Effect of Sperm Cryopreservation on miR-34c and miR-15b Expression Level and Their Correlation with Sperm Parameters and Oxidative Stress in Oligoasthenoteratozoospermia Men
- 1. Department of Biology, Arak University, Iran
- 2. Department of Reproductive Biology, Culture and Research, Iran
ABSTRACT
Background: The purpose of this study was to investigate the changes and relationship of the expression of miRNAs 34c (mir 34C) and miRNAs 15b (mir15b) with sperm parameters in infertile oligoasthenoteratozoospermia men during the sperm freezing-thawing process.
Materials and Methods: In this experimental study, 25 semen samples were collected of oligoasthenoteratozoospermia men, who referred to infertility treatment Center Qom. Each sample was divided into two, non-frozen (Fresh) and frozen groups. Sperm parameters were evaluated using WHO criteria. Oxidative stress markers (ROS), total antioxidant capacity (TAC) and Malondialdehyde (MDA) level, were determined by ELISA kit. The expression level of miRNAs (34c and 15b) was assessed by Real-time PCR technique.
Results: The expression of miRNAs 34c decreased and miRNAs 15b increased significantly in the frozen group compared to the fresh group (P<0.01). There was a significant decrease in motility, morphology, and viability in the frozen group compared to the fresh group (P<0.05). A significant increase in the level of ROS, MDA, and DNA fragmentation and a decrease in the level of TAC, were observed during the freeze-thaw process in oligoasthenoteratozoospermia (P<0.05). Sperm concentration, motility and morphology as well as oxidative stress factors and DNA integrity were correlated with the expression level of miRNAs (34c and 15b) (P<0.05).
Conclusion: In summary, our study suggested that cryopreservation of sperm can change the expression of miRNAs (miR-34c, and miR15b) could impact fertility potential. In Addition, there was a meaningful correlation between microRNAs and sperm quality post freeze-thawing process in Oligoasthenoteratozoospermia men.
KEYWORDS
- Sperm Cryopreservation
- miRNAs
- Oligoasthenoteratozoospermia
- Oxidative Stress
- DNA fragmentation
CITATION
Mahmoodi M, Jannatifar R, Izadpanah A (2024) Effect of Sperm Cryopreservation on miR-34c and miR-15b Expression Level and Their Cor- relation with Sperm Parameters and Oxidative Stress in Oligoasthenoteratozoospermia Men. JSM Sexual Med 8(3): 1140
INTRODUCTION
Cryopreservation can provoke biochemical and structural damage of spermatozoa, resulting in a deficit in embryo development or fertilization ability [1], Its harmful effects on sperm are a significant concern because it can diminish sperm fertility [2]. Cryopreservation has a negative effect on several sperm parameters, like sperm mitochondrial activities, motility, and viability [3]. On the other hand, the elevated risk of nuclear DNA damage can increase the number of sperms with poor quality. Reactive oxygen species (ROS) caused by cryopreservation can damage epigenome and genome of Sperm [4]. Recent advances such as RNAs and proteins are useful to uncover sperm cellular and molecular alterations in the cryopreservation process [5]. Discovering novel potential markers of sperm freeze/thaw tolerance can optimize current freezing protocols [6]. Transcriptome analysis by RNA sequencing as well as differential expression of miRNAs and mRNAs between fresh and frozen–thawed spermatozoa suggest the cryopreservation ability to change the abundance of sperm transcripts linked to fertility-associated functions [7], Also, mRNA translation and ATP consumption are reduced during cryopreservation [8], MicroRNAs (miR- NAs) affect the mRNA expression of sperm cryopreservation. They are small noncoding RNAs capable of regulating the post-transcriptional stage of target genes[9]. In addition, miRNAs exploration provides useful information regarding sperm cryopreservation as well as the male reproductive system. In freeze-tolerant, miRNAs quickly regulate metabolism-associated signaling pathways and genes and suppress ATP metabolism [10]. The miR-34 group members play a significant role in infertility, like miR-34c, miR-34b, and miR-34a. MiR-34 can be found in the sperm and testis of bovine [11], its highest expression was reported in mouse testis, which is associated with spermatogenesis, as well as in the adult mouse round spermatid and pachytene spermatocytes [12]. The miR-34 family can target many important factors that regulate the cell cycle [13]. This family causes the repression of many target mRNAs, like cell cycle proteins, such as Cyclin D1 and BCL2. Also, it can regulate apoptosis and through its pro- apoptotic and anti-proliferative effects, influences many tissues, like breast cancer cells, and impedes the G2/M phase cell cycle and mortality in breast cancer cells [14]. The atopic expression of miR-34 arrests the cell cycle in both tumor-derived and primary cells [15], miR-34 plays a vital role in the signaling pathway of the p53 gene. DNA damage can directly stimulate P53, which causes the up-regulation of miR-34b and miR-34c expression [16], MiR-15b targets the coding region of mRNA for isocitrate dehydrogenase 3 (NADþ) alpha (IDH3A). Low IDH3A expression levels disrupt sperm motility through changes in sperm energy metabolism [17]. We investigated the effect of Sperm Cryopreservation on miR-34c and miR-15b expression level and their correlation with sperm parameters and oxidative stress in oligoasthenoteratozoospermia men
MATERIALS AND METHODS
Patient Selection
This study was carried out according to the Ethical Committee of Arak University (IR.ARAKU.REC.1401.136), and informed consent was acquired from 25 infertile men. The infertile patients (24–40 years) were detected as Oligoasthenoteratozoospermia (OAT) based on their spermiogram. Each sample was divided into two, non-frozen (Fresh) and frozen groups. Males who had cancer, smoking history, alcohol, gland / systematic disease, and genetic abnormality were excluded.
