Impact of Aloe buettneri Extract on Reproductive Physiology and Fertility in Female Rats
- 1. Department of Biochemistry, University of Douala, Cameroon
- 2. Department of biomedical sciences, University of Ngaoundere, Cameroon
- 3. Department of Biochemistry, University of Dschang, Cameroon
Citation
LIENOU LL, SIMO RT, GOKA MSC, DONGHO DONGMO FF, NGONO NGANE RA, et al. (2025) Impact of Aloe buettneri Extract on Repro ductive Physiology and Fertility in Female Rats. JSM Sexual Med 9(1): 1151.
ABBREVIATIONS
ADHJ: Mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus, and Justicia insularis; AEAb: Aqueous extract of Aloe buettneri; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; PCOS: Polycystic Ovarian Syndrome; PMSG: Pregnant-Mare Serum Gonadotriphin
INTRODUCTION
Aloe species have been utilised worldwide and specifically in Africa for medicinal purposes for several years [1]. Aloe, an herbaceous plant belonging to the Liliaceae family, features thorny-edged green leaves [2]. Traditionally and empirically, Aloe has been employed for its anti-inflammatory, antidiabetic, neuroprotective, anti-cancer, and anti-ulcer properties, as well as its immunomodulatory effects on gastrointestinal function [3,4], reviewed recent pharmacological studies on Aloe species, focusing on its anti-cancer action, skin and digestive protective activity, and antimicrobial properties. They also discussed the potential clinical applications of the plant and its main compounds. Other researchers have shown the potential use of the plant’s aqueous and ethanol extracts for contraceptive purposes as demonstrated in Wistar rats [5], or the potential of the plant extract in restoring fertility in Polycystic ovarian syndrome (PCOS) induced Swiss albino mice [6], revealing the high potential of Aloe species in curing reproductive ailments.
In the western region of Cameroon, Aloe buettneri leaves are combined with those of three other plants: Dicliptera verticillata, Hibiscus macranthus, and Justicia insularis (ADHJ) to treat dysmenorrhoea and certain cases of female infertility [7-10], conducted a series of studies demonstrating the inductive effect of this aqueous extract mixture on ovarian steroidogenesis and folliculogenesis in female rats. Furthermore, investigations have confirmed the mixture’s FSH-like effect and its ability to enhance puberty stimulation in PMSG-primed animals [11-13]. The other plants within the mixture have undergone pharmacological and pre-clinical trials to elucidate their specific contributions [14-17].
The present study aimed to explore the effects of the aqueous extract of Aloe buettneri, administered individually, on folliculogenesis and fertility in female rats.
METHODOLOGY
Extract preparation
In February 2019, fresh leaves of Aloe buettneri were meticulously collected from the botanical garden at the University of Dschang, Cameroon. These leaves had previously been identified and catalogued as voucher specimen code 59062/HNC at the National Herbarium of Cameroon [8]. After collection, the leaves were carefully washed and dried at room temperature. Subsequently, the dried leaves were ground into a fine powder using a mortar.
To prepare the aqueous extract, 100 grams of the powdered Aloe buettneri leaves were added to 1.5 litres of boiling distilled water. The mixture was boiled for 30 minutes. After cooling, the extract was filtered and dried in a ventilated oven at 45°C. The resulting powder was weighed to determine the extraction yield, which was calculated to be 31.73%.
The Aloe buettneri powder extract was stored at -20°C and later used to prepare the extract administered to animals at specific concentrations: 12.5, 50, and 100 mg/kg of body weight. Notably, the dose of 12.5 mg/kg was derived from the traditional healer’s main recipe, as documented in ethnopharmacological research conducted in the western region of Cameroon, while the other two doses were multiples thereof.
Animals
In this study, we utilised immature albino Wistar female rats aged 21-22 days and weighing 30-45 grams. These rats were bred in the animal house of the Biochemistry Department at the University of Dschang in Cameroon. They were housed under natural light conditions (12-hour cycles) and maintained at a temperature of 22 ± 2°C. Their diet consisted of a standard laboratory feed, and they had access to tap water ad libitum. This study was performed according to the internationally accepted standard ethical guidelines for laboratory animal use and care as described in the European Community guidelines [18]. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant institutional guides on the care and use of laboratory animals.
