Managing Low Libido in Female Adult Cancer Survivors: A Review of the Latest Evidence
- 1. Faculty of Health Sciences, Queen’s University, Canada
- 2. Department of Medical Oncology & Hematology, University of Toronto, Canada
Citation
Bhinder JK, Al-Khaifi M (2025) Managing Low Libido in Female Adult Cancer Survivors: A Review of the Latest Evidence. JSM Sexual Med 9(3): 1161.
INTRODUCTION
Over the past several decades, survival rates for female cancer survivors have improved significantly, and as a result, there is an increasing need for comprehensive survivorship care [1-4]. Among these needs, sexual health has been recognized as a prevalent concern, with many female cancer survivors experiencing sexual dysfunction that negatively impacts their physical intimacy, emotional wellbeing and overall Quality of Life (QoL) [5-8]. Sexual dysfunction may manifest in various domains, including, but not limited to, vaginal dryness, low sexual desire, pain during intercourse (dyspareunia), and psychological concerns [6-9] Female cancer survivors experience changes in sexual health at high rates across various tumor types [5-11]. A recent systematic review and meta-analysis by Esmat Hosseini et al. found that up to 66% of women with cancer report sexual dysfunction, with rates as high as 90% among gynecologic cancer survivors and 75–90% among Breast Cancer Survivors (BCS) [5-10]. Additionally, 77% of female lung cancer survivors and 75% of colorectal cancer survivors report sexual health symptoms [10 12]. A systematic review by Guedes et al. reported that the incidence of sexual dysfunction among female cancer survivors ranges from 30% to 80%, with the risk being 2.7 times higher in women with cervical cancer and 3.5 times higher in those with breast cancer compared to women without cancer [13]. Moreover, female cancer survivors are at increased risk of experiencing sexual dysfunction at all stages of survivorship [14-16]. A recent systematic review by Rodrigues-Machado et al. (2025), which examined 16 studies involving 4,058 women, found that the prevalence of sexual dysfunction ranged from 17.5% prior to diagnosis to 86.0% after six months of hormone therapy, and the most commonly affected domains were sexual desire, arousal, lubrication, orgasm, satisfaction, and dyspareunia [15].Low libido is one of the most frequently reported and distressing concerns, often including decreased interest in sex, reduced arousal, difficulty initiating or maintaining desire during sexual activity, and challenges in achieving orgasm [10-19]. Also known as Hypoactive Sexual Desire Disorder (HSDD), it is characterized by a persistent or recurrent lack of motivation for sexual activity, which may present as reduced or absent interest in initiating sex or difficulty maintaining desire during sexual encounters [13-22]. For instance, in a survey among 1,775 BCS found that 85.5% reported a decreased interest in sex, 69.2% reported reduced arousal, and 41.0% experienced difficulty achieving orgasm [17].Low libido results from a complex interplay of biological, psychological, relational, and treatment-related factors that considers both physical and psychological factors [23 25]. The four domains of sexual function of desire, arousal, orgasm, and pain are closely interconnected [15-26]. For example, pain during sex can lower desire, while reduced desire can impact arousal and orgasm, and difficulties with arousal or orgasm can increase pain or lead to avoidance of intimacy [15-24]. Treatments such as chemotherapy, radiation, ovarian suppression, and endocrine therapy can disrupt hormone levels, which are important for maintaining libido, arousal, and lubrication [27,28]. In a 2025 narrative review, Rossi et al. noted that anatomical changes, hormonal treatments, and chronic inflammation related to cancer can contribute to loss of libido, often perpetuating a cycle of sexual avoidance [24]. Additionally, fatigue, surgical changes affecting body image or sensation, and other physical symptoms may limit sexual activity [29-31]. These are often compounded by psychological distress such as anxiety and depression, and by medications used to manage them [24-32]. Relationship challenges, poor communication, coexisting medical conditions, certain medications, and past trauma may also contribute to low sexual desire [18-33].Given the high prevalence and multifactorial nature of low libido among female cancer survivors, this review synthesizes current evidence on its management, including both non-pharmacological and pharmacological interventions, while highlighting existing research gaps.