Semen Sample Collection
This study was conducted at the Infertility Research Center, the Academic Center for Education, Culture, and Research (ACECR), Qom, Iran. The samples were donated through masturbation following 2–5 days of abstinence and kept in an incubator (37 0C). The initial semen assessment was done 30 min following liquefaction and the ejaculated specimens were located in non-toxic and sterile tubes (Oosafe tube, Denmark). Then washing was performed to obtain sperm pellets. Respectively, centrifugation 3000 rpm in 5 min was carried out by adding the sperm preparing media (Nutrient Mixture Ham’s F-10, United Kingdom), 1:1 (v/v). Sperm suspension was divided into two aliquots (fresh and cryopreserved), each containing 300 µl of samples.
Freeze-Thawing Process
Cryopreservation was done as instructed and routine cryopreservation technique of the Infertility Research Center at the ACECR, Qom, Iran. Spermatozoa solution was washed with Quinn’s Advantage sperm freezing medium (ART-8022, SAGE media, Denmark) for 30 s to generate the ratio of 1:1 in the cryo tubes (1.8 ml). Following cooling (0.50 C/min; 25 to – 50 C), we kept the cryo container at - 5°C for 3 min, and this step was done again for 7 min. Following cooling (10 0C /min; - 5 to - 80 0C), immersion of straws into liquid nitrogen was done (- 196 0C). Cryopreservation was done for two weeks [18]. Cryovials underwent agitation in the water bath at 30 to 35 C. The freezing- thawing was followed by a washing process to assess the sperm parameters following cryopreservation. Cryovials underwent agitation in the water bath at 30 to 35 0C. The freezing-thawing was followed by a washing process to assess the sperm parameters following cryopreservation.
Sperm Parameters Assay
Semen parameters were analyzed according to WHO recommendations after full liquefaction [19]. The sperm motility categorized as non-progressive, progressive, or immotile. Papanicolaou staining [20], was used to assess normal morphology. In Addition, Sperm viability was evaluated via eosin-nigrosin stain. Briefly, approximate 10 μl of the sample was mixed with an equal volume of the staining solution in 370 C temperature. About 200 sperm were counted and differentiated according to coloring with a light microscope. Unstained spermatozoa were considered to live while pink sperm were dead [21].
Sperm DNA Fragmentation Assay
The Sperm DNA fragmentation assay (SDFA) kit (Gena Medical, IRAN, Cat#080910) was applied for DNA fragmentation before and following cryopreservation. Samples were diluted considering their level in Ham’s F-10 and the agarose tube was located in the water bath (90–100 0C / 5 min), followed by adding diluted samples to the agarose tube. Then, pipetting of 50 µl of the agarose-sperm mixture was done into kits’ slides and the slides were located in the slide’s coverslip. After placing the slides on a smooth plate in the refrigerator (40 C), the sperm microgel was formed after 5 min. The slides’ coverslips were removed at ambient temperature and they were rapidly positioned horizontally. The sperm agarose layer was added to lysing solution A and maintained in a dark place, followed by incubation in denaturation solution B after 7 min, and for 15 min. After washing the slides, in distilled water (DW) for 2 min, dehydration in ethanol 70, 90, and 100% was done for 2 min, respectively. Then, slides underwent incubation respectively in staining solutions C, D, and E for 75 Seconds, 3 min, and 2 min. The specimens were washed using the indirect flow of water and using a light microscope (Olympus, Japan), about 300 sperm cells were counted. DNA Fragmentation Index (DFI) reflects the sperm percentage characterized by DNA breaks. The SDF frequency presents the fertility potential [22] .
Sperm Plasma Membrane Integrity (PMI) Assay
PMI was assessed via Hypo-osmotic swelling (HOS) test [23], Approximately 20μl of the specimen was added to 200μl of hypo- osmotic solution (100 mOsm/kg; sodium citrate 1.9 mM, fructose 5.0 mM)) followed by incubation at 37 °C for 30 min. Then about 200 sperm were calculated and distinguished considering appearance. The swollen-coiled sperm indicates the presence of sperm having a functional and intact plasma membrane.
