Endometrial Immune Profiling in Patients with Endometriosis Associated-Repeated Implantation Failures
- 1. MatriceLAB Innove, 2 rue Antoine Etex, France
- 2. Centre d’assistance médicale à la procreation, Hopital des Bluets, France
Abstract
Problem: Strong evidence suggests that the immune system plays a critical role in the progression and development of endometriosis. This study aims at assessing the endometrial immune profile in patients with endometriosis-associated recurrent implantation failures (RIFs) following in-vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI), in comparison to women with male infertility-associated RIFs.
Method of Study: This case-control study compared the endometrial immune profile in women with endometriosis-associated RIFs (case group) versus those with male infertility-associated RIFs (control group). The profile was evaluated using the ratio of IL-18/TWEAK mRNA expression levels (a biomarker for angiogenesis and Th1/Th2 balance), the ratio of IL- 15/Fn14 mRNA expression levels (a biomarker for uNK cell activation/maturation), uNK cell counts, and CD56 mRNA expression levels (a marker for uNK cell mobilization).
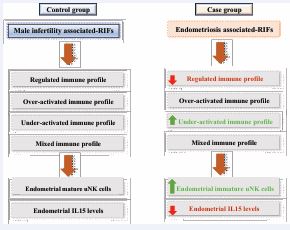
Results: The distribution of immune profiles significantly differed between the case group and the control group. The case group had fewer patients with a regulated profile (18.9% vs. 24.3%, P value 0.01) and more patients with under-activated profiles (34.2% vs. 28.4%, P value < 0.0001). Additionally, the case group had a higher proportion of immature uNK cells (46.2% vs. 39.2%, P value 0.007). The immaturity of uNK cells in endometriosis-associated RIFs appeared to be mediated by decreased IL-15 expression levels.
Conclusions: The study highlights unique immune characteristics in the endometrial environment of women with endometriosis-associated RIFs, emphasizing the role of immune dysregulation in the development and progression of endometriosis.
Graphical Abstract
• The case group is characterized by enhanced proportion of patients with an under- activated endometrial immune profile and decreased proportion of those with a regulated profile when compared to the control group. These endometrial immune particulates are mediated by increased endometrial uNK cells immaturity and decreased IL15 mRNA levels.
Keywords
• Endometriosis
• Embryo Implantation
• Endometrium
• Immunology
• Cytokines
Citation
Habeichi NJ, Chenière S, Petitbarat M, Prat-Ellenberg L, Lédée N, et al. (2025) Endometrial Immune Profiling in Patients with Endometriosis Associated-Repeated Implantation Failures. JSM Sexual Med 9(3): 1160.
INTRODUCTION
Endometriosis is a major cause of infertility affecting millions of women worldwide (Practice Committee of the American Society for Reproductive Medicine 2012). It has been documented that 10-15% of reproductive aged women suffer from endometriosis increasing infertility rate substantially; as twice as the rate observed in women without endometriosis [1,2]. Endometriosis is a complex clinical syndrome that is characterized by the presence of endometrium-like tissue grows outside the uterine cavity that undergoes a cyclic growth, proliferation, and breakdown in response to hormonal changes during the menstrual cycle. This internal bleeding, however, remains in the body and leads to an inflammatory response resulting eventually in scar formation and adhesion during the repair phase [3]. Although multiple hypotheses have been implicated in the etiology of endometriosis associated-infertility, the exact mechanism is yet to be fully understood. Laux-Biehlmann et al., elucidate that the inflammatory response plays a major role in the development and progression of endometriosis [4]. In that regard, it has been demonstrated that tumor necrosis factor-α is responsible for increased endometrial cell proliferation [5]. Additionally, in an animal model of endometriosis, tumor necrosis factor-α along with estrogen mediated signaling pathway have been shown to suppress apoptosis and enhance proliferation of ectopic region [6]. Furthermore, a local immune dysregulation, mainly detected in the peritoneal fluid in patients with endometriosis, suggested as a key factor in enhanced endometriosis associated-infertility [7-11]. In that regard, a permissive peritoneal environment characterized by an alteration in the function of immune cells has been shown to contribute to the evasion clearance of the endometriotic lesion by the immune system. With respect to this notion, a decrease in macrophage-mediated cytolysis along with altered leukocyte population have been reported in patients with endometriosis, resulting in increased pro inflammatory cytokine release with enhanced growth factors secretion, consequently promoting growth and angiogenic characteristic of the endometriotic lesion [3-12]. In addition to the previous strong published evidence, a dysregulation of systemic and peritoneal expression of pro-inflammatory cytokines such as Interleukin (IL) IL-17, IL-6, IL-8, was also reported in the context of endometriosis [13,14]. Another mechanism has been proposed to play a critical role in the progression and development of endometriosis associated-infertility is the modification of the expression of some hormone receptor genes altering therefore the progesterone / estrogen balance in the endometrium [15,16].
