Factors Influencing Breast Development Following Gender Affirming Hormone Therapy among Transgender Women in China
- #. These authors contributed equally to this work and should be considered co-first authors
- 1. Department of Obstetrics and Gynecology, The First Affiliated Hospital of Jinan University, China
- 2. School of Nursing, Jinan University, Guangzhou, China
- 3. Department of Ultrasonography, The First Affiliated Hospital of Jinan University, Guangzhou, China
Abstract
Purpose: This study aimed to investigate the patterns of breast development and the factors influencing this process following Gender-Affirming Hormone Therapy (GAHT) in Chinese transgender women.
Methods: The clinical data of 113 transgender women from July 2022 to July 2024 were retrospectively analyzed. In addition, in the prospective longitudinal study, 42 transgender women were followed at 4 time points: 0, 3, 6, and 12 months after starting GAHT. Demographic characteristics, Body Mass Index (BMI), Body Fat Percentage (BFP), serum estradiol level, and breast thickness measured by ultrasound were collected. SPSS 25.0 and Python software were used for statistical analysis, and genetic algorithm was used to analyze the related influencing factors.
Results: With the extension of GAHT, mammary gland thickness gradually increased, with the most rapid development occurring during the first six months and stabilization after two years. Mammary gland thickness was positively correlated with treatment duration, BMI, BFP, and serum estradiol levels, with treatment duration having the greatest impact. Over the two years of GAHT, the genetic algorithm predicted that maximum mammary gland thickness (16.35 mm) would be achieved in approximately 22.53 months, corresponding to a BFP of 24.96% and a serum estradiol level of 129.30 pg/ml.
Conclusions: After receiving GAHT, breast development in transgender women was most pronounced during the first six months and stabilized after two years. Increased breast development correlated with treatment duration, BMI, BFP, and serum estradiol levels, providing clinical reference values for treatment optimization. Further studies are necessary to validate long-term outcomes.
Keywords
- Gender-Affirming Hormone Therapy
- Transgender Women
- Breast Development
- Hormonal Factors
Citation
Pei Q, Lin F, Wen Y, Zhang Y, Zhong X, et al. (2025) Factors Influencing Breast Development Following Gender-Affirming Hormone Therapy among Transgender Women in China. JSM Sexual Med 9(5): 1167.
INTRODUCTION
Transgender women frequently experience gender dysphoria due to the discordance between their assigned sex at birth and their self-identified gender [1-4]. To mitigate this dysphoria, many transgender women pursue Gender-Affirming Hormone Therapy (GAHT) and Gender Confirming Surgery (GCS) to modify their physical appearance, thereby aligning their bodies with their self-identified gender [5]. Research indicates that breast development is one of the most sought-after changes for transgender women [6], as breasts are often regarded as a key aspect of femininity [7]. Consequently, the changes in breast development during hormone therapy warrant further investigation. However, the influence of factors such as estrogen and Body Fat Percentage (BFP) on breast development during treatment remains unclear. Research on breast development and the factors influencing it in transgender women undergoing GAHT remains limited. Previous studies have primarily focused on overall changes in breast volume. For instance, the study by de Blok et al. utilized bra cup size as an indicator of breast development and found that significant growth occurred within the first six months of treatment, with a mean increase in breast-chest difference of up to 7.9 centimeters after one year [7].
In another study employing 3D technology to measure breast volume, findings indicated that while the increase in breast-chest difference stabilized after approximately nine months, breast volume continued to rise over a three-year period [8]. However, these studies have not identified factors influencing breast development beyond the duration of hormone treatment. Additionally, there is a lack of data on changes in breast development following hormone therapy specifically for Chinese transgender women. It has been documented that estrogen and BFP increase in transgender women after hormone therapy [9 11].
Given that estrogen and adipose tissue are strongly associated with mammary gland development in cisgender women [12,13], it is plausible to hypothesize that a similar relationship may exist in transgender women undergoing hormone therapy. Furthermore, during hormone therapy, mammary gland tissue proliferation occurs alongside increased fat accumulation in the breasts, enhancing both the mammary glands’ thickness and breast size. Conversely, weight loss leads to a reduction in body fat content, including in the breasts; however, mammary gland tissue is typically not significantly altered by weight loss.