ROS Assay
In this study, the evaluation of ROS positive sperm was done using H2DCFDA dye (DCFH - DA: 2’, 7’-dichlorodihy- drofluoresceindiacetat (Sigma Co, USA) and analysis with fluorescence spectrometer. the dye is actively entered into the cell and by the action of cell esterase, the diacetate groups are split and the non-fluorescent compound DCFH is formed. semen sample suspension was incubated with 10 μM DCFH-DA for 30 min at 37 °C in dark. After centrifugation and resuspension, the fluorescent intensity was detected by a fluorescence spectrometer [24].
Mitochondrial Membrane Potential (MMP (Assay
The semen plasma was centrifuged by PBS at 300g and 5min and supernatants were removed and 5µl Rhodamine 123 (Sigma-Aldrich -62669-70-9-USA) added into the suspension at the 10 min in dark room for evaluation of MMP. After this time the suspension were centrifugal and removed supernatant. Then a thin strain was prepared and evaluated for sperm MMP by a fluorescence microscope (BX51, Olympus, Japan). At least 200 spermatozoa were assessed for each sample. The middle of sperm with normal MMP appeared as bright green and the middle part of sperm with damaged MMP appeared as non-bright.
Biochemical Factors Assay
Semen specimens underwent centrifugation at 1000 g for 10 min to isolate the supernatants, followed by washing the pellets three times using phosphate-buffered saline (PBS). Then, the pellets were subjected to suspending in lysis buffer with 0.2% Triton X-100, and then, incubation was done on ice for 20 min. The collected supernatant underwent biochemical analyses. Total antioxidant capacity (TAC) activity was determined using the spectrophotometric method by commercially available kits (Colorimetric Assay kit ZX-44109-96 BioGmbH, Germany). Malondialdehyde (MDA) levels were determined using the thiobarbituric acid (TBA) approach by the test kits (General MDA/ Malondialdehyde ELISA Kit RK09070 Zell Bio GmbH, Germany). In brief, MDA reacted with TAB and formed a firm chromophoric production with an absorption of up to 532 nm. The MDA levels of spermatozoa specimens were determined at the wavelength and reported as nmol/ml.
Isolation of Total RNA
Total RNA for cryopreserved and fresh specimens was extracted by TRIzol reagent (Cat#15596-026; Invitrogen,). After centrifugation for 7 min at 300 g, the achieved sperm pellets were homogenized using cold TRIzol (1 ml). Then, 200μl Chloroform was added, vortexed for 15s, and kept at ambient temperature (20–25 C) for 2 to 3 min. After centrifugation (12,000g / 15min / 4C), the upper aqueous layer (with RNA) was poured into a tube, including isopropanol (500µl), and 10 min later, centrifugation was done again (12,000g, 4C, 15min,). Then, 75% ethanol (1ml) was added, and following the last centrifugation, the supernatant was isolated and underwent air-drying for 10 min. RNA was then resuspended in Double distilled water (30 µl) and RNA quality was assessed by Nanodrop ND1000 spectrophotometer (Thermo Fisher Scientific).
Quantitative Real Time-PCR (qRT-PCR)
The miRCURY LNA RT Kit (Qiagen, Cat#339,340) was used for the RT reaction. Template RNA was adjusted to 5 ng/µl to be used for RT reaction. Each reaction mixture (10µl) included 2µl reaction buffer, 4.5µl nuclease-free water, 1µl enzyme mix, and 2µL template total RNA, followed by specific temperature protocol (42 0C for 60 min and 950C for 5 min). Then, we set up a 10 µl reaction mix for 45 cycles (950C and 56 0C for 10 and 60s, respectively) using Light cycler 96 Roche (Germany). Also, qRT- PCR for mRNAs was done using RealQ Plus 2x Master Mix Green (Cat#A323499, Amplicon) using StepOnePlus Real-time PCR System (USA). The reaction mixture (10µl) was provided through the addition of the components (0.25 µM forward primer, 5 µl RealQ Plus 2x Master Mix, 5µl cDNA, and 0.25 µM reverse primer). The three-step PCR program was done as follows: keeping at 95 0C, for 10 min, denaturation (45 cycles / 950 C / 15 s, annealing (560 C / 30 s), and extension (720 C / 20 s). Housekeeping genes were U6snRNA and β-actin, respectively for miRNA and mRNA normalization. Also, the relative expression level was assessed by the fold-change technique. Table 1 lists q-RT-PCR primers.
