How Sexual Reproduction Arose
- 1. Department of Ecology, University of Brasília, Brazil
Citation
Gollo G (2025) How Sexual Reproduction Arose. JSM Sexual Med 9(1): 1149.
INTRODUCTION
There is compelling evidence that the first life forms emerged on Earth as soon as the planet’s surface cooled enough to provide the necessary conditions for life to thrive.For instance, a recent study demonstrated the relative ease with which a cell membrane can form [1], while another suggests that LUCA (the Last Universal Common Ancestor) existed around 4.2 billion years ago [2]. This timeframe, in relation to the formation of the Earth and Moon, implies that the emergence of life occurred within a surprisingly brief geological period. Furthermore, these early organisms quickly acquired complexity, approaching the level of today’s prokaryotes in a remarkably short span on an evolutionary timescale .
For approximately 2 billion years after LUCA, however, all life on the planet remained confined to relatively low complexity limits, which still restrict prokaryotes (bacteria and archaea) to this day [3,4].
This limitation was only overcome with the emergence of eukaryotes, which are far more complex than their prokaryotic predecessors. Eukaryotes arose as a result of the endosymbiotic integration between bacteria and archaea [5]. All organisms more complex than bacteria and archaea are eukaryotes.
Another distinctive characteristic of eukaryotes is their mode of reproduction. Sexual reproduction is exclusive to organisms in this group, and although some eukaryotes also undergo certain forms of asexual reproduction, all eukaryotes trace their origins to sexual reproduction carried out by at least part of their ancestral lineage [6].
In fact, the emergence of sexual reproduction, mitosis, meiosis, the cell nucleus, and the alternation of generations together constituted a single phenomenon: the emergence of eukaryotes (Gollo, 2023).
Binary Fission and Sexual Reproduction
Prokaryotes are haploid, unicellular organisms whose cells lack a nucleus or any other structure besides the cell membrane that surrounds them. The characteristic mode of reproduction of these organisms, binary fission, simply consists of cell division following chromosomal duplication.
Paradoxically, this highly simple and efficient mode of reproduction was replaced in eukaryotes by a far more complex, costly, and inefficient mechanism. This apparent contradiction presents a challenge to explanations based solely on traditional Darwinian processes, given the striking inefficiency of the new system.
The cost of sexual reproduction is often euphemistically referred to as “the two-fold cost of sexual reproduction”, drastically and erroneously mitigating an attribution that should be stated as “the two-fold per generation cost of sex.” The distinction between the two formulations is striking and highlights the peculiarity of the process. Notably, the two-fold per generation cost results in a cost of 1000 times over 10 generations, or 1,000,000 times over 20 generations, rather than simply a doubling of cost, as commonly stated. Moreover, this cost pertains only to the investment in the production of males. If the costs per generation for mating were taken into account, as well as the need to support two forms of life in the same cycle (one haploid, the other diploid), the cost of mitosis, meiosis, and all the apparatus and mechanisms necessary for the execution of the bizarre process, the resulting figures would be so odd that one might even question the viability of such a process.
Brief Considerations on Eukaryotes
Even eukaryotes that do not engage in sexual reproduction descend from some ancestor that underwent it. This mode of reproduction is inextricably linked to the advent of mitosis, meiosis, haploid/diploid alternation of generations, and a complex structure including the cytoskeleton and cell nucleus. Each of these characteristics requires the prior acquisition of a series of complex cellular structures, whose function only seems to make sense after the development of sexual reproduction, which constitutes another paradox. This observation has challenged orthodox Darwinian attempts to explain the phenomenon through the accumulation of small modifications across generations. Such explanations would predict the repeated emergence of sexual reproduction—much like the evolution of eyes and other structures—yet sexual reproduction appears to have originated only once. The unorthodox solution to the eukaryote emergence enigma involves a double endosymbiotic event: the acquisition of mitochondria through the incorporation of bacteria by an archaeon, and the coevolution of a host/parasite relationship that gave rise to sexual reproduction.
Origins of Sexual Reproduction
Acquisition of Mitochondria: The saga of eukaryotes is inextricably linked to the emergence of sexual reproduction. The first step involved the incorporation, by an archaeon, of a bacterium that eventually evolved into the mitochondrion. This acquisition led to an extraordinary energy gain for the host cell, as the energy production shifted from the cell membrane (a surface) to the cell’s volume. As a result, energy production, which was previously proportional to the square of the cell’s diameter due to its relation to a surface area—became proportional to the cube of that dimension—due to its distribution throughout the cell’s volume. This shift allowed for a dramatic increase in cell size, with the volume of a typical eukaryotic cell being 15,000 times larger than that of a typical prokaryotic cell [3].