In this study, mammary gland thickness was utilized as an indicator to assess breast development in transgender women undergoing GAHT at a tertiary hospital in Guangzhou, China. A retrospective analysis involved 113 transgender women, followed by the enrollment of 42 patients in a prospective, longitudinal study, with assessments conducted at 0, 3, 6, and 12 months post treatment. The objective of this study was to elucidate the patterns of breast development and the factors influencing these changes in Chinese transgender women following GAHT. It is hoped that it will establish a scientific foundation for enhancing treatment efficacy and devising personalized treatment plans, while also providing insights for the clinical application of GAHT.
METHODS
Ethical Approval and Informed Consent
This study followed the ethical standards of the Declaration of Helsinki revised in 2013 and was approved by the Institutional Review Board (IRB) of the participating tertiary hospital.
Study Population
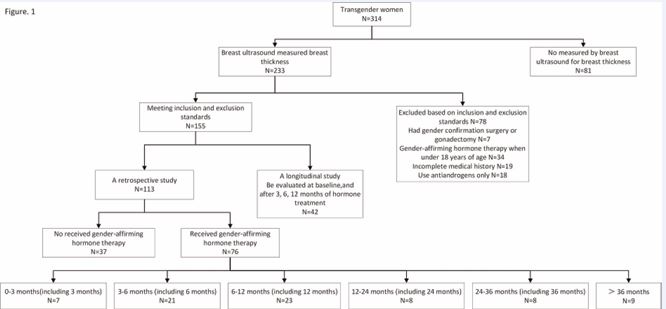
The study population comprised 314 transgender women who attended the outpatient clinic of the reproductive endocrinology department at a tertiary care hospital between July 2022 and July 2024 (Figure 1). Among them, 233 individuals underwent breast ultrasound examinations. A total of 155 patients met the inclusion-exclusion criteria, with 42 of these patients being evaluated at 0, 3, 6, and 12 months post-initiation of GAHT. In the retrospective analysis, 113 transgender women were selected based on a review of available case data, collecting baseline demographics, treatment duration, BFP, serum estradiol levels, and mammary gland thickness measurements. In the prospective longitudinal study, 42 patients receiving GAHT were followed up at 0, 3, 6, and 12 months post-treatment to gather data on demographics, BFP, serum estradiol levels, and mammary gland thickness.
Figure 1 Participant inclusion flowchart.
The inclusion criteria for patients were as follows: (1) All participants were identified as transgender women (individuals assigned male at birth with a female gender identity) by a multidisciplinary team of endocrinologists and psychiatrists specializing in gender confirmation medicine. This identification and the framework for supporting care were guided by the World Professional Association for Transgender Health (WPATH) Standards of Care (Version 8) and the International Classification of Diseases, 11th Revision (ICD-11) [14,15]. Specifically, in China, the clinical assessment process for transgender women seeking medical transition typically involved evaluation based on criteria outlined in the ‘Chinese Classification of Mental Disorders, 3rd Edition (CCMD-3)’ (notably categories F64 ‘Sex identity disorders’ and F65 ‘Disorders of sexual preference’) [16]. Additionally, the conceptual framework and diagnostic criteria for Gender Dysphoria as defined in the ‘Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)’ were considered within the multidisciplinary assessment context [17]. It is important to note that ‘transgender woman’ is an identity descriptor and not a diagnosis within any of these classification systems. The relevant diagnoses (e.g., Gender Dysphoria in DSM-5/ICD-11, F64 in CCMD-3) focus on the presence of clinically significant distress or impairment related to gender incongruence, which was a prerequisite for accessing gender-affirming medical interventions like GAHT in the studied setting; (2) hormones used for GAHT were estrogen and antiandrogen; (3) had mammary gland thickness measurements obtained via ultrasound; (4) age at the start of GAHT was between 18 and 40 years old.
Anthropometric Measurements Data
Basic demographic and clinical data, including age, height, weight, Body Mass Index (BMI), duration of GAHT, BFP, serum estradiol levels, and bilateral mammary gland thickness measurements (right and left), as well as mean mammary gland thickness, were collected from transgender women. The data were categorized into two distinct sets: retrospective and prospective longitudinal (repeated measures), with the retrospective data being analyzed exploratorily and subsequently validated against the longitudinal data.