Table 1. The MiRNA (34b ,15b) and U6 (housekeeping gene) and their product length.
|
Accession numbers |
Gene |
Primer sequence |
product size (bp) |
qPCR efficiencies (%) |
|
NM_ 001291428 |
Mir 34c |
Forward: GCTTCAGGGTTTCATCCAG Reverse: TCGGAAAAAGACCTCTCGG |
19 |
94 |
|
NM_000633 |
Mir 15b |
Forward: CCCTGTGGATGACTGAGTAC ´ |
20 |
96 |
|
Reverse: CGTACAGTTCCACAAAGGCA |
||||
|
NM_032991 |
U6 |
Forward: TCACTGTTCTCTCCCTCCG GACTCA´ |
19 |
97 |
|
|
|
Reverse: GCCCAATACGACCAAATCC |
Statistical Analysis
The data was analyzed as a mean deviation of the standard deviation by SPSS software 21 and all data were exhibited as mean ± SEM. T-Test, and One-way analysis of variance (ANOVA) were used to study the data. The Spearman’s rank correlation test assessed correlations. Value lower than 0.05 was considered as statistically significant.
RESULTS
Semen Analysis
The percentage of fresh and cryopreserved total sperm motility, Progressive motility, Non- Progressive motility, and immotile spermatozoa were estimated by a light microscope (Olympus, Japan). Our data showed that the percentage of Total sperm motility (17.11±2.1 vs. 37±3.2; P=0.0001), and progressively motile spermatozoa (5±3.16 vs. 14.17 ±2.39; P=0.0001) decreased in freezing group as compared to Fresh group. The rate of non-progressive motility (5 ± 3.07vs. 13.33 ± 3.07; P=0.0001), and immotile sperm (83.33±4.77 vs. 63.33±4.77; P=0.0001) enhanced markedly after cryopreservation in Freezing group. On the other hand, In the Freezing group, a significant decrease was observed in the average percentage of sperms with normal morphology compared to the Fresh group (97±0.8 vs. 99±1.0; P=0.01). The average percentage of live sperm (viability) in the Freezing group was significantly reduced compared to the Fesh group (74±7.5 vs. 64±4.1; P=0.001) (Table 2).
Table 2. Comparison of Semen parameters in two groups (freeze and fresh).
|
Parameters |
Fresh group |
Freezing group |
P-Value |
|
Concentration (million/ml) |
10.73±4.2 |
8±2.0 |
NS |
|
Total motility (%) |
37±3.2 |
17.11±2.1 |
0.0001 |
|
Progressive motility (%) |
14.17 ±2.39 |
5 ± 3.16 |
0.0001 |
|
Non- Progressive motility (%) |
13.33 ± 3.07 |
5 ± 3.07 |
0.0001 |
|
Immotile (%) |
63.33±4.77 |
83.33 ± 4.77 |
0.0001 |
|
Normal morphology % |
97±0.8 |
99±1.0 |
0.01 |
|
Viability (%) |
89 ±4.1 |
64±3.5 |
0.001 |
Assessment of Sperm DNA Fragmentation
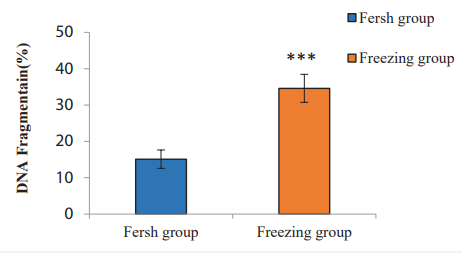
In the present study, Figures 1, 2 demonstrate the results of sperm DNA Fragmentation analysis. According to our finding, the mean percentage of DNA Fragmentation (sperm DNA failure) in the Freezing group (34.21% ±3.40) compared with the Fresh group (15.16%±2.02) (p value= 0.003), showed a significant increase.
Figure 1 Comparison of sperm DNA Fragmentation in the Freezing and the Fresh group. The asterisk (∗) indicates p <0.003.
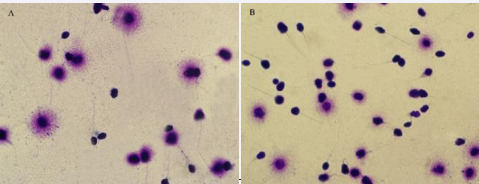
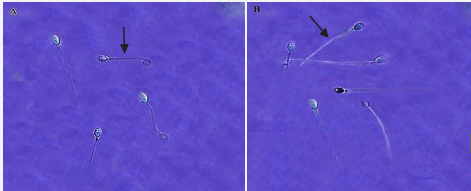
Figure 2 Sperm DNA Fragmentation based on halo formation in two groups - (sperm DNA Fragmentation) Examined by Olympus cx21 light microscope. Magnification 100X A: Fresh group B: Freezing group. Sperm nucleus with large halo (without DNA Fragmentation), sperm nucleus with small halo (with DNA Fragmentation) and sperm nucleus without halo (with severe damage of DNA).
Assessment of Sperm MMP, and Sperm Membrane Integrity
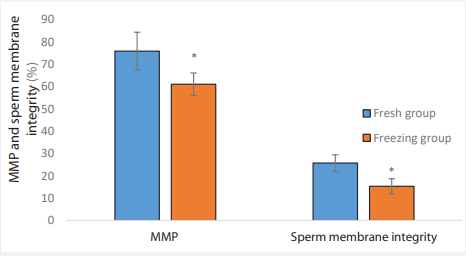
A lower percentage of the sperm membrane integrity was observed in the Freezing group comparing to the Fresh group (25.7±3.67vs. 15.3±3.37; p= 0.01). Also, the mean percentage of MMP in the Freezing group was showed a significant decrease compared to the Fresh group (75.8±8.45 vs. 60.95 ±5.03; p= 0.01) (Figures 3-5).
