dysfunction/fistula |
|
Sexual Health |
Severe (vaginal stenosis, dyspareunia) [8] |
Better preserved vaginal function |
Most severe dysfunction |
|
Ovarian Function |
Always ablated |
Potentially preserved |
Ablated by adjuvant CRT |
|
Lymphedema |
Lower risk |
Moderate risk (from lymphadenectomy) |
Highest risk (synergistic) |
- Best QoL: Successful primary surgery without adjuvant radiotherapy. These patients trade a period of surgical recovery for a high probability of long-term normal bowel, bladder, and sexual function, and preserved ovarian activity [17].
- Intermediate QoL: Primary CRT. Patients avoid major surgery but face a high likelihood of permanent, life-altering side effects, particularly related to bowel and sexual function [4, 8].
- ?Worst QoL: Trimodality therapy. These patients suffer the cumulative morbidity of all treatment modalities, leading to the most profound and permanent negative impact on multiple QoL domains [18].
C. The Peril of Trimodality Therapy: The Central Tenet
The findings from both oncologic and QoL analyses converge on a single, paramount conclusion: the imperative to avoid trimodality therapy. It represents a worst-case scenario, combining the toxicities of all three modalities without a clear survival benefit [16]. Therefore, meticulous patient selection---using high-quality MRI to identify those with a high probability of complete resection without high-risk features---is not just a recommendation but the absolute prerequisite for a surgery-first strategy. The goal must be to select a patient for whom surgery will be the definitive local treatment.
8. The Modern Landscape: Integrating Novel Therapies
A. Immunotherapy and its Potential Synergy with Surgery
The success of the KEYNOTE-A18 trial, which added pembrolizumab to CRT, has fundamentally altered the standard of care for LACC, validating the critical need for effective systemic therapy to combat distant failure [19]. This breakthrough also opens innovative avenues for “immunosurgical” approaches. Here, neoadjuvant immunotherapy is used to prime the immune system in situ, after which radical surgery acts as an in vivo vaccine. The resection releases a burst of tumor antigens, potentially amplifying a systemic immune response to eradicate micrometastases---addressing the key weakness of surgery-alone [20]. While this hypothesis is promising, it remains highly investigational. The optimal sequencing, the risk of delaying definitive local therapy, and the impact of post-surgical stress on the immune response are unknown and must be rigorously tested in clinical trials.
B. Targeted Therapies and Antibody-Drug Conjugates (ADCs)
Beyond immunotherapy, targeted agents offer new paths for neoadjuvant cytoreduction. Antibody-drug conjugates (ADCs) like Tisotumab Vedotin, which delivers a cytotoxic payload directly to tissue factor-expressing tumor cells, have shown significant activity in metastatic disease [21]. Their targeted mechanism offers the potential for profound tumor shrinkage with a toxicity profile that may be more favorable than traditional chemotherapy. As neoadjuvant agents, they could achieve major pathological responses, potentially facilitating less radical surgery or rendering fertility-sparing procedures feasible in previously ineligible patients.
C. Towards an Integrated Future Paradigm
Collectively, these novel therapies are not merely new tools but catalysts for a more integrated treatment model. The historical dichotomy of “surgery versus CRT” is evolving into a paradigm where effective systemic therapy guides local treatment choice. Future strategies may involve neoadjuvant immunotherapy ± ADCs to debulk the tumor and control systemic disease, followed by response-adapted local therapy. This could mean surgery for responders (potentially with de-escalated radicality) or CRT-based approaches for non-responders, moving beyond a one-size-fits-all model to a truly personalized, dynamic sequence.
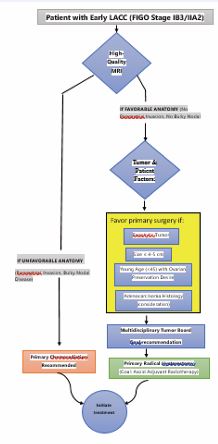
A Framework for Personalization: Who is Best Served by Surgery?
The selection of a primary surgical candidate is an exercise in predicting which patient can be cured with a single local modality, thereby avoiding the long- term toxicity of CRT and the catastrophic morbidity of trimodality therapy. The optimal candidate is defined by a confluence of factors that maximize the likelihood of complete resection without high-risk pathological features. This decision-making process is best guided by a conceptual pathway (Figure 1) integrating the following criteria:
Figure 1 Proposed Decision Pathway for Personalizing Primary Local Therapy in Early LACC.