Despite the great improvement in the treatment of endometriosis, the number of infertile women is still remarkable, posing the necessity to develop new medical and pharmaceutical interventions. The involvement of the endometrium itself as an actor of embryo implantation failures in patients with endometriosis has come recently into focus, however, further investigations are needed to better understand its exact role in this multifactorial disease [17-19].
The endometrial immune reaction occurring in humans during the implantation window is unique and crucial for an adequate placentation. This unique and local immune response is required not only to activate the embryo attachment but also to regulate the invasion phase. During the implantation window, 65 to 70% of the endometrial immune cells are the uterine natural killer (uNK) cells belonging to the innate immunity compartment. Macrophages and dendritic cells are also detected, together with some adaptive immune T cells, such as T regulatory cells (Tregs) [20,21]. The ideal composition of cytokines for adequate functions and differentiations of all these immune cells during the implantation window favors the presence of the T helper 2 (Th2) over T helper 1(Th1) cytokines. This delicate equilibrium would selectively allow the development of local mechanisms that promote immunotrophism and angiogenesis locally at the same time downregulate pro-inflammatory and cytotoxic pathways [22]. Over time, the concept of pregnancy as a Th2 phenomenon has evolved proposing that the absence and excess release of Th1 cytokines are as deleterious as the depletion of Th2 cytokines for implantation and placentation, [23], indicating that the equilibrium between pro-inflammatory and anti-inflammatory Th cells is a key factor for avoiding RIFs.
Based on the strong published evidence and the crucial role of the endometrial immunesystem to facilitate the implantation process, we hypothesis that endometriosis may induce local immune dysregulation resulting in RIFs. In order to assess our aim, we retrospectively compared the endometrial immune profile of women patients with a history of recurrent RIFs after IVF/ICSI associated with endometriosis to pure male infertility. The objective of this retrospective case-control study is to document if patients with endometriosis associated-RIFs exhibit any local endometrial immune response dysregulation: Over activated, under-activated, or a mixed profile during the putative endometrial window of implantation.
MATERIALS AND METHOD
Selection of samples- Inclusion or exclusion criteria
In order to select the samples to compare in a case-control study, we selected between 2012 and 2020 within all the samples received for endometrial immune profiling at the laboratory with the following inclusion criteria:
- Aged from 18 to 40 years old
- With an history of RIFs (defined as the failure to give birth despite the transfer of at least 6 good quality embryos)
- With a well define etiology of their infertility: 1) Case group: 546 women with endometriosis associated RIFs 2) Control group: 1065 women with male infertility associated-RIFs.
Patients in the case group were diagnosed with all types of endometriosis assessed by imaging or surgical exploration.
Any clinical symptoms (like a dysmenorrhea, lower abdominal pain, dyspareunia, urinary et intestinal symptoms and others) or ultrasound suspicion of endometriosis in the control group is excluded from the study. Patients with mixed etiology or in oocytes donation program were also excluded. Additionally, biopsies realized in order to test a treatment or a procedure able to influence the immune profile as scratching, corticoids or luteal hCG were excluded.
Protocol: determination of the immune profile
Patients included in this study underwent endometrial immune profiling prior the IVF, as previously described in detail.
Biopsy and dating of samples
Endometrial biopsies were performed in the mid-luteal phase. The endometrium was gently aspirated by rotating a Cormier pipelle within the endometrial cavity. The pipelle content was divided into two parts, one placed in 4% formol (QPath Formol 4% buffered, VWR Chemicals, Fontenay-sous-Bois, France) for endometrial dating and CD56 immunolabeling.
The second part was placed in RNAlater stabilization solution (Qiagen, Courtabeuf, France) for the molecular analysis (MatriceLab Innove, France).