Figure 3 Comparison of MMP, and sperm integrity in the Freezing and Fresh groups. * Indicate P< 0.01. MMP: Mitochondrial membrane potential.
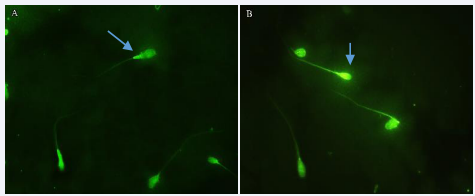
Figure 4 Evaluation of mitochondrial membrane potential of sperm using rhodamine 123 staining in two groups: A, Fresh group B, Freezing group. Healthy sperm with a bright green middle piece. Damaged sperm, lacking a bright green middle pice. (Olympus, DP71, Japan, 100x magnification).
Figure 5 Evaluation of the integrity of the plasma membrane of human sperm using the HOS test method in two groups (Olympus, DP71, Japan) 100x magnification. A: Fresh group B: Freezing group. Sperm with a healthy plasma membrane with curved tail. Sperm with a damaged plasma membrane with a smooth tail.
Assessment of Sperm ROS, TAC, and MDA
Among two groups, the ROS level in the Fresh group (204.1 ± 16.10) was statistically lower than the Freezing group (185 ± 6.77) (P= 0.04). Also, MDA concentration in sperm increased after cryopreservation (0.62±0.13) compared to the Fresh group (0.40±0.04) (P= 0.03). According to our finding, TAC level decreased in Freezing group (0.24± 0.09), compared to the Fresh group (0.073 ± 0.01) (P= 0.03) (Table 3).
Table 3. Comparison of ROS, and Biochemical factors between two groups.
|
Parameters |
Fresh group |
Freezing group |
P-Value |
|
ROS (%) |
204.1 ± 16.10 |
185 ± 6.77 |
0.04 |
|
TAC |
0.074 ± 0.01 |
0.24 ± 0.09 |
0.03 |
|
MDA |
0.404±0.04 |
0.62±0.13 |
0.03 |
Assessment of the Expression Levels of microRNAs (34c, and 15b)
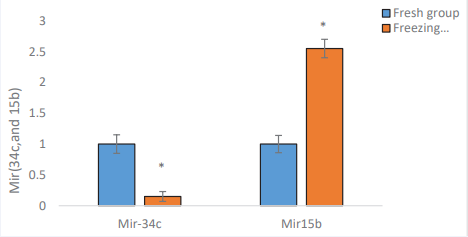
Quantitative RT-PCR indicated considerably decreased expression level of MiR-34c in Freezing group (0.15±0.08 vs. 1±0.15; p= 0.01). On the other hand, the expression level of miR- 15b in the Freezing group was higher than Fresh group (2.550 ± 14 vs. 1±0.15; p= 0.01) (Figure 6).
Figure 6 The evaluation of the miR-34c, and 15 b level expression in Freezing group compared with the Fresh group. p-value <0.01.
Assessment of the Correlation
According to correlation analysis, in the Freezing group, there was a positive correlation between miR-34c, sperm parameters such as motility, viability, normal morphology, sperm MMP, Sperm Integrity, and TAC (P<0.05), But a negative correlation with Severe DNA Fragmentation, ROS, and MDA (P<0.05). Our results showed a negative relationship between miR-15b, sperm motility, viability, normal morphology, sperm MMP, Sperm membrane integrity, and TAC. There was also a positive correlation between miR-15b and severe DNA Fragmentation, ROS, and MDA (P<0.05) (Table 4).
Table 4. Correlations between miR-34c, and miR-15b, and sperm parameters.