- Stage and Resectability: The most critical factor. Surgery is most favorable for FIGO Stage IB3/IIA2, provided high-resolution MRI shows no radiological evidence of parametrial invasion or bulky nodal disease [6].
- Tumor Characteristics: Exophytic tumors ≤4-5 cm in size are more amenable to complete resection with clear margins. A “barrel-shaped” cervix or extensive LVSI are unfavorable characteristics that favor CRT.
- Histology: Adenocarcinoma, particularly gastric type, may be less radiosensitive, a perception that often pushes multidisciplinary teams towards surgery in otherwise eligible patients, though conclusive evidence is lacking.
- Patient Factors: Young age (<45) is a major driver, as primary surgery allows for ovarian transposition and preservation, avoiding immediate surgicalinferior to CRT for selected patients with stage IB3/ IIA2 cervical cancer?”---has never been answered in a large, randomized controlled trial. A well-designed RCT with co-primary endpoints of overall survival and quality of life is urgently needed.
inferior to CRT for selected patients with stage IB3/ IIA2 cervical cancer?”---has never been answered in a large, randomized controlled trial. A well-designed RCT with co-primary endpoints of overall survival and quality of life is urgently needed.
2. Refining Patient Selection: Prospective studies are required to validate and refine patient selection criteria, including the development of imaging and molecular biomarkers to better predict which patients will have low-risk pathology and excel with surgery alone.
3. Integrating Novel Therapies: The future lies in “immunosurgical” and targeted paradigms. Key research directions include:
- Testing neoadjuvant and perioperative immunotherapy to reduce distant failure after surgery.
- Investigating whether deep responses to novel agents like ADCs can facilitate less radical surgery or eliminate the need for adjuvant radiotherapy.
DISCUSSION
This critical reappraisal reveals that the management paradigm for LACC is in a state of necessary and productive evolution. The historical primacy of concurrent chemoradiation (CRT), while built upon a foundation of robust Level 1 evidence [1,2], is being rightfully challenged by the principles of personalized oncology. The central finding of this review is that the question is no longer if surgery has a role, but rather for whom it represents the optimal strategy to maximize both survival and quality of life.
The limitations of the CRT paradigm are now undeniable. The stagnation in survival outcomes despite two decades of research [9,10], coupled with the significant and permanent burden of long-term toxicity [4,8], creates a compelling rationale for re-evaluation. The shift in the pattern of recurrence to distant failure [11] underscores that the principal challenge is now systemic control---a challenge not adequately met by a single radiosensitizing agent. Furthermore, the “one-size-fits-all” application of CRT across a clinically heterogeneous disease spectrum fails to acknowledge that a patient with a bulky but resectable Stage IB3 tumor has vastly different risks and priorities than a patient with fixed Stage IIIB disease [6,12].
Concurrently, the resurgent interest in surgery is not a return to an outdated approach but is built upon the foundation of modern surgical oncology. The evolution from the Piver to the Querleu-Morrow classification [5,13], has been pivotal, providing a standardized, nerve sparing framework that reduces morbidity. Furthermore, the field has been refined by evidence establishing open surgery as the standard of care for radical hysterectomy in this setting. This technical progress is reflected in contemporary guidelines that now endorse radical hysterectomy for early LACC (IB3/IIA2) [6]. The collective evidence from retrospective studies and meta-analyses, while inherently reflecting the selection bias of choosing optimal candidates, consistently suggests that for these carefully selected patients, oncologic outcomes are comparable to those achieved with CRT [14,15].
The critical comparison of these modalities hinges on a fundamental trade-off: the acute, but often recoverable, morbidity of major surgery versus the chronic, life-altering sequelae of pelvic radiation. The hierarchy of quality-of life outcomes is clear: successful surgery without adjuvant radiotherapy offers the most favorable long-term profile, while the worst outcomes are unequivocally associated with trimodality therapy [16,18]. This makes meticulous patient selection the cornerstone of a successful surgical strategy. The failure of the EORTC 55994 trial to demonstrate non-inferiority for NACT-S [16], is not an indictment of surgery per se, but rather a stark warning about the perils of poor patient selection that leads to trimodality treatment.
The landscape is further complicated and energized by the integration of novel therapies. The success of immunotherapy in the KEYNOTE-A18 trial [19], has finally broken the survival plateau and sets a new standard against which all future treatments must be measured. More importantly, it opens a new frontier for synergistic “immunosurgical” approaches [20], where the combination of effective systemic therapy and radical surgery could potentially overcome the weaknesses of both standalone modalities---although this strategy remains largely hypothetical and must be validated in clinical trials.