GAHT Programs
In this study, the primary hormones utilized for GAHT were estrogens and antiandrogens. Estrogen therapy primarily consisted of estradiol valerate tablets (2–4 mg/ day) or estradiol gel (1.5–3 mg/day), while antiandrogen therapy mainly involved cyproterone acetate tablets (6.25–25 mg/day). In the retrospective analysis, patients were categorized based on the duration of their GAHT treatment: 0 represented no GAHT treatment; 1 represented treatment duration of up to three months; 2 represented treatment duration between three and six months (including six months); 3 represented treatment duration between six and twelve months (including twelve months); 4 represented treatment duration between twelve and twenty-four months (including twenty-four months); 5 represented treatment duration between twenty-four and thirty-six months (including thirty-six months); and 6 represented treatment duration exceeding thirty six months. Patients receiving GAHT were required to have follow-up assessments every six months, which included medical examinations such as liver and kidney function tests, breast ultrasounds, and bone densitometry assessments.
Mammary gland Thickness Measurement
All subjects underwent breast ultrasonography on a Sonic Aixplorer ultrasonic diagnostic machine with a line array SL15-4 probe at a frequency of 10 MHz, in a supine position with the arms naturally raised at the sides, fully exposing the breasts bilaterally for breast ultrasonography. The BI-RADS classification of the breast tissue was confirmed, and the Region of Interest (ROI) was selected to measure the thickness of the breast gland. All measurements were performed by two trained attending sonographers.
Serum estradiol level testing and BFP measurement
Serum estradiol levels were assessed from blood samples using a fully automated chemiluminescence analyzer (Beckman Coulter DX1800). BFP was calculated utilizing Dual-Energy X-ray Absorptiometry (DXA) technology with a Lunar Prodigy system (GE Healthcare, Chicago, IL, USA).
Statistical Analyses
Excel software was employed to construct the database, and statistical analyses were conducted using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and Python (Python Software Foundation, Delaware, USA). All tests were two sided, with a significance level set at 0.05. Data conforming to a normal distribution were described using mean ± standard deviation and analyzed using analyses of Analysis of Variance (ANOVA) and Pearson’s correlation. Data not conforming to a normal distribution were described using median (range) and analyzed with the rank-sum test and Pearson correlation analysis. Multifactorial analyses were performed using multiple linear regression, and generalized estimating equations were utilized to analyze repeated measures data. Genetic algorithms were employed to calculate the maximum mammary gland thickness and the corresponding predictor values.
Consent to Participate
All individual participants included in this study provided written informed consent before participation in the study.
RESULTS
Characteristics of 113 transgender women
A total of 113 transgender women were included in this retrospective study. The mean age of participants was 24 years, with a mean height of 172.51 cm, a mean BMI of 20.94 kg/m², and a mean weight of 62.38 kg. The median BFP was 25.60%, with a median estradiol level of 34.07 pg/mL. The median thickness of the right mammary gland was 9.90 mm, while the median thickness of the left mammary gland was 10.00 mm. The average mammary gland thickness was also 9.90 mm. Regarding treatment duration, 37 participants (32.74%) had received no GAHT, 7 (6.19%) were in the 0-3 month category, 21 (18.58%) in the 3-6 month category, 23 (20.35%) in the 6-12 month category, 8 (7.08%) in the 12-24 month category, 8 (7.08%) in the 24-36 month category, and 9 (7.96%) had received GAHT for more than 36 months. The detailed characteristics of the participants are summarized in Table 1.
Table 1: Characteristics of 113 transgender women.
|
Variables |
Total (N= 113) |
|
Age (years) |
24 ± 5 |
|
Height (cm) |
172.51 ± 6.89 |
|
BMI (kg/cm2) |
20.94 ± 3.27 |
|
Weight (kg) |
62.38 ± 10.90 |
|
treatment duration, N (%) |
|
|
0 |
37 (32.74) |
|
1 |
7 (6.19) |
|
2 |
21 (18.58) |
|
3 |
23 (20.35) |
|
4 |
8 (7.08) |
|
5 |
8 (7.08) |
|
6 |
9 (7.96) |
|
BFP (%) |
25.60 (20.10, 29.90) |
|
Estradiol (pg/ml) |
34.07 (23.57, 63.01) |
|
Right mammary gland thickness (mm) |
9.90 (5.60, 13.80) |
|
Left mammary gland thickness (mm) |
10.00 (6.00, 13.90) |
|
Average mammary gland thickness (mm) |
9.90 (5.55, 13.85) |
Note: Data are presented as mean ± SD, median (range), or percentages. treatment duration: based on 0.0: No received gender-affirming hormone therapy; 1: 0-3 months (including 3 months); 2: 3-6 months (including 6 months); 3: 6-12 months (including 12 months); 4: 12-24 months (including 24 months); 5: 24-36 months (including 36 months); 6: > 36 months.