|
Correlation |
miR- 34c |
miR-15b |
||
|
r |
P-value |
r |
P-value |
|
|
Total motility |
0.613* |
0.024 |
- 0. 698* |
0.035 |
|
Viability |
0.705* |
0.024 |
- 0.681* |
0.015 |
|
Normal morphology |
0.432* |
0.033 |
-0.484 |
0.023 |
|
MMP |
0.537 * |
0.034 |
- 0.587* |
0.033 |
|
Sperm Integrity |
0.498* |
0.018 |
- 0.511 * |
0.021 |
|
DFI |
-0.642* |
0.03 |
0.632* |
0.022 |
|
TAC |
0.595* |
0.043 |
-0.607* |
0.012 |
|
ROS |
-0.486* |
0.049 |
0.522* |
0.028 |
|
MDA |
-0.546* |
0.023 |
0.641* |
0.031 |
DISCUSSION
In the current study, we investigated the available literature on molecular, structural, and functional damage in cryopreserved spermatozoa in humans. Our results demonstrated that motility, DNA health, Mitochondrial Membrane Potential, and membrane Integrity in sperm decreased following cryopreservation. Also, ROS and MDA levels increased following the freeze-thawing process. In addition, the evaluation of microRNAs indicated that cryopreservation was linked to a decrease in the expression of miR-34c and an increase in the expression of miR-15b. Interestingly, novel meaningful correlations between these microRNAs, and sperm quality were found in the cryopreserved group. Transcriptome analysis by RNA sequencing indicated differential expression of miRNAs and mRNAs between frozen-thawed fresh boar and bull sperm, indicating that cryopreservation and process can change the abundance of sperm transcripts associated with fertility function [25]. There is limited information on sperm RNAs after cryopreservation in humans. In a study on the miRNAs profiling of frozen human spermatozoa from sperm donors published by Wang et al., [26] twenty-seven differentially expressed miRNAs, and proteins were identified, mainly related to acrosome integrity, viability, capacitation, sperm motility, and mitochondrial activity. Further studies using high-throughput technologies showed a greater number of differentially expressed proteins and miRNAs [10,27]. The identity of the majority of down- and up-regulated miRNAs and proteins found under cryopreserved conditions differed in these studies. They are mostly associated with significant sperm functions to achieve egg fertilization, like apoptosis, motility, energy production, and DNA damage [28], In our study, it appears that the change in the expression level of miRNAs could affect sperm DNA health. There are several genes regulated by miR-34c and miR-15 in some cancers [29], The effect of these microRNAs on sperm disorders is not clear in infertile men. A recent study showed that the expression of miR-34c changed during cryopreservation and played an important role in the regulation of oxidative stress, the P53 gene, and DNA fragmentation in the sperm of infertile men [30], which is consistent with the results of our research. Different mechanisms can cause an increase in sperm DNA damage following cryopreservation. There is an elevation in caspase activity during the cryopreservation process, suggesting the involvement of apoptotic pathways in sperm DNA cryo-injury [5,31] . Adult round spermatids and pachytene spermatocytes have a high level of miR-34c, which is important for the first cell division by modulating caspase expression as a direct target of miR-34c [32], Since caspase is as target gene of miRNA34c, it can be concluded that the change of expression of these miRNAs in the freezing process increases the expression of caspase and then sperm DNA damage [33]. On the other hand, miR-15, as an apoptosis-inducing miRNA, targets the BCL2 gene and leads to the induction of apoptosis by reducing the expression of the BCL2 gene [34], Taheri et al reported in 2019 that the increase in miR-15a/16 expressions and the decrease in BCL2 gene expression increased DAN damage and decreased the growth of embryos resulting from cytoplasmic sperm injection [35], during the freezing and thawing of sperm, it is known to increase the production of ROS and decrease the antioxidant level [36]. This imbalance in the redox state may have deleterious effects on DNA following these procedures. Alterations in mitochondrial membrane fluidity can lead to excessive ROS release and increased oxidative stress, resulting in sperm damage [4], an excessive amount of ROS production during cryopreservation will impair miRNAs structure and function for several reasons, including mitochondrial membrane damage, and sperm membrane integrity [30], our data demonstrate that miR-34c, and miR15b significantly correlated with oxidative stress factors [37], According to our results, Mostafa et al showed that in seminal specimens of oligoasthenoteratozoospermia men who had varicocele than controls, miR-34c and GPx decreased, while Bax and MDA showed a marked increase [38]. The miR- 34c plays an important role in oxidative stress, suppressing the SIRT1 protein in the testis [37]. The oxidative stress induction in the mouse testis up-regulates miR-34c and down-regulates SIRT1 [37], Following oxidative stress, a higher apoptotic index and expression of apoptotic markers in male germ cells are observed in the testis, suggesting oxidative stress is the main cause of male infertility [39]. The miR-34c exerts significant anti- apoptotic and anti-oxidant effects and is important for testicular homeostatic mechanisms [40]. The miR-34c up-regulation after oxidative stress shows that through their unique actions, miR- 34c members ensure the maintenance of spermatogenesis when the testicular microenvironment is compromised [41].
CONCLUSIONS
Sperm cryopreservation is an effective and suitable treatment; nonetheless, it can impress fertility rate because of cryo damage. Sperm motility is possibly an important parameter affected by different factors-mediated apoptosis. Cryopreservation is accompanied by mitochondrial-associated apoptosis. Also, miR-34c and miR-15b affect sperm parameters and DNA fragmentation. Hence, increasing TAC following that decrease of ROS, and MDA was correlated with low severe DNA fragmentation spermatozoa to increase survival spermatozoa after the freeze- thawing procedure.