Non-Pharmacological Interventions
A growing body of evidence supports the use of non pharmacological interventions as first line interventions to improve sexual desire, function, and emotional intimacy, summarized in Table 1.
Table 1: Summary of Non-Pharmacological Interventions.
|
Intervention |
Considerations |
|
Counselling & Psychosexual Therapy |
ncludes individual, couple-based, or sex therapy with professionals (e.g., psychologists, marriage counselors, sex therapists). Helps survivors and partners communicate about needs, fears, and expectations. Couple-based interventions show strong evidence for improving satisfaction and intimacy. May include psychoeducation, intimacy training, and relationship skills building. Should ideally begin before treatment and continue through survivorship. |
|
Sex Therapy (Sensate Focus, Erotic Reframing) |
Focuses on reducing anxiety, improving body image, and enhancing pleasure. Techniques like sensate focus help reduce performance anxiety and build trust/intimacy. Often includes both survivor and partner in guided exercises. |
|
Mindfulness-Based Interventions (MBIs) |
Practices include sitting meditation, body scans, and yoga. Enhances awareness of bodily sensations, reduces anxiety, and improves sexual arousal and satisfaction. Improves alignment between psychological and physiological arousal. |
|
Cognitive Behavioural Therapy (CBT) |
Aims to reframe negative thoughts, reduce anxiety, and increase self-efficacy. Addresses avoidance, maladaptive beliefs, and body image issues. Strong evidence for improving desire, arousal, and orgasm. Effective both individually and in groups. |
|
Use of Sexual Aids |
Helps enhance genital blood flow, arousal, and orgasm. Recommended by clinical guidelines; evidence supports use over 8–12 weeks. Can be self-directed or partner-assisted. EROS-CTD (FDA-approved) may aid women with arousal/orgasmic disorders. |
|
Directed Masturbation & Physical Stimulation |
Behavioural therapy involves progressive genital stimulation over sessions. Improves orgasm consistency and enhances self-knowledge. Encourages comfort with one's body and sexual responsiveness. May include erotic media, journaling, or role-playing to rebuild desire. |
Counselling: Individual, psychosocial, couple based, counselling and sex therapy, has demonstrated considerable effectiveness in improving sexual health outcomes in female cancer survivors [34,35]. Professionals, such as marriage and family counselors, sex therapists, and psychologists, consistently show evidence in addressing these concerns [36,37], and it is recommended to consult support before anti-cancer treatment starts and continue until symptoms are resolved [25-36].Among these approaches, studies show that couple based therapy has been particularly impactful, as it combines education on cancer and sexual health with communication skills training [34-39]. For example, a Randomized Controlled Trial (RCT) by Reese et al. (2025) evaluated the intimacy enhancement intervention, a four session couple-based program for BCS and their partners [39]. The intervention group showed significantly greater short-term improvements in sexual function, satisfaction, arousal, lubrication, and orgasm compared to controls [39,40]. Involving a partner is strongly recommended, as it facilitates mutual understanding, support, and improved relationship dynamics [34-39]. Another systematic review and meta-analysis from Li et al. of couple-based intervention on sexuality and the QoL of cancer patients and their partners reported that nine studies, assessed sexual relationships among partners, and among these couple-based interventions showed significantly greater improvements compared to control groups (p = 0.03; effect size = 0.52; 95% CI: 0.06–0.98) [41]. Couple-based psychosexual interventions typically include educational components, such as information on how cancer and its treatments can affect sexuality and sexual response [34-39]. They also provide training in coping strategies, including communication and physical intimacy skills [38,39].Further, sex therapy is a form of psychotherapy that aims to enhance erotic experiences and reduce anxiety or self-consciousness related to sexual performance [42-44]. For instance, sex therapists often provide a framework for women to explore their sexuality and address problematic behaviors and beliefs [43-45]. Another well-known technique is sensate focus, developed by Masters and Johnson, which involves a structured series of partner exercises designed to increase awareness of pleasurable sensations and personal preferences for sexual touch while gradually reducing anxiety [44-48]. Sensate focus and nonjudgmental sensual touching are commonly used in therapy and are typically taught to both the survivor and their partner, and it continues to be widely cited in medical and social science literature, with evidence supporting its effectiveness in treating a broad range of sexual concerns, including those with complex or combined causes [46-50].