Experimental protocols
Puberty onset and Fertility assays: A total of sixty (60) immature female rats were randomly assigned to four groups, based on their body weight, with fifteen animals per group. These rats received either distilled water or varying doses of the Aloe buettneri aqueous extract (AEAb) orally for twenty consecutive days. Throughout the experimental period, their weights were monitored every two days. After two weeks of treatment, daily checks were conducted to observe the occurrence of vaginal opening in each rat. On the 21st day, five (5) animals from each group were randomly sacrificed via intra-abdominal injection of thiopental sodium (80 mg/kg of body weight). Their liver, heart, kidneys, lungs, spleen, ovaries, suprarenal glands, and uteri were removed, blotted, weighed, and stored at -20°C for subsequent analysis.
The remaining rats (10 per group) were mated with proven fertile males over a two-week period starting from the day of mating. Daily vaginal smears were collected to detect sperm presence. Ten days after mating, laparoscopy was performed under anesthesia using a mixture of diazepam (5 mg/ml, 5 mg/kg) and ketamine (50 mg/ ml, 80 mg/kg) to count the number of implantation sites in the uterine horns and the number of corpora lutea in the ovaries. After delivery, the fetuses were weighed, and their numbers recorded. Based on this data, several indices were calculated: the number of resorption sites (number of implantation site – number of live fetuses), implantation index [(total number of implantation sites/ number corpora lutea) × 100], resorption index [(total number of resorption sites/total number of implantation sites) × 100], pre-implantation loss [(number of corpora lutea – number of implantations/number of corpora lutea) × 100], post-implantation loss [(number of implantations × number of live fetuses/ number of implantations) × 100], antifertility activity [(number of females without live fetuses/total number of females) × 100], anti-implantation activity [(number of females without implantation sites/ total number of females) × 100], and gestation rate [(number of females with live fetuses at birth/total number of gestational females) × 100] [19].
Organs extraction and biochemical analysis: The ovaries and uteri were homogenised in a Tris-sucrose buffer containing 0.25 M sucrose, 1 mM EDTA, and 10 mM Tris HCl (pH 7.4) at concentrations of 1% and 2%, respectively. Subsequently, the homogenates were centrifuged at 6000 rpm and 4°C (using a Beckman model J2–21 centrifuge) for 15 minutes. The resulting supernatants were utilised for protein quantification following the Bradford method [20] and cholesterol assays based on methods established by [21,22].
Serum samples were analysed for total proteins using the method described by the Gornall biuret method [23]. Additionally, creatinine levels, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities were assessed using specific commercial diagnostic kits (Fortress Diagnostics, London, UK). For liver analysis, a 20% homogenate was prepared in a 50 mM Tris-HCl, 150 mM KCl buffer at pH 7.4. After centrifugation at 4000 rpm for 15 minutes, the supernatant was assayed for protein content following the biuret method.
Statistical analysis
The data obtained from biological assays were expressed as Mean ± s.e.m (standard error of the mean). To assess statistical differences between the values, we employed an ANOVA (Analysis of Variance) test. For pairwise comparisons of means, the Fisher LSD (Least Significant Difference) test was utilised. Percentages were analysed using the X² (Chi-square) test. Non-parametric data were evaluated using the Kruskal-Wallis test, while the Mann-Whitney test was applied when significant differences were observed [24].
RESULTS
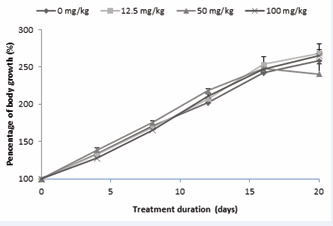
Effect of AEAb on the animal growth
In Figure 1,
Figure 1 : Evolution of the body masses of the immature female rats treated with AEAb throughout the extract administration period.
Each dot represents the average ± s.e.m. of 5 animals (ANOVA and Fisher LSD).
the progression of animal weights throughout the treatment period is observed. Notably, the animal masses exhibited a consistent and significant increase (p < 0.0001) from the beginning to the end of the treatment. Interestingly, there were no significant differences observed between the groups on any specific day, including during the post-treatment mating period.