AUTHOR CONTRIBUTION
M. Mahmoodi conceived, designed and supervised the study. M. Mahmoodi and R. Jannatifar: analyzed and interpreted the data and prepared the manuscript. A. Izadpanah: performed the experiments. M. Mahmoodi and R. Jannatifar reviewed during the manuscript preparation and revised the manuscript.
ETHICS APPROVAL
This study was approved by the research ethics committee (Arak University Ethical Committee [IR.ARAK.REC.1401.136]).
REFERENCES
- Ali Erdem Öztürk, Mustafa Numan, Mustafa Bodu, Nuri Ba?p?n, Ilhami Celik, Zhiquan Shu, et al. Cryobiology and cryopreservation of sperm, in Cryopreservation-Current Advances and Evaluations. Intech Open. 2019.
- Julia Kopeika, Alan Thornhill, Yacoub Khalaf. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015; 21: 209-227.
- D Pabón, M Meseguer, G Sevillano, A Cobo, J L Romero, J Remohí, et al. A new system of sperm cryopreservation: evaluation of survival, motility, DNA oxidation, and mitochondrial activity. Andrology, 2019; 7: 293-301.
- R Chianese, R Pierantoni. Mitochondrial reactive oxygen species (ROS) production alters sperm quality. Antioxidants. 2021; 10: 92.
- L Tamburrino, G Traini, A Marcellini, L Vignozzi, E Baldi, S Marchiani, et al. Cryopreservation of Human Spermatozoa: Functional, Molecular and Clinical Aspects. Int J Mol Sci. 2023; 24: 4656.
- M ?ura?ka, F Benko, E Tvrdá Molecular Markers: A New Paradigm in the Prediction of Sperm Freezability. Int J Mol Sci. 2023; 24: 3379.
- Muhammad Khan, Z Cao, H Liu, A Khan, S Ur Rahman, MZ Khan, et al. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance omics to assess sperm cryo-tolerance in farm animals. Frontiers in Veterinary Science. 2021; 8: 609180.
- S Chamayou, G Bonaventura, C Alecci, D Tibullo, F Di Raimondo, A Guglielmino, et al. Consequences of metaphase II oocyte cryopreservation on mRNA content. Cryobiology. 2011; 62: 130-134.
- Esin Keles, Eleni Malama, Siyka Bozukova, Mathias Siuda, Sarah Wyck, Ulrich Witschi, et al. The micro-RNA content of unsorted cryopreserved bovine sperm and its relation to the fertility of sperm after sex-sorting. BMC genomics. 2021; 22: 1-19.
- Aishao Shangguan, Hao Zhou, Wei Sun, Rui Ding, Xihe Li, Jiajia Liu, et al. Cryopreservation induces alterations of miRNA and mRNA fragment profiles of bull sperm. Front Genet. 2020; 11: 419.
- Linlin Zhang, Tiantian Ma, Qibing Tao, Wushuang Tan, Huatao Chen, Wei Liu, et al. Bta-miR-34b inhibits proliferation and promotes apoptosis via the MEK/ERK pathway by targeting MAP2K1 in bovine primary Sertoli cells. J Anim Sci. 2020; 98: 313.
- Xiujuan Z, Wenhua W, Jiabin Z, Linmiao L, Haiying J, Jinping C. MiR- 34b/c play a role in early sex differentiation of Amur sturgeon, Acipenser schrenckii. Front Zool. 2022; 19: 23.
- Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010; 17: 193-199.
- Chandrani A, Sofia W, Yvonne C, Christer L. Expression of miR-34c induces G2/M cell cycle arrest in breast cancer cells. BMC Cancer. 2014; 14: 1-9.
- S Rahbar, MG Novin, E Alizadeh, V Shahnazi, F Pashaei-Asl, Y A AsrBadr, et al. New insights into the expression profile of MicroRNA- 34c and P53 in infertile men spermatozoa and testicular tissue. Cellular and Molecular Biology. 2017; 63: 77-83.
- Matjaz R, Huihui L, Longchang J, Heiko H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014; 6: 214-230.
- Masood A, Mohamad H, Jana S, Petra L, Andreas K, Eckart M, et al. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil Steril. 2013; 99: 1249-1255.
- N Ziarati, TR Topraggaleh, P Rahimizadeh, L Montazeri, S Maroufizadeh, MAS Gilani, et al. Micro-quantity straw as a carrier for cryopreservation of oligozoospermic semen samples: effects of storage times and cryoprotectant. Cryobiology. 2019; 86: 65-70.
- Organization WH. World health statistics. 2010; WHO.
- Nikolaos C, Amber DD, Maheswari M, Mathilde EB, RM Austin. Inception and development of the papanicolaou stain method. Acta cytological. 2017; 61: 266-280.
- Björndahl L, I Söderlund, U Kvist. Evaluation of the one-step eosin- nigrosin staining technique for human sperm vitality assessment. Hum Reprod, 2003; 18: 813-816.