Mindfulness-Based Interventions: Mindfulness based therapies are increasingly recognized as effective for addressing sexual dysfunction in female cancer survivors [51-53]. These interventions typically include techniques such as sitting meditation, body scans, Hatha yoga, and walking meditation [52-54]. A systematic review of 15 studies found that MBTs led to significant improvements in subjective arousal, desire, satisfaction, and reduced fear associated with sexual activity [51]. They also improved the alignment between perceived arousal and physiological genital response [51]. Similarly, a systematic review by Banbury et al., examined the effectiveness of mindfulness for sexual functioning in women with cancer, which included both brief and longer-term interventions and found that five out of ten studies reported low baseline sexual desire, all of which showed increases in sexual interest and desire following the intervention [55].Further evidence from Chang et al. on evaluating mindfulness-based stress reduction, where participants who completed six consecutive weeks of intervention demonstrated statistically significant improvements in arousal (p ≤ .001), lubrication ( p ≤ .001), orgasm (p = .002), satisfaction (p = .007), and pain (p = .012) [56]. In another study, 52 participants were randomly assigned to intervention and control groups, receiving an eight-session mindfulness-based stress reduction program [57]. Sexual function, measured using the FSFI, showed significant improvements in sexual desire and arousal at follow-up in the intervention group (p = .021 for both), based on data from 46 participants [57]. Building on these findings, a pilot study by Gorman et al. (2022) assessed Mindful After Cancer, an 8-week virtual mindfulness program for female breast and gynecologic cancer survivors, focused on sexual health. The program included guided meditations, group discussions, and home-based practices, and participants reported high engagement and found it relevant and manageable [58].
Cognitive Behavioural Therapy (CBT): CBT is believed to improve sexual health by reducing anxiety, challenging maladaptive beliefs, and enhancing communication [59]. CBT has also shown promise in enhancing sexual function among female cancer survivors, and specifically, cognitive behavioural sex therapy modifies one’s thought patterns or belief that may interfere with one’s sexual intimacy and/or sexual pleasure [43-60].
A systematic review and meta-analysis by Ko et al. (2025) included a study involving women with cervical or endometrial cancer who participated in three monthly 90-minute sessions delivered by a psychologist or sex therapist, and the brief mindfulness-based cognitive behavioral intervention was found to be effective in improving sexual functioning [61]. Similarly, a systematic review by Carney et al. (2023), reported that four out of nine included studies assessed specific domains of the FSFI, including arousal, desire, lubrication, orgasm, satisfaction, and pain [62]. Among these, all four studies found significant improvements in arousal, three reported improvements in desire, and two observed improvements in orgasm [62]. The authors emphasized the need for future research to include active control groups and investigate mechanisms of change. Additionally, one RCT (n = 100) involving BCS delivered ten one-hour CBT sessions over five weeks [59]. Four weeks post-intervention, participants in the CBT group demonstrated statistically significant improvements in sexual desire (p = .033), arousal (p = .018), orgasm (p = .031), satisfaction (p = .047), and overall sexual function (p = .001), as measured by the FSFI [59].