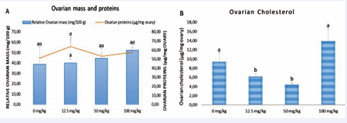
Effect of AEAb on ovarian weight, proteins and cholesterol levels of treated animals
The physiological and biochemical changes observed in ovaries after twenty days of oral administration of various doses of AEAb to immature female rats are presented in Figure 2.
Figure 2 : Effect of different doses of AEAb on the ovarian relative weight and proteins concentration (A) and ovarian cholesterol level (B).
Each histogram represents the average ± s.e.m. of 5 animals. The values with different letters are significantly different at p < 0.05 (ANOVA and Fisher LSD).
There was no significant effect on mass and proteins, but a significant decrease was noted in ovarian cholesterol levels at 12.5 mg/kg (p < 0.05) and 50 mg/kg (p < 0.001) dosages.
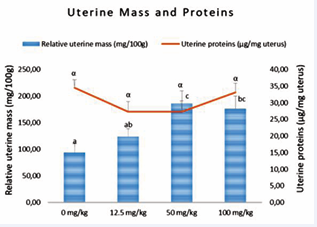
Effect of AEAb on uterine weight and proteins
Rats’ uterine proteins were not significantly affected by AEAb, while masses were significantly increased in all treated animals compared to the controls, with the highest value observed in the 50 mg/kg treated animals (Figure 3).
Figure 3 : Effect of various doses of AEAb on the relative uterine masses and proteins level
Each histogram or dot represents the average ± s.e.m. of 5 animals. The values with different letters are significantly different at p < 0.05 (ANOVA and Fisher LSD).
Effect of AEAb on organs weights and biochemical parameters of treated animals
Different dosages of AEAb were tested to assess their impact on the central physiology of the animals by modifying the functioning or weight of various visceral organs. The results presented in Table 1 indicate that many parameters remained unaffected by AEAb administration.
Table 1: Effect of AEAb on visceral organ weight and biochemical parameters in treated animals AST, Aspartate AminoTransferase; ALT, Alanine AminoTransferase; Each value represents the average ± s.e.m. of 5 animals. The values with different letters in lines are significantly different at p < 0.05 (ANOVA and Fisher LSD).
|
Parameters |
|
|
Dose |
|
|
|
0 mg/kg |
12.5 mg/kg |
50 mg/kg |
100 mg/kg |
|
Biochemical parameters |
||||
|
Serum proteins (mg/ml) |
70.46 ± 7.15a |
74.87 ± 4.92a |
68.56 ± 4.03a |
66.92 ± 8.70a |
|
Liver proteins (mg/g liver) |
174.39 ± 19.17a |
129.78 ± 11.43a |
165.85 ± 29.52a |
171.25 ± 14.86a |
|
AST (UI/L) |
57.35 ± 9.90a |
54.92 ± 10.26a |
42.58 ±12.10a |
65.20 ± 12.10a |
|
ALT (UI/L) |
38.42 ± 8.42a |
27.47 ± 5.44a |
34.35 ± 6.07a |
47.54 ± 8.29a |
|
Serum creatinine (mg/dL) |
0.17 ± 0.03a |
0.18 ± 0.02a |
0.12 ± 0.02a |
0.15 ± 0.02a |
|
Urine creatinine (mg/dL/day) |
1.23 ± 0.25a |
1.43 ± 0.19a |
1.59 ± 0.17a |
2.28 ± 0.25b |
|
Organs relative masses |
||||
|
liver relative mass (g/100 g) |
3.99 ± 0.28a |
4.49 ± 0.18ab |
4.99 ± 0.29b |
4.47 ± 0.17ab |
|
suprarenal gland relative mass (mg/100 g) |
37.92 ± 2.28a |
46.22 ± 3.37b |
43.44 ± 2.36ab |
44.44 ± 1.74ab |
|
heart relative mass (mg/ 100 g) |
408.52 ± 9.50a |
393.32 ± 45.16a |
492.79 ± 23.38b |
424.75 ± 9.10ab |
|
kidney relative mass (mg/ 100 g) |
969.59 ± 60.73a |
1118.93 ± 86.05ab |
1190.18 ± 66.13b |
954.18 ± 58.16a |
|
spleen relative mass (mg/ 100 g) |
270.74 ± 5.83a |
381.22 ± 74.49a |
380.86 ± 27.91a |
343.05 ± 53.22a |
|
lungs relative mass (mg/ 100 g) |
734.15 ± 62.49a |
797.70 ± 45.20a |
880.22 ± 59.41a |
798.45 ± 45.85a |