- HA Beydoun, AL Broussard, R Colver, B Leader, H Russell, EE Tirado., et al. High sperm DNA fragmentation index (DFI) on the day of oocyte retrieval is correlated with lower fertilization and slower development to the blastocyst stage for trophectoderm biopsy. Fertil Steril. 2018; 110: 295.
- AT Quintela, IRS Oliveira, AO Souza, Alberto LG, Alexandre RS. Water-induced hypo-osmotic test for the evaluation of canine sperm membrane integrity. Anim Reprod. 2018; 7: 70-74.
- Valeria P, Maria N, Francesca DS, Elena B, Fiammetta B. An Overview on Assay Methods to Quantify ROS and Enzymatic Antioxidants in Erythrocytes and Spermatozoa of Small Domestic Ruminants. Animals. 2023; 13: 2300.
- Ding-Hui D, Izhar HQ, Ming-Xia R, Kai L, Yan Z, Ming Z, et al. Exploration of miRNA and mRNA profiles in fresh and frozen-thawed boar sperm by transcriptome and small RNA sequencing. Int J Mol Sci. 2019; 20: 802.
- Shangqian W, Wei W, Yang X, Min T, Jianzheng F, Hongyong S, et al. Proteomic characteristics of human sperm cryopreservation. Proteomics. 2014; 14: 298-310.
- Yan Z, Dinghui D, Yu C, Yuan L, Ming Z, Guangbin Z, et al. Cryopreservation of boar sperm induces differential microRNAs expression. Cryobiology. 2017; 76: 24-33.
- Anne B, Anna G, Alice F-H, Tiffany M, Solenne B, Amaël P, et al. Cell-free and intracellular nucleic acids: new non-invasive biomarkers to explore male infertility. Basic Clin Androl. 2017; 27: 1-8.
- Xu-Bao S, Clifford G T, Ralph W dV W. Cancerous miRNAs and their regulation. Cell cycle. 2008; 7: 1529-1538.
- Maryam E, Dariush S, Behzad B, Kobra H, Maryam P. Investigation of molecular cryopreservation, fertility potential and microRNA- mediated apoptosis in Oligoasthenoteratozoospermia men. Cell Tissue Bank. 2021; 22: 123-135.
- Doaa H Elsayed, Ayat A El-Shamy, Heba MA Abdelrazek, DA El-Badry. Effect of genistein on semen quality, antioxidant capacity, caspase-3 expression and DNA integrity in cryopreserved ram spermatozoa. Small Rumin Res. 2019; 177: 50-55.
- Benancy PCW, Kai-Fai L, Kaiqin L, Philip CNC, Ronald TKP, Wei-Min L, et al. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc Natl Acad Sci USA. 2012; 109: 490-494.
- Ahlem BS, Chantal P, Fatma E, Imen F, Mohamed B, Slim AK, et al. Protective role of Spirulina platensis against bifenthrin-induced reprotoxicity in adult male mice by reversing expression of altered histological, biochemical, and molecular markers including microRNAs. Biomolecules. 2020; 10: 753.
- Sahar H, Soheila A, Shohreh ZK. Evaluation of BCL2 and its regulatory miRs, miR-15-b and miR-16 expression changes under the exposure of extremely low-frequency electromagnetic fields on human gastric cancer cell line. Radiat Prot Dosimetry. 2021; 197: 93-100.
- Hossein T, Sara H, Mohammad S. The relationship between sperm DNA fragmentation and differential expression of human sperm pro- apoptotic miR-15a/16 and anti-apoptotic BCL-2 gene. The Iranian Journal of Obstetrics, Gynecology and Infertility. 2019; 22: 42-48.
- Bansal AK, G Bilaspuri. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. 2010; 2010.
- Amir MG, Alireza S, Rafighe G, Bagher P, Naser K, Roya N. Cyclosporine A induces testicular injury via mitochondrial apoptotic pathway by regulation of mir-34a and sirt-1 in male rats: The rescue effect of curcumin. Chem Biol Interact. 2020; 327: 109180.
- Taymour M, Laila A, Nashaat I, Ihab O, Rashad M, Mohamed F. Seminal miRNA relationship with apoptotic markers and oxidative stress in infertile men with varicocele. Biomed Res Int. 2016; 2016: 4302754.
- Emokpae MA, Chima HN. Effect of senescence on some apoptosis and oxidative stress markers in infertile normozospermic and oligospermic men: A cross-sectional study. Int J Reprod Biomed. 2018; 16: 435-442.
- Konstantinos Pantos, Sokratis Grigoriadis, Penelope Tomara, Ioanna Louka, Evangelos Maziotis, Agni Pantou, et al. Investigating the role of the microRNA-34/449 family in male infertility: a critical analysis and review of the literature. Front Endocrinol. 2021; 12: 709943.
- JR Baker, C Vuppusetty, T Colley, Andriana I Papaioannou, P Fenwick, Louise Donnelly, et al. Oxidative stress dependent microRNA-34a activation via PI3Kα reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci Rep. 2016; 6: 35871.