Retroparasitism
It can be assumed that, after a long coevolutionary period, much like sperm cells, the parasite inoculated the host with its genome, along with what would eventually become the mitotic apparatus— material originally used by the parasite to form the cyst in which it remained embedded. This assumption helps explain the emergence of the cell nucleus, mitosis, and meiosis. Under this interpretation, the cell nucleus originates from the parasitic cyst, which explains, for example, a certain paradoxical feature of this structure: the RNA transport system from the cell nucleus to the cytosol, which was originally used by the parasite to exploit the host cell. This inference resolves the paradox surrounding the origin of such complex structures that were thought to had no prior function.
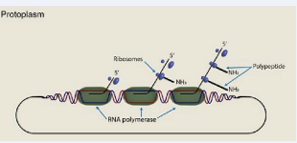
According to Bell
The change from the coupled system of transcription and translation found modern archaea (Figure 1),
Figure 1 : Change from the coupled system of transcription and translation found in modern archaea
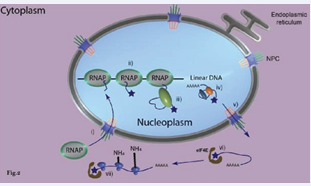
to the uncoupled eukaryotic system found in LECA (Figure 2),
Figure 2 : Change from the uncoupled eukaryotic system found in LECA
required the evolution of a complex molecular system involving hundreds of genes acting in concert. (...) Paradoxically, the high level of complexity and the integrated nature of the cap-based system of uncoupling transcription from translation suggest a long evolutionary history, yet no transitional cellular forms linking the prokaryotic (Figure 1), and eukaryotic systems (Figure 2,3),
Figure 3 : Transitional cellular forms linking the prokaryotic and eukaryotic systems
have been described [7].
The paradox is not limited to the time it takes to form such complex structures. Since their formation, nucleated organisms, such as eukaryotes, have relied on a transport mechanism that prevents complete isolation resulting from the confinement of their chromosomes within the nucleus.
Another radically unusual episode occurred when, in the scenario described above, the host’s genome was engulfed by the cyst formed after the inoculation of the genome and mitotic apparatus by the parasite, causing the entanglement of the genomes inside. The result of this phenomenon was retroparasitism, in which the parasite became parasitized by its own host! In this way, the host became the parasite of its own parasite, while their genomes became inextricably intertwined. From then on, each new infection inoculated the double genome of the chimeric creature.
Another radically unusual event occurred when, in the scenario described above, the host’s genome was engulfed by the cyst formed after the inoculation of the parasite’s genome and mitotic apparatus, resulting in the entanglement of the host and parasite genomes. The outcome of this phenomenon was retroparasitism, where the parasite became parasitized by its own host. In this way, the host became the parasite of its own parasite, with their genomes becoming inextricably intertwined. From then on, each new infection inoculated the double genome of the chimeric organism.
The Emergence of Sexual Reproduction: The new scenario then included the presence of a chimeric parasite, carrying a dual genome, infecting hosts that had already reached an advanced stage of coevolution. Over generations,the parasitic relationship had become significantly attenuated, allowing the parasite to survive encysted in its host for extended periods. One way for the host to rid itself of the parasite—which, although attenuated, continued to usurp its resources—was to trigger the cellular division mechanism after infection, thereby freeing one of the resulting cells from the infection. The parasite’s counterstrike came in the form of cyst duplication, thereby preventing half of the host cell from escaping its domain, a strategy that ultimately resulted in mitosis. However, before this process emerged in its contemporary form, the host introduced a new strategy that further attenuated the relationship. This forced the encysted parasite to remain for increasingly longer periods in a latent, practically inert, state, with no significant energy expenditure, postponing or even preventing its multiplication in the form of infecting BALOs (Bdellovibrio And Like Organisms) [8,9]. In this way, the host reduced the damage caused by the parasite to almost zero. The strategy led to a multitude of hosts infected by nearly harmless parasites, long encysted within them.