However, urine creatinine levels were significantly higher (p < 0.05) in animals treated with 100 mg/kg compared to the control and other treated groups. Regarding organ masses, suprarenal glands showed hypertrophy in animals treated with 12.5 mg/kg relative to the control group. Additionally, heart and kidney masses were significantly higher (p < 0.05) in animals treated with 50 mg/kg compared to both the control and the 12.5 mg/kg dosage. Similarly, liver mass was significantly higher (p < 0.05) in the same group compared to all other experimental groups. Different dosages of AEAb were tested to assess their impact on the central physiology of the animals by modifying the functioning or weight of various visceral organs. The results presented in Table 1 indicate that many parameters remained unaffected by AEAb administration. However, urine creatinine levels were significantly higher (p < 0.05) in animals treated with 100 mg/kg compared to the control and other treated groups. Regarding organ masses, suprarenal glands showed hypertrophy in animals treated with 12.5 mg/kg relative to the control group. Additionally, heart and kidney masses were significantly higher (p < 0.05) in animals treated with 50 mg/kg compared to both the control and the 12.5 mg/kg dosage. Similarly, liver mass was significantly higher (p < 0.05) in the same group compared to all other experimental groups.
Effect of AEAb on some fertility and gestational parameters
The impact of AEAb on gestational and fertility parameters in treated animals is summarised in Table 2.
Table 2: Effect of AEAb on fertility and gestational parameters of treated animals Each value represents the mean ± s.e.m of ten (10) animals. Values affected with different letters are significantly different (p<0.05) in the same line (Fisher LSD, X2, Kruskall-Wallis tests).
|
Parameters |
Dose (mg/kg) |
|||
|
0 |
12.5 |
50 |
100 |
|
|
Number of IS |
9.14 ± 0.456a |
9.71 ± 0.42a |
9.18 ± 0.53a |
9.44 ± 0.29a |
|
Number of CL |
9.33 ± 0.49a |
10.14 ± 0.59a |
9.70 ± 0.39a |
9.67 ± 0.33a |
|
Number of LF |
9.00 ± 0.41a |
9.00 ± 0.41a |
8.87 ± 0.64a |
9.33 ± 0.49a |
|
Weight of LF (g) |
4.98 ± 0.07a |
4.59 ± 0.33a |
4.81 ± 0.14a |
5.25 ± 0.12a |
|
Number of DF |
0.00 ± 0.00a |
0.75 ± 0.48b |
0.00 ± 0.00a |
0.00 ± 0.00a |
|
Number of RS |
4.00 ± 1.81a |
4.14 ± 1.87a |
2.72 ± 1.42a |
3.22 ± 1.53a |
|
IR (%) |
96.66 ± 3.33a |
96.43 ± 2.48a |
99.09 ± 0.90a |
97.98 ± 2.02a |
|
FR (%) |
43.75 (7/16)a |
43.75 (7/16)a |
68.75 (11/16)a |
56.25 (9/16)a |
|
GR (%) |
57.14 (4/7)a |
57.14 (4/7)a |
72.73 (8/11)a |
66.66 (6/9)a |
|
AIA (%) |
56.25 (9/16)a |
56.25 (9/16)a |
31.25 (5/16)a |
43.75 (7/16)a |
|
PrI loss (%) |
3.33 ± 3.33a |
3.57 ± 2.47a |
0.91 ± 0.91a |
2.02 ± 2.02a |
|
RI (%) |
43.75 (28/64)a |
41.17 (28/68)a |
29.70 (30/101)a |
34.12 (29/85)a |
|
AFA (%) |
75 (12/16)a |
75 (12/16)a |
50 (8/16)a |
62.50 (10/16)a |
|
PoI loss (%) |
44.28 ± 19.74a |
48.47 ± 18.32a |
27.27 ± 14.08a |
34.56 ± 16.40a |
The number of corpora lutea, implantation sites, and live born foetuses, as well as the implantation rate, fertility rate, gestation rate, resorption index, and anti-implantation and anti-fertility activities, remained unchanged after twenty days of oral AEAb administration compared to control animals. However, a notable increase in stillborn foetuses was observed in female rats receiving the 12.5 mg/kg dosage.