RNA extraction and reverse transcription
After confirming the histological dating of the endometrial samples, biopsies were incubated in the RNAlater (Qiagen) then RNA was extracted. In order to perform the RNA extraction, samples were incubated in a lysis buffer consisting of the RNeasy Plus kit (QIAGEN) and TissueLyser LT (QIAGEN). From 2012 to 2014, RNA was extracted by the RNeasy Plus kit, according to the manufacturer’s instructions, whereas, from 2015 to 2020, MagNA Pure 96 Cellular RNA LV Kit (Roche, Meylan, France) was used to extract RNA. Until 2015, the First Strand cDNA Synthesis Kit for RT-PCR (Roche, Meylan, France) was used to reverse-transcribed RNA into cDNA, whereas, starting from 2015, RNA was reverse-transcribed using the transcriptor first strand cDNA synthesis (Roche, France) The cDNAs were then stored at –20°C for the molecular analysis.
Quantitative RT-PCR
Quantitative RT-PCR was performed using the Light Cycler 480 instrument (Roche Diagnostic) and Light Cycler 480 SYBR Green I Master mix (Roche Diagnostic). Forward and reverse primers were used with a final concentration of 0.5 µM. Each quantitative RT-PCR assay included a negative control (H2 O) and a positive control; inter-run calibrator (IRC). Of note, the cDNA samples and the positive control were diluted 1/20. Cycling conditions were as follows: denaturation (95°C for 5 min), amplification and quantitation (95°C for 10 s, 60°C for 10 s and 72°C for 15 s) repeated 40 times, melting curve (65-95°C with a ramp rate of 2.2°C/s) followed by the cooling step to 4°C. The IRC was obtained from pools of endometrium samples with respect to all the studied genes. PCR efficiency for each quantified target and reference was calculated with known serial dilutions of each specific cDNA. LightCycler®480 Software release 1.5.0 was used to analyze the data, and each specific target transcription level was normalized to the geometric mean of the reference genes; ribosomal protein L13 (RPL13A) and beta-2-microglobulin (β2M), with the software’s advanced relative quantification workflow. Gene amplification efficiency was specifically determined. For each sample, the results were expressed as the ratio of target/reference cDNA. Primer sequences are detailed in Table 1.
Table 1: List of primers used for RT-qPCR analysis
|
Target |
Reverse Primer |
Forward primer |
|
IL-15 |
TAC-TTG-CAT-CTC-CGG-ACT- CA |
GAA-GCC-AAC-TGG-GTG- AAT-GT |
|
IL-18 |
ATA-AAG-ATG-GCT-GCT-GAA- CC |
TCA-AAT-AGA-GGC-CGA- TTT-CC |
|
Fn-14 |
TTT-CTG-GCT-TTT-TGG-TCT- GG |
GGC-ACA-TTG-TCA-CTG- GAT-CA |
|
TWEAK |
TGC-ACC-TAA-AGG-CCG-GAA- AAC-ACG |
CAG-CGC-AGG-GCC-AGC- ACA-CCA-TCC |
|
RPL13A |
CCT-GGA-GGA-GAA-GAG-AGA- AAG-AGA |
TTG-AGG-ACC-TCT-GTG-TAT- TTG-TCA-A |
|
β2M |
TGC-TGT-CTC-CAT-GTT-TGA- TGT-ATC-T |
TCT-CTG-CTC-CCC-ACC-TCT- AAG-T |
|
CD56 |
5' AGA-CCC-CAT-TCC-CTC- CAT 3' |
5' ATG-TGC-CCA-TCC-AGA- GTC-TT 3' |
Immunohistochemistry (IHC) of uNK cells
The uNK cell count was measured as the mean of CD56+ cells in 4 representative fields at ×400 magnification using IHC. Briefly, endometrium tissues were fixed in 4% formalin dehydrated, and embedded in paraffin. Paraffin embedded endometrium tissues were sectioned into 5-µm thickness, with an automated streptavidin-biotin method (Benchmark GX, Ventana Medical Systems). The prediluted anti-CD56 (clone 123C3) murine monoclonal primary antibody (Ventana Medical Systems®, Roche Diagnostics) was applied according to the manufacturer’s instructions. Briefly, after deparaffinization of the slides, antigen retrieval buffer was used for 60 min in (pH 8.4). The CD56 primary antibody was then applied for 32 min. Slides for negative controls were prepared by replacing the primary antiserum with non-immune IgG. Slides were then incubated for 8 min with a biotinylated anti-mouse secondary antibody. Diaminobenzidine or 3-amino-9 ethylcarbazole was used as the chromogen (iVIEW DAB detection kit, Ventana Medical Systems) and slides were counterstained with hematoxylin for 2 min, incubated in bluing reagent (for 2 min), and mounted. Between each step, slides were rinsed with reaction buffer. uNK cells mobilization was initially evaluated using IHC labelling positive CD56 cells and is now evaluated directly by quantitative RT-PCR [24-26]. A low recruitment was defined by CD56 positive cells less than 10 by the field, whereas, a high recruitment was defined by CD56 positive cells over 100 by Field.