A second infection, however, put the host at a numerical disadvantage in the battle against its parasites, which, by combining their forces, managed to overcome the host’s control, freeing themselves from the inert state previously imposed in order to trigger their own multiplication and subsequent hatching, in the form of BALOs. What happened next has been recapitulated to this day during fertilization. Just as pronuclei merge to form the single nucleus of an embryonic cell, the cysts injected by the BALOs also fused, generating diploid nuclei that gave rise to diploid BALOs. Successive reinfections of such organisms eventually resulted in a genomic imbalance caused by uncontrolled polyploidy. The solution to this imbalance involved the execution of meiosis. After this final step, infections no longer differed from fertilizations, with the infecting BALOs no longer distinguishable from sperm, and the host cells resembling eggs. Thus, sexual reproduction had emerged—this evolutionary kludge that loudly highlights the parasitic origin of sexual reproduction.
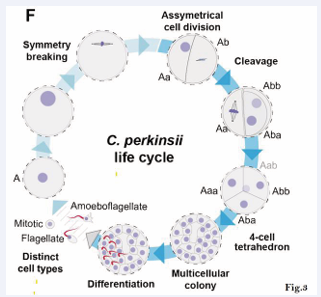
The ichthyosporean Chromosphaera perkinsii – a close relative that diverged from animals around 1 billion years ago – undergoes symmetry breaking and develops through cleavage divisions, forming a prolonged multicellular colony with distinct, coexisting cell types [10,11]. This provides a model for the life cycle of early eukaryotes.
It is worth noting that the narrative presented above also describes the emergence of eukaryotes, the most extraordinary phenomenon in the entire history of living beings.
Additionaly, the reasons above suggest that the answer to the Fermi paradox is this: although life is a rather common phenomenon in the universe, as suggested by Moody et al., the emergence of complex life depended on a series of rare and extremely unlikely events, which is why intelligent life is exceedingly rare.
CONCLUSIONS
The sperm cell is an endosymbiont.
The account above unifies the emergence of sexual reproduction, mitosis, meiosis, the cell nucleus, and eukaryotes into a single complex phenomenon.
Sexual reproduction is a glaring evolutionary kludge and should be viewed as such, along mitosis, meiosis, and the alternation of generations—all of which are evolutionary kludges revealing the same unified phenomenon.
REFERENCES
- Cho CJ, An T, Lai YC. Protocells by spontaneous reaction of cysteine with short-chain thioesters. Nat Chem. 2024.
- Moody ERR, Álvarez-Carretero S, Mahendrarajah TA, James WC, Holly CB, Nina D, et al. The nature of the last universal common ancestor and its impact on the early Earth system. Nat Ecol Evol. 2024; 8: 1654-1666.
- Lane N, Martin W. The energetics of genome complexity. Nature. 2010; 467: 929-934
- Bengtson S, Rasmussen B, Ivarsson M, Muhling J, Broman C, Marone F, et al. Fungus-like mycelial fossils in 2.4-billion-year-old vesicular basalt. Nat Ecol Evol. 2017; 1: 141,
- Margulis L. Archaeal-eubacterial mergers in the origin of Eukarya: phylogenetic classification of life. Proceedings of the National Acad Sci. 1996; 93: 1071-1076.
- Colnaghi M, Lane N, Pomiankow A. Genome expansion in early eukaryotes drove the transition from lateral gene transfer to meiotic sex. Elife. 2020; 29: e58873.
- Bell PJL. Evidence supporting a viral origin of the eukaryotic nucleus. Virus Res. 2020; 289: 198168.
- Jurkevitch E, Davidov Y. Phylogenetic Diversity and Evolution of Predatory Prokaryotes. In: Jurkevitch, E. (eds) Predatory Prokaryotes. Microbiology Monographs, Springer Berlin Heidelberg. 2006; 4: 11-15.
- Rotem O, Pasternak Z, Jurkevitch E. The prokaryotes – deltaproteobacteria and ep-silonproteobacteria. In: Rosenberg, E., et al. (Eds.), The Genus Bdellovibrio and like Organisms. Springer- Verlag Berlin Heidelberg. 2014.
- Olivetta M, Bhickta C, Chiaruttini N, John B, Omaya D. A multicellular developmental program in a close animal relative. Nature. 2024; 635: 382-389.
- Martin WF, Garg S, Zimorski V. Endosymbiotic theories for eukaryote origin Phil. Trans R Soc. 2015; 370: 20140330.s