Abbreviation: N, sample size; BFP, Body Fat Percentage; BMI, Body Mass Index; SD, Standard Deviation.
Correlation of mean mammary gland thickness with other variables
Mean mammary gland thickness was significantly correlated with medication duration, age, BMI, body weight, BFP, and serum estradiol levels. Among these variables, the strongest positive correlation was observed between mean mammary gland thickness and medication duration (ρ = 0.526, P < 0.01). Detailed correlations are presented in Table 2.
Table 2: Correlation of mean mammary gland thickness with other variables.
|
Variables |
Right mammary gland thickness (mm) |
Left mammary gland thickness (mm) |
Average mammary gland thickness (mm) |
|||
|
ρ |
P |
ρ |
P |
ρ |
P |
|
|
Treatment duration |
0.529 |
< 0.01 |
0.397 |
< 0.01 |
0.526 |
< 0.01 |
|
Age (years) |
0.182 |
0.018 |
0.201 |
0.009 |
0.18 |
0.02 |
|
Height (cm) |
-0.089 |
0.251 |
-0.08 |
0.3 |
-0.086 |
0.266 |
|
BMI (kg/cm2) |
0.367 |
< 0.01 |
0.407 |
< 0.01 |
0.377 |
< 0.01 |
|
Weight (kg) |
0.323 |
< 0.01 |
0.358 |
< 0.01 |
0.330 |
< 0.01 |
|
BFP (%) |
0.461 |
< 0.01 |
0.439 |
< 0.01 |
0.462 |
< 0.01 |
|
Estradiol (pg/ml) |
0.283 |
< 0.01 |
0.284 |
< 0.01 |
0.285 |
< 0.01 |
Changes in mammary gland thickness after receiving different durations of GAHT
The differences in right mammary gland thickness, left mammary gland thickness, and mean mammary gland thickness among the seven groups were statistically significant (P < 0.05). Mammary gland thickness increased progressively with the duration of GAHT, with the most notable increase occurring approximately six months after the initiation of treatment. Detailed information regarding these changes is provided in Table 3.
Table 3: Changes in mammary gland thickness with treatment duration
|
Variables |
Total (N = 113) |
0 (N = 37) |
1 (N = 7) |
2 (N = 21) |
3 (N = 23) |
4 (N = 8) |
5 (N = 8) |
6 (N = 9) |
F |
P value |
|
Right mammary gland thickness (mm) |
9.26 ± 5.61 |
2.96 ± 3.56 |
8.86 ± 2.23 |
13.42 ± 2.65 |
12.45 ± 3.56 |
13.20 ± 3.33 |
11.88 ± 2.64 |
11.74 ± 4.84 |
33.37 |
< .001* |
|
Left mammary gland thickness (mm) |
9.47 ± 5.83 |
2.92 ± 3.69 |
9.76 ± 3.18 |
14.11 ± 2.71 |
12.70 ± 3.74 |
13.38 ± 3.21 |
11.68 ± 1.68 |
11.66 ± 5.28 |
33.25 |
< .001* |
|
Average mammary gland thickness (mm) |
9.36 ± 5.67 |
2.94 ± 3.59 |
9.31 ± 2.64 |
13.76 ± 2.53 |
12.57 ± 3.55 |
13.29 ± 3.24 |
11.78 ± 2.05 |
11.70 ± 5.02 |
34.72 |
< .001* |
*Statistical significance at a level of 0.05.