The range of interpretation was defined in a fertile cohort study. The fertile group contained 45 women who underwent an endometrial sampling 3 months before the embryo transfer (ET) and who all successfully gave birth at the first attempt of fresh or frozen-thawed ET, without any personalized treatment of local immune dysregulation. Notably, all participated women had a normal ovarian reserve and the main cause of infertility was only related to the male counterpart. Their mean age patients were 32 years. The interpretations evolve as new methodologies are refined but were performed in the same cohort.
Outcome measurement
Determination of uterine immune profile
The local immune profile was defined in function of the local equilibrium of the mRNA expression of the two ratios IL-18/TWEAK and IL-15/Fn-14 as well as CD56 immunostaining or mRNA expression levels [25,26]. Four types of local immune profile were diagnosed:
• An endometrial regulated immune profile characterized by IL-18/TWEAK and IL- 15/Fn-14 mRNA ratios and CD56+ cell count within the same range that previously defined in the fertile cohort study.
• An endometrial under-activated immune profile is characterized by low IL-18/TWEAK (enhanced Th2 cytokines depletion) mRNA ratios and/ or low IL-15/Fn-14 (reflecting immature uNK cells) or an absence of uNK mobilization (low CD56+ cell count).
• An endometrial over-activated immune profile is characterized by high IL- 18/TWEAK (excess Th-1 cytokines secretion) and/or IL-15/Fn-14 mRNA ratios of and/or a high uNK mobilization (CD56+ cell count).
• An endometrial mixed profile is characterized by a high mRNA ratio of IL-18/TWEAK (excess of Th-1 cytokines) simultaneously with low IL-15/Fn-14 ratio (reflecting immature uNK cells).
Statistical analysis
Statistical analysis was performed with medcalc Statistical Software version 12.7.2 (MedCalc Software, Ostend, Belgium; http://www.medcalc.org; 2013). We compared the repartition of each immune profiling in RIF patients with infertility related either to endometriosis or pure male infertility using the chi-square test. For quantitative variables, the Student’s t test was used. A P value below 0.05 was considered significant.
RESULTS
Study population
Between 2012 and 2020, 546 women with endometriosis associated RIFs and 1065 women with male infertility associated-RIF were included in this case – control cohort analysis. Their history of implantation failures is summarized in Table 2.
Table 2: Clinical context and characteristic of the case (RIF cohort with endometriosis) versus control (RIF cohort with pure male infertility)
|
|
Group Endometriosis (N = 546) |
Group Pure male infertility (N = 1065) |
P value |
|
Age (y) |
35.1 (25-40) |
35.1 (22-40) |
0.99 |
|
Duration of prior infertility (y) |
5 (2-13) |
5(1-17) |
0.60 a |
|
Type of infertility Primary Secondary |
54,5% (96/176) 45,5% (80/176) |
57,2% (299/523) 42,8% (224/523) |
0.75b 0.71b |
|
Ovarian reserve AMH (ng/mL) |
2,88 (4-7,5) |
2,74 (1-9,29) |
0.68a |
|
Previous IVF Range of attempts No. of embryos transferred |
3 (2-8)
8 (6-27) |
3 (2-11)
8 (6-35) |
0.14 a
0.71a |
Footnotes: Data are mean (min – max) or n (min - max) or percentage % (n/N) unless otherwise specified. AMH: Antimullerian hormone; IVF: In vitro fertilisation; No: Numbre; Y: Years.
a student’s t test
b Pearson’s chi-square test
Both groups were comparable for the maternal age (P value 0.99), duration of infertility (P value 0.60), primary or secondary type of infertility (P value 0.70). Both were comparable for their range of attempts but more embryos have been replaced in the pure male group than in the endometriosis group. Such difference reflects the impact of endometriosis on the ovaries reserve.