Other Interventions: Clinical guidelines for cancer care recommend physical interventions as a core component of managing sexual dysfunction in female cancer survivors [9]. Professional cancer clinical guidelines, including those from the American Society of Clinical Oncology, suggest that any form of regular sexual stimulation, such as masturbation, can help improve sexual response, regardless of the method used [63,64]. The use of sexual aids, including hand-held vibrators and clitoral stimulators, is commonly recommended to support arousal and address orgasmic dysfunction [38-65]. Survivors are encouraged to use these devices themselves or with a partner, directing stimulation to areas that are pleasurable and avoiding those that may be tender or uncomfortable [37-64]. Also, directed masturbation is a behavioral technique that involves graded genital stimulation to facilitate heightened arousal and orgasm and is conducted over multiple weekly therapy visits [66,67]. Success rates can be as high as 90% and are best when coupled with CBT [66,67]. Hand-held vibrators, in particular, are supported by moderate evidence for their ability to enhance arousal and orgasm [68,69]. Studies have shown that consistent use over 8 to 12 weeks can improve both subjective and physiological sexual responses [68]. For example, a psychophysiology study evaluating premenopausal breast cancer patients undergoing antihormonal therapy found that both patients and healthy controls exhibited greater genital response to clitoral vibrator use than to visual stimuli, highlighting the effectiveness of direct physical stimulation [69].A scoping review of sexual dysfunction among cervical cancer survivors, included that for the Difficulty in experiencing orgasm, the FDA-approved EROS-Clitoral Therapy Device (EROS-CTD) is another tool with potential utility in female cancer survivors [19-70]. This device uses gentle suction to increase blood flow and sensation in the clitoral area, specifically improving arousal, lubrication, and orgasm [19-64]. Clinical evidence supports its use at least three times per week for optimal benefit [19-71]. While its efficacy has been demonstrated in women with surgical menopause, it may also be a suitable intervention for cervical cancer survivors, though more cancer-specific evidence is needed [19]. Additional treatment methods to support sexual function in survivors include extended foreplay, erotic literature or visual aids, and structured sexual rehabilitation plans that involve communication and exploration between partners [19-73]. A scoping review from Misha et al. on sexual dysfunction in cervical cancer survivors noted these approaches as viable options for managing difficulty in achieving orgasm [19]. When used in combination, these interventions offer a multimodal, individualized approach to improving sexual health and quality of life among female cancer survivors [19-69].
Pharmacological Interventions
If nonpharmacologic approaches are insufficient in managing low sexual desire, pharmacologic options, including both non-hormonal and hormonal therapies, may be considered. However, this remains an area in need of further research and development. A summary of pharmacological treatments are shown in Table 2.
Table 2: Summary of Pharmacological Treatments to Treat Low Libido in Female Cancer Survivors.
|
Medication |
Mechanism of Action |
Evidence in Female Cancer Survivors |
FDA Approval |
Guideline Recommendations |
|
Flibanserin |
5-HT1A agonist, 5-HT2A antagonist; modulates serotonin to increase dopamine/ norepinephrine |
Retrospective study (Javaid): 75% reported improved desire, 30% reported mood/sleep benefits. Prospective trial (Goldfarb): FSFI improved from 11 to 17–18; expanding to larger cohort (NCT03707340). |
Approved (2015) for premenopausal women with HSDD |
NCCN: Small studies with BCS on adjuvant endocrine therapy may be effective in this population.
ASCO: Flibanserin may be offered to premenopausal women who are experiencing hypoactive sexual desire disorder. Flibanserin has not been evaluated in women with a history of cancer or those on endocrine therapy.
SOGC: Flibanserin is a moderately safe and well-tolerated option for low libido and arousal, though clinical benefits are modest. |
|
Bremelanotide |
Melanocortin receptor agonist; centrally increases sexual desire |
No published studies in BCS. Not evaluated in survivors or those on endocrine therapy. |
Approved (2019) for premenopausal women with HSDD |
NCCN: Not studied in cancer survivors, but panel believed appropriate for HSDD survivors.
ASCO: Insufficient evidence to support.
SOGC: Bremelanotide is a moderately safe and well-tolerated option for low libido and arousal, though clinical benefits are modest. |
|
Bupropion |
Norepinephrine- dopamine reuptake inhibitor (NDRI); enhances reward/ motivation pathways |
Phase II RCT (NRG-CC004): No statistically significant improvement over placebo in BCS with low desire; safe and well tolerated. |
Not FDA-approved for HSDD (approved as antidepressant) |
NCCN: Considered off-label treatment for HSDD, despite limited efficacy and safety data.