Multiple linear regression results of factors
influencing mean mammary gland thickness
The multiple linear regression analysis was conducted using treatment duration, age, BMI, body weight, BFP, and serum estradiol levels as independent variables, with average mammary gland thickness as the dependent variable. The results indicated a significant linear regression relationship between changes in mean mammary gland thickness and treatment duration, BMI, and BFP. Specifically, changes in mean mammary gland thickness were positively correlated with treatment duration, BMI, and BFP, with treatment duration exerting the greatest influence on mean mammary gland thickness. The most significant difference in mean mammary gland thickness was observed between treatment durations of 6-12 months versus no receiving treatment. Additionally, the greatest change in mammary gland thickness occurred during the 3–6-month period following the initiation of treatment compared to other time intervals. Detailed data are provided in Tables 4, 5, and 6.
Table 4: Independent variable assignment method
|
Independent variable |
Assignment method |
|
Age |
Original value input |
|
BMI |
Original value input |
|
Weight |
Original value input |
|
BFP |
Original value input |
|
Estradiol |
Original value input |
|
treatment duration |
No received GAHT=0 |
|
|
0-3 months (including 3 months)= 1 |
|
|
3-6 months (including 6 months)= 2 |
|
|
6-12 months (including 12 months)= 3 |
|
|
12-24 months (including 24 months)= 4 |
|
|
24-36 months (including 36 months)= 5 |
|
|
> 36 months= 6 |
Abbreviation: GAHT: Gender-Affirming Hormone Therapy. Note: Treatment duration
Table 5: Multiple linear regression results of factors influencing mean breast thickness factors1
|
Variables |
B |
standard error |
β |
t |
P |
95% Confidence Interval |
|
|
lower-bound |
upper-bound |
||||||
|
(constant) |
-8.052 |
1.781 |
|
-4.52 |
< 0.01 |
-11.584 |
-4.519 |
|
BMI (kg/cm2) |
0.346 |
0.107 |
0.2 |
3.239 |
0.002 |
0.134 |
0.557 |
|
BFP (%) |
0.184 |
0.057 |
0.204 |
3.213 |
0.002 |
0.07 |
0.297 |
|
treatment duration |
|
|
|
|
|
|
|
|
1 |
5.014 |
1.166 |
0.214 |
4.299 |
< 0.01 |
2.701 |
7.326 |
|
2 |
8.976 |
0.803 |
0.618 |
11.184 |
< 0.01 |
7.384 |
10.567 |
|
3 |
9.203 |
0.778 |
0.656 |
11.832 |
< 0.01 |
7.661 |
10.746 |
|
4 |
8.769 |
1.118 |
0.398 |
7.846 |
< 0.01 |
6.553 |
10.986 |
|
5 |
7.809 |
1.105 |
0.355 |
7.068 |
< 0.01 |
5.618 |
10 |
|
6 |
7.464 |
1.082 |
0.358 |
6.9 |
< 0.01 |
5.319 |
9.609 |
1-0: with 0 as the reference point in time, 1 compared to 0;
Table 6: Multiple linear regression results of factors influencing mean breast thickness factors 2
|
Variables |
B |
standard error |
β |
t |
P |
95% Confidence Interval |
|
|
lower-bound |
upper-bound |
||||||
|
(constant) |
-1.314 |
1.819 |
|
-0.722 |
0.472 |
-4.921 |
2.293 |
|
BMI (kg/cm2) |
0.329 |
0.106 |
0.19 |
3.09 |
0.003 |
0.118 |
0.539 |
|
BFP (%) |
0.195 |
0.057 |
0.216 |
3.429 |
0.001 |
0.082 |
0.307 |
|
treatment duration |
|
|
|
|
|
|
|
|
1—0 |
6.528 |
0.524 |
0.653 |
12.459 |
< 0.01 |
5.489 |
7.567 |
|
2—1 |
7.694 |
0.874 |
0.657 |
8.802 |
< 0.01 |
5.961 |
9.427 |
|
3—2 |
5.201 |
0.877 |
0.574 |
5.929 |
< 0.01 |
3.462 |
6.94 |
|
4—3 |
2.521 |
0.838 |
0.226 |
3.009 |
0.003 |
0.86 |
4.182 |
2-1: with 1 as the reference point in time, 2 compared to 1;
3-2: with 2 as the reference point in time, 3 compared to 2;
4-3: with 3 as the reference point in time, 4 compared to 3.