DISCUSSION
Numerous studies have highlighted the role of Aloe species in addressing reproductive issues in women. In the western region of Cameroon, traditional healers combine Aloe buettneri leaves with three other medicinal plants-Justicia insularis, Dicliptera verticillata, and Hibiscus macranthus—to treat menstrual disturbances and functional sterility in women [8,10]. Recent research continues to support the efficacy of these combinations in traditional medicine [13].
In this study, the inductive effects of Aloe buettneri leaves on ovarian folliculogenesis and fertility were evaluated using immature female rats, a well-established model for investigating these issues [25]. Ovarian follicle development is intricately regulated by pituitary hormones and various growth factors. Among these, FSH (follicle-stimulating hormone) plays a crucial role in follicle recruitment and selection during their final growth stage [26,27].
Following daily oral administration of AEAb over twenty days, a slight but non-significant increase in ovarian protein levels was observed; ovarian weight slightly increased as well. Interestingly, the ovarian cholesterol level decreased at the lowest doses of AEAb. This reduction may be attributed to an increase in steroidogenesis in the ovary by compounds found in the plant extract, which would have resulted in an increase in steroid hormone production and action in these organs, responsible for the anatomical and physiological changes observed in the reproductive organs, even though the effect is of less extent [28,29], the increase in uterine mass would also result from that effect.
In addition to ovarian and suprarenal gland oestrogens, phytoestrogens from plants can exert similar effects on the uterus [30-32]. The significant increase in uterine weight observed in animals treated with a 50 mg/kg dose likely results from the combined effects of these chemical compounds on ovaries and suprarenal glands. Notably, the weight of these glands significantly increased (p < 0.05) in animals treated with a 12.5 mg/kg dose, potentially influencing their production. Alternatively, the compounds may directly impact the uterine muscle and mucosa. Experimental models have previously demonstrated the oestrogenic effect of ADHJ mixture on uterine smooth muscle in immature female rats during the estrous phase [33,34].
Furthermore, the weak stimulation of folliculogenesis was observed at the lowest doses of AEAb, as evidenced by a slight increase in haemorrhagic points, corpora lutea, and implantation sites at the 12.5 mg/kg dose. Importantly, normal gestation progression and physiological parameters related to nidation, embryogenesis, and organogenesis did not indicate foetal toxicity associated with Aloe buettneri aqueous extract. However, the increased number of stillborn baby rats at the 12.5 mg/kg dose and the elevated post-implantation percentage raise concerns about the potential risks of long-term and repeated use of this extract during pregnancy [35]. Shah et al., [5] reported an increase in resorption index and anti-implantation effects following the administration of aqueous and ethanolic extracts of Aloe vera, confirming the result presented. This obviously shows that the plant extract should not be consumed alone during pregnancy and that the mild abortifacient effect of the plant would be attenuated by the presence of the other plants in ADHJ mixture as previously reported, evidencing the beneficial impact of plant mixtures in alternative medicine in curing reproductive ailments [36-39].
CONCLUSION
In summary, our study reveals a weak inductive effect of AEAb at the lowest doses on fertility and ovarian folliculogenesis. Fortunately, the extract does not exhibit foetal toxicity at the doses used. Nevertheless, further investigations into its effects during different gestational stages are necessary to fully understand its impact on reproductive parameters.
ETHICAL STATEMENT
This research was approved by the scientific committee of the Faculty of Science at the University of Dschang, Cameroon, and strictly conformed to the internationally accepted standard ethical guidelines for laboratory animal use and care as described in the European Community guidelines. The rules of the ARRIVE guidelines 2.0 of the National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs) were carefully respected throughout this research (Persie du Sert et al., 2020).
ACKNOWLEDGEMENTS
The authors express their gratitude to the traditional medicine practitioner for generously sharing his recipe.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: LLL, PBT. Methodology: LLL, RTS. Software: PBT. Validation: MSCG, PBT. Formal analysis: RTS, PBT. Investigation: LLL, RTS. Resources: PBT, RANN. Data curation: PBT. Writing – Original Draft: LLL, RTS. Writing – Review & Editing: LLL, RTS, MSCG, FFDD. Visualization: PBT. Supervision: RANN. Project administration: PBT. Funding acquisition: All the authors.