ASCO: Bupropion at doses of either 150 mg or 300 mg, though there is limited evidence supporting its use. SOGC: May be used off-label; limited number of trials outside of oncology settings. |
|
Testosterone |
Central and peripheral androgen replacement |
2024 pilot study in 29 premenopausal BCS on AIs: FSFI improved from 11.7 to 19.1 over 3 months. Estradiol levels remained <2.7 pg/mL. No serious adverse events. No RCTs in BCS population. |
Not FDA-approved for use in women |
NCCN: Limited data regarding use of androgen therapy in survivors of hormonally mediated cancers; more info needed for BCS on AIs. Small studies report it is beneficial and safe.
ASCO: Not specifically recommended. SOGC: Contraindicated or discouraged in ER+ survivors; off-label use requires extreme caution. |
|
Vaginal DHEA (Prasterone) |
Local androgen precursor; acts on vaginal tissue to improve GSM symptoms and possibly libido |
Approved by Health Canada; used in BCS with vaginal symptoms. Retrospective study in BCS: improved GSM symptoms after 8 weeks of 6.5 mg vaginal DHEA. No significant estradiol elevation. Ongoing trial: PROPOSE (NCT06611514). |
FDA-approved (2016) for moderate-to-severe dyspareunia |
NCCN: Small studies with BCS on adjuvant endocrine therapy may be effective in this population.
ASCO: Three RCTs investigating intravaginal dehydroepiandrosterone (prasterone) in postmenopausal women all showed improvements in the signs and symptoms of vaginal atrophy. SOGC: Not FDA approved for postmenopausal women; more research needed. |
BCS: Breast Cancer Survivors; HSDD: Hypoactive Sexual Desire Disorder; FSFI: Female Sexual Function Index; MBSR: Mindfulness-Based Stress Reduction; CBT: Cognitive Behavioral Therapy; NCCN: National Comprehensive Cancer Network; ASCO: American Society of Clinical Oncology; ESMO: European Society for Medical Oncology; SOGC: Society of Obstetricians and Gynaecologists of Canada; FDA: Food and Drug Administration; RCT: Randomized Controlled Trial; AI: Aromatase Inhibitor; DHEA: Dehydroepiandrosterone; GSM: Genitourinary Syndrome of Menopause; PROMIS-SF: Patient-Reported Outcomes Measurement Information System – Short Form; BMT: Bremelanotide; NDRI: Norepinephrine-Dopamine Reuptake Inhibitor; SSRI: Selective Serotonin Reuptake Inhibitor; PDE5: Phosphodiesterase Type 5.
Bupropion: A randomized, controlled Phase II trial (NRG-CC004) evaluated two dose levels of extended release bupropion (150 mg or 300 mg once daily) versus placebo for the treatment of low sexual desire in 230 breast or gynecologic cancer survivors with baseline FSFI desire scores <3.3 (74). At nine weeks, all three groups, including placebo, showed improvement in sexual desire scores; however, there were no statistically significant differences between the bupropion and placebo groups in change in the FSFI desire subscale [74]. No grade 4 or 5 treatment related adverse events were reported [74]. The study notes limitations, including a predominantly white, non-Hispanic population, which may limit the generalizability of the findings, as well as the relatively short follow-up period [74]. Nevertheless, the National Comprehensive Cancer Network (NCCN) notes that bupropion and buspirone may still be considered as off-label options for HSDD in female cancer survivors, acknowledging the limited safety and efficacy data [37]. Also, bupropion, has associated with a lower incidence of sexual dysfunction, and is noted as a potentially more suitable option for cervical cancer survivors experiencing depressive symptoms, especially since SSRIs, have been linked to reduced libido and anorgasmia, but such side effects can contribute to the persistence or worsening of depressive symptoms [9-69].The biological rationale for using bupropion as an intervention stems from established evidence linking dopaminergic agents to increased sexual desire [75]. Bupropion, a dopaminergic medication approved by the FDA for the treatment of depression, seasonal affective disorder, and smoking cessation, has shown promise in preliminary studies suggesting potential benefits in enhancing sexual energy among female cancer survivors [37-75]. However, it is important to note that bupropion is not FDA-approved for this specific indication, and the current evidence supporting its use for sexual dysfunction in this population remains limited.