Characteristics of 42 Transgender women Before GAHT
At baseline, the median age of the participants was 24 years, with a mean height of 172.25 cm, a mean weight of 61.89 kg, a BMI of 20.84 kg/m², and a median BFP of 24.60%. Secondary outcome indicators revealed a median estradiol level of 29.79 pg/ml. Among the primary outcome indicators, the median right mammary gland thickness was 7.45 mm, while the median left mammary gland thickness was also 7.45 mm, resulting in a mean mammary gland thickness of 7.65 mm. Overall, the patients were generally young and of lower body weight. Hormone levels and mammary gland thickness variability were high. The specific data are provided in Table 7.treatment duration of 22.53 months, a BFP of 24.96%, and a serum estradiol level of 129.30 pg/ml.
Table 7: Characteristics of 42 transgender women Before gender-affirming hormone therapy
|
Variables |
0 (N = 42) |
|
Age (years) |
24.24 (21.25,25.75) |
|
Height (cm) |
172.25 ± 4.74 |
|
Weight (kg) |
61.89 ± 8.14 |
|
BMI (kg/cm2) |
20.84 ± 2.96 |
|
BFP (%) |
24.60 (18.35,26.31) |
|
Estradiol (pg/ml) |
29.79 (20.49,62.13) |
|
Right mammary gland thickness (mm) |
7.45 (0.00,10.84) |
|
Left mammary gland thickness (mm) |
7.45 (0.00,10.87) |
|
Average mammary gland thickness (mm) |
7.65 (0.00,10.86) |
Note: Data are presented as mean ± standard deviation, median (range). Only BFP is presented as percentages.
DISCUSSION
Current research shows that after receiving GAHT, mammary gland development occurs primarily in the earliest 6 months, with the greatest changes occurring at 3-6 months, with significant mammary gland development at 1 year, and stabilization of mammary gland development at roughly 2 years. As the duration of treatment increases, rising BMI, BFP and estrogen levels promote an increase in mammary gland thickness.
In prior studies, breast development has been evaluated through measures such as breast volume, Tanner staging [18], and chest measurements to calculate bra cup size. These studies have found that mammary gland development predominantly occurs during the first six months of GAHT, with complete breast development potentially requiring more than two years of continuous hormone therapy [18-20]. The present study utilized ultrasound to measure mammary gland thickness as a means of assessing breast development.
The evaluation results aligned with previous research, indicating that the rate of breast development is most rapid during the first six months of GAHT. Subsequently, the rate of development gradually decreases, leveling off after 24 months. During puberty in cisgender women, mammary gland thickness increases with age due to hormonal influences, transitioning from basic to mature mammary glands [21].
Nevertheless, the current study did not observe an effect of age on mammary gland thickness, likely due to the smaller patient sample size, the shorter observation period, and minimal age variation among patients during the one-year cycle. Previous research has indicated a strong correlation between body weight and breast mass in cisgender women, with heavier individuals typically exhibiting larger breast masses, primarily attributed to differences in fat content [22]. However, this study measured only mammary gland thickness and did not account for adipose tissue within the breasts, thus precluding any observed effect of body weight on breast development. Additionally, two prominent studies on breast development in transgender women did not find age or weight to be predictive of breast development [7,8].
Histologically, the breast tissue of transgender women undergoing GAHT exhibits chemotaxis and develops ducts, lobules, and alveoli, analogous to the mammary gland development observed in cisgender women [20]. This process occurs during puberty and is hormone-dependent, with estrogen playing a critical role [23-25]. Consequently, this study incorporated serum estradiol levels into the generalized estimating equation for analysis, revealing a positive correlation between estradiol levels and the thickness of mammary gland development. Hormone therapy elevates estrogen levels in transgender women, thereby stimulating breast tissue growth and development.
However, it is important to note that breast development may not depend solely on estrogen levels. Our findings suggest that BFP is also a significant predictor of mammary gland thickness. Adipocytes can participate in the synthesis and storage of estrogen; as BFP increases, the number of adipocytes rises, allowing for greater estrogen storage, which can further stimulate the proliferation and development of mammary glands. Additionally, adipose tissue secretes various growth factors, such as insulin- like growth factor-1 (IGF-1), which may be implicated in mammary gland development [19].