REFERENCES
- Anywar G, Tugume P, Kakudidi EK. A review of Aloe species used in traditional medicine in East Africa. South Afr J Botany. 2022; 147: 1027-1041.
- Mulu T, Teshale F, Gemeda S, Sahu O. Medicated Evaluation of Aloe vera: Overview on Characteristics and Application. World J Nutr Health. 2015; 3: 1-7.
- Sharma AK, Madaan V, Singh Y, Soni N, Sharma L, Jain R, et al. Aloe vera crude drug: a review. Int J Gender Science Technol. 2023; 12.
- Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological update properties of Aloe vera and its major active constituents. Molecules. 2020; 25: 1324.
- Shah SK, Jhade D, Chouksey R. Antifertility activity of ethanolic and aqueous extracts of Aloe vera Mill on female Wistar rats: Rising approaches of herbal contraception. J Pharmaceutical Sci Res. 2016; 8: 952-957.
- Ghagane SC, Toragall MM, Akbar AA, Hiremath MB. Effect of Aloe vera (Barbadensis Mill) on Letrozole induced polycystic ovarian syndrome in Swiss albino mice. J Human Reproductive Sci. 2022; 15: 126-132.
- Telefo PB. Contribution à l’étude des plantes médicinales au Cameroun: Toxicité biochimique d’un extrait de plantes médicinales et effet sur le taux d’œstradiol chez la rate. Mémoire de Maîtrise en Biochimie. Université de Yaoundé. 1992 : 57.
- Telefo PB, Moundipa FP, Tchana A, Tchouanguep DC, Mbiapo FT. Effect of aqueous extract of Aloe buettneri, Justicia insularis, Hibiscus macranthus, Dicliptera verticillata on some physiological and biochemical parameters of reproduction in immature female rats. J Ethnopharmacol. 1998; 63:193-200.
- Telefo PB, Moundipa FP, Tchouanguep FB. Oestrogenicity and effects on hepatic metabolism of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus, and Justicia insularis. Fitoterapia. 2002; 73: 472-478.
- Telefo PB, Moundipa FP, Tchouanguep FM. Inductive effects of the leaf mixture extract of Aloe buettneri, Justicia insularis, Dicliptera verticillata, and Hibiscus macranthus on in vitro production of estradiol. J Ethnopharmacology. 2004; 91: 225-230.
- Goka CMS, Telefo PB, Mbemya TG, Awouafack MD, Lienou LL, Yemele MD, et al. Potentialisation of pregnant mare serum gonadotropin inducing effect on ovarian follicles growth by the aqueous extract of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis leaves in immature rats. Pharmacologia. 2016; 7: 328-336.
- Goka CMS, Lienou LL, Njina NS, Fekam BF, Telefo PB. Effect of four Cameroonian medicinal plants on sexual maturation of immature female rats. Afr J Integrated Health. 2017; 7: 54-60.
- Goka CMS, Awouafack MD, Lamshöft M, Lienou LL, Mbemya TG, Fekam BF, et al. Comparative effect of the aqueous extracts of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis on the sexual maturation of pregnant mare serum gonadotrophin-primed immature female rats. J Basic Clin Physiol Pharmacology. 2018: 9.
- Mbemya GT, Guerreiro DD, Donfack NJ, Silva LM, Vieira LA, Sousa GFC, et al. Justicia insularis improves the in vitro survival and development of ovine preantral follicles enclosed in ovarian tissue. J Pharmaceutical Sci Res. 2017; 5: 668-680.
- Mbemya GT, Cadenas J, Ribeiro de Sá NA, Damasceno Guerreiro D, Donfack NJ, Alberto Vieira L, et al. Supplementation of in vitro culture medium with FSH to grow follicles and mature oocytes can be replaced by extracts of Justicia insularis. PLoS One. 2018; 13: e0208760.
- Meguem SO, Lienou LL, Goka CMS, Tagne SR, Yemele MD, Telefo PB. Effects of the aqueous extract of Dicliptera verticillata on fertility and different stages of gestation in female rats. Zygote. 2021; 1-7.
- Meguem SO, Lienou LL, Goka CMS, Telefo PB. Effects of the aqueous extract of Justicia insularis on reproductive parameters in testosterone propionate-induced hyperandrogenic female albino Wistar rats. Asian J Emerging Res. 2024; 6: 66-73.