Endometrial immune profiles repartition in endometriosis associated-RIFs and pure male related RIFs patients
The repartition of the distinct endometrial immune profiles (regulated, under-activated, over-activated, and mixed profiles) between women with endometriosis associated-RIFs and women with male infertility associated-RIFs was significantly different (P value 0.0096) (Table 3). Digging more into the realized statistic, our findings showed a significant less percentage of patients with regulated endometrial immune profile in women with endometriosis associated-RIFs group when compared to women with male infertility associated RIFs: 18.9% vs. 24.3%, respectively (P value 0.0131). Additionally, a marked higher percentage of patients with under- activated endometrial immune profile in women with endometriosis associated-RIFs was observed when compared to women with male infertility associated-RIFs: 34.2% vs. 28.4%, respectively (P value < 0.0001).
Table 3: Repartition of the Immune profiles in RIF patients with endometriosis associated infertility vs. pure male related- infertility
|
|
Group Endometriosis (N = 546) |
Group Pure male infertility (N = 1065) |
P value |
|
Regulated profiles |
18.9% (103/546) |
24.3% (259/1065) |
0.0131 a |
|
Over-activated profiles |
31.5% (144/546) |
29.4% (313/1065) |
0.2038 a |
|
Under- activated profiles |
34.2% (187/546) |
28.4% (302/1065) |
<0.0001a |
|
Mixed profiles |
20.5% (112/546) |
17.9% (190/1065) |
0.190 a |
Footnotes: Data are percentage % (n/N). The repartition of the distinct immune profiles (normal / under- activated / over-activated /mixed profiles) was significantly different (P value 0.0096).
a student’s t test
Immunoregulated equilibrium between cytotoxic versus angiogenic/ immunotrophic cytokines
IL-18/TWEAK ratio was used as a biomarker to assess the local Th-1/Th-2 equilibrium and more specifically the local immunoregulated equilibrium between cytotoxic cytokines (Th-1) versus angiogenic/ immunotrophic cytokines (Th-2). Th-1/Th-2 equilibrium was defined below the normal range when a local Th-2 depletion was observed and over the normal range when Th-1 dominant environment was detected. Our findings revealed no difference in Th1/Th-2 equilibrium between women with endometriosis associated-RIFs and women with male infertility associated-RIFs (P value 0.6354) as detailed in (Table 4).
Table 4: Immunoregulated Th-1/ TH- 2 uterine equilibrium
|
|
Group Endometriosis (N = 546) |
Group Pure male infertility (N = 1065) |
P value |
|
Normal Th-1/ Th-2 equilibrium |
44.0 % (240/546) |
46 % (490/1065) |
0.6354 a |
|
Excess of Th-1 cytokines |
43.8 % (239/546) |
43 % (458/1065) |
0.7684 a |
|
Depletion of Th-2 cytokines |
12.3 % (67/546 |
11 % (117/1065) |
0.4428 a |
Footnotes: Data are percentage % (n/N). a student’s t test. Th1: T helper 1; Th2: T helper 2
Mobilization of CD56 positive cells
The mobilization of CD56 positive cells was comparable between women with endometriosis associated-RIFs and women with male infertility associated-RIFs (41 CD56+/ field and 42 CD56+ cells/ field, respectively) (P value 0.0849).
Maturity of uterine NK cells or hyper-activation through IL-15
The ratio IL-15/ Fn-14 was used to evaluate the local maturity of uNK cells. A low IL-15/Fn-14 ratio reflected uNK cells immaturity. In contrast a high IL-15/ Fn-14 ratio was interpreted as an IL-15 related uNK over-activation. A substantial higher percentage of patients with a low IL-15/ Fn-14 ratio in women with endometriosis associated-RIFs when compared to women with male infertility associated RIFs was observed (46.2% vs. 39.2%, respectively), (P value 0.0070), interpreted as an uNK cells immaturity (Table 5).