Research indicates that during the first year of treatment, increases in BFP and serum estradiol levels promote the thickening of breast tissue. Throughout the long-term treatment process, BMI and BFP are positively correlated with changes in mammary gland thickness. However, excessive estradiol intake can lead to numerous side effects and increase the risk of various conditions, such as Venous Thromboembolism (VTE) [26], hepatotoxicity, cholelithiasis, and hyperprolactinemia [27]. Furthermore, a high BFP can also increase the risk of cardiovascular disease.
Additionally, this study demonstrates that changes in breast development primarily occur during the first two years of treatment. Therefore, we utilized a model to calculate the optimal serum estrogen levels and BFP values during this critical period of breast development, providing clinicians with reference indices for blood estrogen levels and BFP when administering hormone therapy to transgender women. Consequently, we propose that during the early stages of hormone therapy, when breast development is most pronounced, estrogen dosages should be maintained at a higher value within the normal range to facilitate optimal breast growth, followed by a reduction in estrogen dosage. Such a treatment strategy may help mitigate the side effects associated with exogenous estrogen.
Limitations
While this study elucidates the effects of hormone therapy on breast development and identifies various factors influencing this process, it has notable limitations. The small sample size and the one-year observation period of the longitudinal study restrict our ability to understand the long-term changes in breast development. To obtain a more comprehensive understanding of breast development changes, future studies should aim to increase the sample size, extend the observation period, and employ more robust methods for assessing breast development. Furthermore, it would be valuable to investigate whether varying dosages of estrogen, different administration methods, and the use of estrogen alone versus in combination with cyproterone acetate impact breast development.
CONCLUSIONS
This study investigated the patterns of breast development and the influencing factors in Chinese transgender women following GAHT. The findings indicated that transgender women experienced the most rapid breast development during the first six months of GAHT, primarily concentrated within the first two years of treatment, after which development stabilized.
During the first year of treatment, increased BFP and elevated serum estradiol levels contributed to increased breast development. Throughout the long-term treatment period, BMI and BFP were positively correlated with changes in breast development. Thus, estrogen appears to significantly influence breast development in the early stages of treatment, with this effect diminishing as hormone levels stabilize over time. By utilizing genetic algorithm prediction, this study provided clinicians with reference values for serum estradiol levels and BFP to optimize breast development outcomes, thereby reducing the side effects associated with exogenous estrogen and enhancing treatment efficacy.
Although this study offers important insights into breast development processes in transgender women, the limitations of a small sample size and a short observation period must be acknowledged. Future research should aim to increase the sample size, extend the observation period, and employ more comprehensive assessment methods to further refine hormone treatment regimens and improve medical care for transgender women.
ACKNOWLEDGMENTS
The authors would like to acknowledge the support provided by the Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University and the Science and Technology Program of Guangzhou.
Author contribution(s)
Q.P.: Writing – original draft (equal); Formal analysis (equal); Investigation (equal). F.L.: Writing – original draft (equal). Y.W.: Writing – original draft (equal). Y.Z.: Data curation (lead); Validation (lead); Investigation (supporting); Methodology (supporting). X.Z.: Project administration (equal); Supervision (equal); Writing – review & editing (equal). X.T.: Project administration (equal); Supervision (equal); Writing – review & editing (equal). L.G.: Project administration (lead); Supervision (lead); Writing – review & editing (lead).
Funding Information
This study was supported by the Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University, China (No. JNU1AF-CFTP-2022-a01227) and Science and Technology Program of Guangzhou, China (No. 2023A03J0602).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Shumer DE, Nokoff NJ, Spack NP. Advances in the care of transgender children and adolescents. Adv Pediatr. 2016; 63: 79-102.
- Cohen-Kettenis PT, Klink D. Adolescents with gender dysphoria. Best Pract Res Clin Endocrinol Metab. 2015; 29: 485-495.
- Safer JD, Tangpricha V. Care of the Transgender Patient. Ann Intern Med. 2019; 171: ITC1-ITC16.
- Puckett JA, Cleary P, Rossman K, Newcomb ME, Mustanski B. Barriers to Gender-Affirming Care for Transgender and Gender Nonconforming Individuals. Sex Res Social Policy. 2018; 15: 48-59.