Therefore, the future of LACC management lies in a risk-adapted, multimodal, and patient-centric paradigm. The choice between primary CRT and primary surgery must be guided by a sophisticated assessment of stage, tumor volume, histology, nodal status, and patient factors like age and fertility desires. This decision is best made by a high-volume multidisciplinary team. The most significant knowledge gap remains the lack of a direct, randomized comparison between modern primary surgery and modern image-guided CRT in the era of immunotherapy. Answering this question is the essential next step to replacing a one-size-fits-all tradition with a truly personalized and optimized future for all women with LACC.
CONCLUSION
The management of LACC is evolving beyond a rigid, universal paradigm. While CRT remains essential for advanced local disease, for a well-defined subset of patients with early LACC---selected through meticulous imaging and multidisciplinary review---primary radical hysterectomy offers a compelling path to preserve quality of life and ovarian function without compromising survival. The role of surgery is thus to complement CRT within a new, personalized framework, a paradigm further advanced by the integration of novel systemic therapies. The critical question is no longer which modality is universally superior, but rather, “For which patient is this specific approach the optimal choice?” Embracing this nuanced paradigm is the key to advancing both survival and the quality of survival for all women with LACC.
REFERENCES
- Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001; 358: 781-786.
- National Cancer Institute. NCI Clinical Announcement: Concurrent Chemoradiation for Cervical Cancer. Bethesda, MD: National Cancer Institute. 1999.
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019; 393: 169-182.
- Kirchheiner K, Nout RA, Tanderup K. Health-related quality of life and patient-reported symptoms after combined radiochemotherapy of locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2017; 98: 843-853.
- Querleu D, Morrow CP. Classification of radical hysterectomy. LancetOncol. 2008; 9: 297-303.
- Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie- Meder C, et al. The European Society of Gynaecological Oncology/ European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Virchows Arch. 2018;472(6):919-936.
- Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomised trials. J Clin Oncol. 2008; 26: 5802-5812.
- Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999; 340: 1383-1389.
- Dueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011; 29: 1678-1685.
- Potter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC- ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018; 9: 48-60.
- Tanderup K, Fokdal LU, Sturdza A. Effect of tumor dose, volume and disease on local control after radiochemotherapy in MRI-based brachytherapy in locally advanced cervical cancer. Lancet Oncol. 2016; 17: 538-548.
- Frigerio L, Gallo G, Corrado G. Avoiding Radiotherapy: A Rationale for the Primary Surgical Treatment of Bulky Early-Stage Cervical Cancer. Curr Oncol Rep. 2020; 22: 100.
- Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974; 44: 265-272.
- Wang Y, Li Z, Wang Y. Comparison of survival outcomes between radical hysterectomy and definitive radiochemotherapy in stage IB2 and IIA2 cervical cancer: A systematic review and meta-analysis. Front Oncol. 2021; 11: 785092.
- Roh JW, Lee DO, Suh DH. Efficacy of neoadjuvant chemotherapy followed by radical hysterectomy versus concomitant chemoradiation for stage IB2-IIA2 cervical cancer: a systematic review and meta- analysis. J Gynecol Oncol. 2019; 30: e86.
- Vergote I, Lacave AJ, Fagö-Olsen C. Neoadjuvant chemotherapy followed by radical surgery versus concomitant radiotherapy and chemotherapy in patients with stage IB2-IIB cervical cancer: long- term results of the randomized EORTC 55994 trial. Eur J Cancer. 2018; 101: 56-66.
- Ramirez PT, Frumovitz M, Pareja R. Management of patients with early-stage cervical cancer: a Society of Gynecologic Oncology (SGO) clinical practice update. Gynecol Oncol. 2020; 158: 341-347.
- Duyn AV, Kenter GG, Zwinderman AH. Quality of life after neoadjuvant chemotherapy or primary radiotherapy for stage IB2-IIB cervical cancer: results of the EORTC 55994 trial. Eur J Cancer. 2010; 46: 2753-2762.
- Lorusso D, Xiang Y, Hasegawa K. Pembrolizumab plus chemoradiotherapy for high-risk locally advanced cervical cancer: A randomized, double-blind, phase III ENGOT-cx11/GOG-3047/ KEYNOTE-A18 study [abstract]. Presented at: ESMO Congress; Madrid, Spain. 2023.
- Zamarin D, Deng W, Lankes HA. A phase II trial of neoadjuvant pembrolizumab for advanced cervical cancer. J Clin Oncol. 2022; 40: 5505.
- Coleman RL, Lorusso D, Gennigens C, González-Martín A, Randall L, Cibula D, et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021; 22: 609-619.