Table 5: Maturity of uterine NK cells or hyper-activation through IL-15
|
uNK maturity |
Group Endometriosis (N = 546) |
Group Pure male infertility (N = 1065) |
|
|
|
P value |
||
|
Mature uNK |
45.2 % (247/546) |
48.7 % (519/1065) |
0.12 a |
|
Immature uNK |
46.2 % (252/546) |
39.2 % (417/1065) |
0.0070 a |
|
IL-15 related uNK over-activation |
8.6 % (47/546) |
12.1% (129/1065) |
0.0324 a |
|
Footnotes: Data are percentage % (n/N). uNK: Uterine Natural Killer cells, IL15: Interleukin 15 a student’s t test |
|||
DISCUSSION
Endometriosis is often perceived as a chronic, cyclic pro-inflammatory, estrogen-dependent disease, increasing infertility rate significantly (Practice Committee of the American Society for Reproductive Medicine 2012). There is a consensus among authors that the immune composition of the peritoneal fluid of women with endometriosis is different from healthy fertile controls with enhanced pro-inflammatory environment [7-11]. In that regard, it has been demonstrated that immune cells are a key player in the pathogenesis of endometriosis. Neutrophils and peritoneal macrophages have been shown to produce biochemical factors that enhance angiogenesis,endometriotic cell growth, and invasion. Additionally, it has been indicated that peritoneal macrophages and NK cells in endometriosis have a reduced capacity to eliminate endometrial cells in the peritoneal cavity. Furthermore, the imbalance between Th1/Th2 cells result in abnormal cytokine release and inflammation, exacerbating subsequently lesion progression [27,28].
Concerning the role of endometrium itself in the pathogenesis of endometriosis associated-RIFs, no conclusive conclusion has yet been obtained and further investigations are warranted. To the best of our knowledge, this is the first study to highlight the predominant under activated endometrial immune profile in patients with endometriosis associated-RIFs when compared to the male infertility associated-RIFs, in contrast to the findings observed in peritoneal fluid in women patients diagnosed with endometriosis [25]. Exploring therefore the role of the endometrial immune system in the etiology of endometriosis will guide future diagnosis and treatment of this disease. Strong evidence has demonstrated an atypical distribution of macrophages within the lesions of women with endometriosis [29-31]. In fact, macrophages are shown to exert a critical role in the pathogenesis of endometriosis [32,33]. In this study, we demonstrated that the distribution of distinct immune profiles in the context of endometriosis-related RIFs differs from those in women with male infertility-associated RIFs. The difference is mainly characterized by a reduced presence of regulated endometrial immune profiles and a higher proportion of women with under-activated endometrial immune profiles in the endometriosis-related group. Our extended analysis indicated that this difference is primarily characterized by more immature uNK cells and less endometrial IL-15 related over-activation in endometriosis-associated RIFs. These data are in line with the previous investigations describing a defect in uNK local activity [34,35]. Of note, IL-15 is known as a pleiotropic cytokine with several key functions, such as enhancing the production of Th1-predominant pro-inflammatory cytokines, inducing the proliferation of T cells and NK cells [36,37], and regulating the differentiation, development, and cytotoxic activity of NK cells [38,39]. Differing from prior findings that highlighted the pro-inflammatory characteristics of endometriosis based on the peritoneal environment analysis, our data indicated no access endometrial pro inflammatory Th-1 release along with a dominant under activated endometrial immune profile.
LIMITATIONS
This study has certain limitations that should be acknowledged, including its retrospective analysis design that, leads to miss some data such as endometriosis categories.
According to the American society of reproductive medicine, endometriosis is classified under four categories: minimal, mild, moderate, and severe based on the size and localization of implants and the extent of adhesion. This classification may have some prognostic significance concerning the prediction of women’s infertility [40, 41]. Additionally, the retrospective design did not allow us to differentiate patients with isolated endometriosis from those with endometriosis associated with adenomyosis. Furthermore, macrophages, T regulatory cells, mesenchymal stem cells and Th-17 cells are not covered by the test, therefore further investigations are needed to have a complete story of the endometrial immune environment at the time of implantation in the context of endometriosis associated-RIFs.
CONCLUSION
Progress has been made in the exploration of endometriosis pathogenesis in terms of immune regulation. However, the exact molecular pathways implicated in the progression and development of endometriosis is yet to be fully elucidated, resulting in hindering the development of personalized therapy for endometriosis in the immune direction.