- de Blok CJ, Dijkman BA, Wiepjes CM, Konings IR, Dreijerink KM, Barbé E, et al. Frequency and outcomes of benign breast biopsies in trans women: A nationwide cohort study. Breast. 2021; 57: 118-122.
- Masumori N, Baba T, Abe T, Niwa K. What is the most anticipated change induced by treatment using gender-affirming hormones in individuals with gender incongruence? Int J Urol. 2021; 28: 526-529.
- de Blok CJM, Klaver M, Wiepjes CM, Nota NM, Heijboer AC, Fisher AD, et al. Breast Development in transwomen after 1 Year of cross-sex hormone therapy: Results of a Prospective Multicenter Study. J Clin Endocrinol Metab. 2018; 103: 532-538.
- de Blok CJM, Dijkman BAM, Wiepjes CM, Staphorsius AS, Timmermans FW, Smit JM, et al. Sustained breast development and breast anthropometric changes in 3 years of gender-affirming hormone treatment. J Clin Endocrinol Metab. 2021; 106: e782-e790.
- Yun Y, Kim D, Lee ES. Effect of Cross-Sex Hormones on Body Composition, Bone Mineral Density, and Muscle Strength in Trans Women. J Bone Metab. 2021; 28: 59-66.
- Sumerwell C, Carlin K, Walsh E, Hodax JK. Serum hormone concentrations in transgender youth receiving estradiol. Endocr Pract. 2024; 30: 155-159.
- Pei Q, Song Y, Huang Z, Yu H, Xu H, Ye X, et al. Effects of gender- affirming hormone therapy on body fat: A retrospective case?control study in Chinese transwomen. Lipids Health Dis. 2024; 23: 146.
- Fu NY, Nolan E, Lindeman GJ, Visvader JE. Stem cells and the differentiation hierarchy in mammary gland development. Physiol Rev. 2020; 100: 489-523.
- Biswas SK, Banerjee S, Baker GW, Kuo CY, Chowdhury I. The Mammary Gland: Basic structure and molecular signaling during development. Int J Mol Sci. 2022; 23: 3883.
- Coleman E, Radix AE, Bouman WP, Brown GR, de Vries ALC, Deutsch MB, et al. Standards of care for the health of transgender and gender diverse people, Version 8. Int J Transgend Health. 2022; 23: S1-S259.
- World Health Organization. International classification of diseases, 11th revision: ICD-11 for mortality and morbidity statistics. Geneva, Switzerland: World Health Organization; 2025.
- Chinese Society of Psychiatry. Chinese classification of mental disorders. 3rd ed. Jinan, China: Shandong Science and Technology Press; 2001
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013
- Nolan BJ, Frydman AS, Leemaqz SY, Carroll M, Grossmann M, Zajac JD, et al. Effects of low-dose oral micronised progesterone on sleep, psychological distress, and breast development in transgender individuals undergoing feminising hormone therapy: A prospective controlled study. Endocr Connect. 2022; 11: e220170.
- Berliere M, Coche M, Lacroix C, Riggi J, Coyette M, Coulie J, et al. Effects of hormones on breast development and breast cancer risk in transgender women. Cancers (Basel). 2022; 15: 245.
- Lienhoop T, Green L. Breast imaging in transgender women: A review. Clin Imaging. 2021; 80: 283-289.
- Reisman T, Goldstein Z, Safer JD. A REVIEW OF BREAST DEVELOPMENT IN CISGENDER WOMEN AND IMPLICATIONS FOR TRANSGENDER WOMEN. Endocr Pract. 2019; 25: 1338-1345.
- Brown N, White J, Milligan A, Risius D, Ayres B, Hedger W, et al. The relationship between breast size and anthropometric characteristics. Am J Hum Biol. 2012; 24: 158-164.
- Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999; 20: 358-417.
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995; 9: 2266-2278.
- Wierckx K, Gooren L, T’Sjoen G. Clinical review: Breast development in trans women receiving cross-sex hormones. J Sex Med. 2014; 11: 1240-1247.
- ACOG committee opinion no. 556: Postmenopausal estrogen therapy: Route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013; 121: 887-890.
- Oh JW, Yun Y, Lee ES. A Review of gender-affirming hormone therapy for transgender and gender diverse adults in South Korea. J Menopausal Med. 2022; 28: 92-102.