- Radzikowski C. Protection of animal research subjects. Science andEngineering Ethics. 2006; 1: 103-110.
- Costa-Silva JH, Lyra MMA, Lima CR, Arruda VM, Araujo AV, Ribeiro E, et al. A toxicological evaluation of the effect of Carapa guianensis Aublet on pregnancy in Wistar rats. J Ethnopharmacol. 2007; 112: 122-126.
- Kruger NJ. The Bradford Method for Protein Quantitation. In: J.M. Walker (ed.), The Protein Protocols Handbook, Springer Protocols Handbooks, Humana Press, Totowa, NJ. 2009.
- Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clinical Chemistry. 1974; 20: 470-475.
- Roeschlau P. Enzymatic determination of total cholesterol in serum. Clinical Chemistry and Clinical Biochemistry. 1974; 12: 226-229.
- Sapan CV, Lundblad RL. Review of methods for determination of total protein and peptide concentration in biological samples. Proteomics Clinical Applications. 2015; 9:268-276. Mackenzie G, Peng D. Statistical modelling in biostatistics and bioinformatics. The Centre for Biostatistics, University of Limerick, Limerick, Ireland. 2014: 1-6.
- Bachman S, Hellwig J, Jackh R, Christian MS. Uterotrophic assay of two concentrations of migrates of 23 polystyrenes administered orally (by gavage) to immature female Wistar rats. Drug Chem Toxicol. 1998; 21: 1-30.
- Webb R, Buratini J, Hernandez-Medrano JH, Gutierrez CG, Campbell BK. Follicle development and selection: past, present and future. Animal Reproduction. 2016; 13: 234-249.
- Casarini L, Crépieux P. Molecular mechanisms of action of FSH. Front Endocrinol. 2019; 10: 305.
- Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. In: R. Piprek (ed.), Molecular Mechanisms of Cell Differentiation in Gonad Development, Results and Problems in Cell Differentiation. Springer, Cham. 2016; 58.
- Miller WL. Steroidogenesis: unanswered questions. Trends Endocrinol Metabolism. 2017; 28: 771-793.
- Whitten PL, Russell E, Naftolin F. Effects of a normal, human- concentration, phytoestrogen diet on rat uterine growth. Steroids. 1992; 57: 98-106.
- Diel P, Schmidt S, Vollmer G. In vivo test systems for the quantitative and qualitative analysis of the biological activity of phytoestrogens. J Chromatography B. 2002; 777: 191-202.
- Mariotte AM. Répertoire des phyto-estrogènes. Sécurité et bénéfices des phyto-œstrogènes apportés par l’alimentation. Maisons-Alfort, AFSSA. 2005: 13-17.
- Guemo TC. Effets comparés des extraits aqueux de feuilles d’Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus, Justicia insularis et de leur mélange sur le muscle lisse utérin de la rate en œstrus. Mémoire de maîtrise en Biochimie, Université de Dschang. 2002.
- Njindikouyou I. Effets œstrogéniques et antioestrogéniques de l’extrait aqueux du mélange de feuilles d’Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus, et Justicia insularis chez les rattes ovariectomisées. Thèse de Master of Science, Université de Dschang. 2006.
- Lima A, Batista-Santos P, Veríssimo E, Rebelo P, Ferreira RB. Differential inhibition of gelatinase activity in human colon adenocarcinoma cells by Aloe vera and Aloe arborescens extracts. BMC Complement Med Ther. 2020; 20: 379.
- Telefo PB, Lienou LL, Yemele MD, Lemfack MC, Mouokeu C, Goka CS, et al. Ethnopharmacological survey of plants used for the treatment of female infertility in Baham, Cameroon. J Ethnopharmacol. 2011; 136: 178-187.
- Slighoua M, Mahdi I, Amrati F, Boukhira S, EL Hamsas EL Youbi A, A Bari, et al. Ethnopharmacological survey of medicinal plants used in the traditional treatment of female infertility in Fez region, Morocco. Phytothérapie. 2020; 18: 321-339.
- Hyun JY, Jung HS, Park JY. Herbal therapeutics for female infertility: A systematic review and meta-analysis. J Ethnopharmacol. 2024; 319: 117258.
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020; 18: e3000410.