Our findings reveal an alteration in the endometrial immune profile in women diagnosed with endometriosis when compared to the control group. This alteration is mainly characterized by a higher percentage of patients with under-activated endometrium immune profile along with a lower percentage of patients with a regulated profile. The observed differences seem to be at least partially mediated at the level of uNK cells, with more immature uNK cells, and less IL-15 related uNK over activation. The altered endometrial immune profile that has been demonstrated in this study may help optimize existing methods and play a crucial role in understanding the etiology of endometriosis to develop new personalized treatment against this disease based on the endometrial immune profiling in the future, aim at increasing consequently LBR substantially.
ACKNOWLEDGMENTS
We would like to thank the patients who consented to the retrospective collection of their pregnancy outcomes. We also extend our thanks to the physicians who took part in this innovative procedure and who carefully supplied us with the follow-up information on their patients over the years. This study was supported by MatriceLab Innove for the diagnostic tests and the prospective collection of data for the follow-up.
CONFLICT OF INTEREST STATEMENT
NL initiated the MatriceLAB Innove company and holds a patent covering the endometrial immune tests and the appended recommendations (PCT/EP2013/065355). The remaining authors declare no conflict of interest regarding the publication of this article
AUTHOR CONTRIBUTION STATEMENT
NH wrote the manuscript, collected the clinical data, performed the immune analysis and created tables. SC selected the patients with endometriosis, performed the immune profiling. NL and LPE performed the endometrial biopsy and provided comprehensive supervision over the overall logistics of the project with MPB. NL was the project leader and principal investigator, spearheading various aspects of the study such as ethical approval, study design, data analysis, and supervised the discussions and the writing of the manuscript. All authors contributed to the article and approved the submitted version.
ETHICS STATEMENT
In 2011, the Institutional Review Board and the Ethical Committee of St. Louis Hospital approved the prospective follow-up of a cohort after immune profiling in order to document their outcome and a potential benefit (ref. 2011- A00994-37).
REFERENCES
- Polat M, Yaral? ?, Boynukal?n K, Yaral? H. In vitro fertilization for endometriosis-associated infertility. Womens Health (Lond). 2015; 11: 633-641.
- Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012; 39: 535-549.
- Klemmt PAB, Starzinski-Powitz A. Molecular and Cellular Pathogenesis of Endometriosis. Curr Womens Health Rev. 2018; 14: 106-116.
- Laux-Biehlmann A, d’Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol Sci. 2015; 36: 270-276.
- Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic
stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab. 2000; 85: 824-829.
- Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell. 2015; 163: 960-974.
- Monsanto SP, Edwards AK, Zhou J, Nagarkatti P, Nagarkatti M, Young SL, et al. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril. 2016; 105: 968-977.e5.
- Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. 1996; 174: 1522- 1526.
- Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, et al. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997; 176: 593-597.
- Yoshino O, Osuga Y, Koga K, Hirota Y, Tsutsumi O, Yano T, et al. Concentrations of interferon-gamma-induced protein-10 (IP-10), an antiangiogenic substance, are decreased in peritoneal fluid of women with advanced endometriosis. Am J Reprod Immunol. 2003; 50: 60- 65.
- Tariverdian N, Siedentopf F, Rücke M, Blois SM, Klapp BF, Kentenich H, et al. Intraperitoneal immune cell status in infertile women with and without endometriosis. J Reprod Immunol. 2009; 80: 80-90.
- Jones RK, Bulmer JN, Searle RF. Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum Reprod Update. 1998; 4: 702-709.
- Ponce C, Torres M, Galleguillos C, Sovino H, Boric MA, Fuentes A, et al. Nuclear factor kappaB pathway and interleukin-6 are affected in eutopic endometrium of women with endometriosis. Reproduction. 2009; 137: 727-737.
- Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed Res Int. 2015; 2015: 795976.
- Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002; 83: 149-155.
- Bedaiwy MA, Dahoud W, Skomorovska-Prokvolit Y, Yi L, Liu JH, Falcone T, et al. Abundance and Localization of Progesterone Receptor Isoforms in Endometrium in Women With and Without Endometriosis and in Peritoneal and Ovarian Endometriotic Implants. Reprod Sci. 2015; 22: 1153-1161.
- Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. 2017; 108: 19-27.
- Garcia-Velasco JA, Fassbender A, Ruiz-Alonso M, Blesa D, D’Hooghe T, Simon C. Is endometrial receptivity transcriptomics affected in women with endometriosis? A pilot study. Reprod Biomed Online. 2015; 31: 647-654.
- Miravet-Valenciano J, Ruiz-Alonso M, Gómez E, Garcia-Velasco JA. Endometrial receptivity in eutopic endometrium in patients with endometriosis: it is not affected, and let me show you why. Fertil Steril. 2017; 108: 28-31.
- Loke YW, King A, Burrows TD. Decidua in human implantation. Hum Reprod. 1995; 10: 14-21.
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006; 12:
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993; 14: 353-356.
- Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007; 29: 95-113.
- Abbondanzo SJ, Cullinan EB, McIntyre K, Labow MA, Stewart CL. Reproduction in mice lacking a functional type 1 IL-1 receptor. Endocrinology. 1996; 137: 3598-3601.
- Abbondanzo SJ, Cullinan EB, McIntyre K, Labow MA, Stewart CL. Reproduction in mice lacking a functional type 1 IL-1 receptor. Endocrinology. 1996; 137: 3598-3601
- Lédée N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, et al. The Uterine Immune Profile May Help Women With Repeated Unexplained Embryo Implantation Failure After In Vitro Fertilization. Am J Reprod Immunol. 2016; 75: 388-401.
- Lédée N, Prat-Ellenberg L, Chevrier L, Balet R, Simon C, Lenoble C, et al. Uterine immune profiling for increasing live birth rate: A one-to- one matched cohort study. J Reprod Immunol. 2017; 119: 23-30
- Podgaec S, Abrao MS, Dias JA Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007; 22: 1373-1379.
- Abramiuk M, Grywalska E, Ma?kowska P, Sierawska O, Hrynkiewicz R, Nied?wiedzka-Rystwej P. The Role of the Immune System in the Development of Endometriosis. Cells. 2022; 11: 2028.
- Scheerer C, Bauer P, Chiantera V, Sehouli J, Kaufmann A, Mechsner S. Characterization of endometriosis-associated immune cell infiltrates (EMaICI). Arch Gynecol Obstet. 2016; 294: 657-664.
- Hutter S, Heublein S, Knabl J, Andergassen U, Vrekoussis T, Makrigiannakis A, et al. Macrophages: are they involved in endometriosis, abortion and preeclampsia and how? J Nippon Med Sch. 2013; 80: 97-103.
- Karin Wickström, Anneli Stavréus-Evers, Olivier Vercauteren, Matts Olovsson, Greta Edelstam. Effect of lignocaine on IL-6, IL-8, and MCP-1 in peritoneal macrophages and endometriotic stromal cells. Reproductive Sci. 2017; 24: 382-392.
- Xiaocui L, Wei H, Yunlang C, Zhenzhen Z, Min A. CSF-1-induced DC-SIGN+ macrophages are present in the ovarian endometriosis. Reprod Biol Endocrinol. 2022; 20: 48.
- Haomeng Zhang, Shuman Sheng, Zhengwu Pan, Lanlan Zhao, Chunrun Yang, Changzhong Li, et al. Immune and Endocrine Regulation in Endometriosis: What We Know. J Endometriosis and Uterine Disorders. 2023; 4: 100049.
- Jørgensen H, Fedorcsak P, Isaacson K, Tevonian E, Xiao A, Beste M, et al. Endometrial cytokines in patients with and without endometriosis evaluated for infertility. Fertil Steril. 2022; 117: 629-640.
- Reis JL, Rosa NN, Ângelo-Dias M, Martins C, Borrego LM, Lima J. Natural Killer Cell Receptors and Endometriosis: A Systematic Review. Int J Mol Sci. 2022; 24: 331.
- Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up- regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002; 169: 3600-3605.
- Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002; 13: 169-183.
- Barreira da Silva R, Graf C, Münz C. Cytoskeletal stabilization of inhibitory interactions in immunologic synapses of mature human dendritic cells with natural killer cells. Blood. 2011; 118: 6487-6498.
- Yang M, Li D, Chang Z, Yang Z, Tian Z, Dong Z. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J Exp Med. 2015; 212: 253- 265.
- Guzick DS, Silliman NP, Adamson GD, Buttram VC Jr, Canis M, Malinak LR, et al. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine’s revised classification of endometriosis. Fertil Steril. 1997; 67: 822-829.
- Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand. 2017; 96: 659-667.