Flibanserin: In August 2015, the FDA approved flibanserin for the treatment of acquired, generalized HSDD in premenopausal women [76]. Meta-analyses in the general population suggest its use is associated with approximately one additional satisfying sexual event every two months [77,78]. However, evidence supporting its use in female cancer survivors remains limited and has only been investigated in BCS [37].In a retrospective chart review by Javaid et al. of women with low libido and a history of breast cancer who had received at least one prescription for flibanserin [79]. Among the 20 participants, 75% reported improved sexual desire, and 30% noted additional non-sexual benefits such as better sleep or mood [79]. Adverse events occurred in 40% of participants, most commonly insomnia, mood changes, and headache [79]. The authors noted that while flibanserin was generally well tolerated and perceived benefits were common, these findings may not be directly attributable to the drug alone, and results may not generalize to all clinical practices [79]. Additionally, a prospective, longitudinal trial (NCT03707340) by The Goldfarb et al. followed 43 women with stage 0–III ER+ BC on endocrine therapy for at least three months who had HSDD. All participants received flibanserin 100 mg nightly for 24–36 weeks and were followed for up to 52 weeks. Objective measures showed significant improvement in FSFI total scores (from 11 at baseline to 17 by week 8 and 17–18 at week 36) and in domains including desire, arousal, satisfaction, and lubrication, although PROMIS-SF scores suggested a temporary decrease in lubrication at week 8. Common side effects included nausea, somnolence, and dizziness, with no severe adverse events reported. The authors conclude that data from this pilot study is currently informing a larger randomized placebo-controlled study evaluating the efficacy of flibanserin in women with BC [80].While both studies suggest potential benefits of flibanserin in BCS, important limitations remain, including small sample sizes, lack of placebo control, and risk of bias. Further high-quality randomized trials are needed to confirm the efficacy and safety of flibanserin in this population, particularly among individuals receiving endocrine therapy [27-79].Bremlanotide: In June 2019, the FDA approved bremelanotide for the treatment of acquired, generalized HSDD in premenopausal women [81]. It is a melanocortin agonist that is self-injected, and has limited evidence in female cancer survivors [82-84]. Bremelanotide is administered via a subcutaneous injection and is designed to be used on a needed basis, about 45 minutes before anticipated sexual activity [82-84]. It does not interact significantly with other drugs or with alcohol, making it a relatively safe option for most users [85,86]. Its safety and efficacy were demonstrated in two phase 3 randomized, placebo-controlled trials (RECONNECT-301 and RECONNECT-302) [84]. Participants receiving bremelanotide as needed reported statistically significant improvements in sexual desire ( p < .001) and reductions in distress related to low desire (p = .005), compared to placebo [84]. Common side effects included mild-to moderate nausea, flushing, and headache [84]. Additionally another ongoing trial of a Phase 3, multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Trial to Evaluate the Efficacy and Safety of Subcutaneously Administered Bremelanotide in Premenopausal Women With Hypoactive Sexual Desire Disorder (With or Without Decreased Arousal) is being investigated (cite). Although bremelanotide has not been studied specifically in cancer survivors, NCCN considers it a potential option for BCS with HSDD [37].
Hormonal: ThereA 2024 pilot study found that low-dose topical testosterone gel (3 mg/day) significantly improved are limited data regarding the use of androgen therapy in survivors of hormonally mediated cancers, with small studies suggesting that it may be beneficial and safe [37-87]. The literature has noted that testosterone improved the frequency of sexually satisfying events, desire, arousal, and orgasm in several randomized, controlled studies of surgically and naturally postmenopausal women [88]. However, some cancer organizations, such as the NCCN, emphasize that more information is needed to ensure safety, particularly for some cancer survivors on aromatase inhibitors [37-88].sexual function in premenopausal, high-risk hormone receptor-positive breast cancer survivors undergoing ovarian suppression, with FSFI scores rising from 11.7 to 19.1 over three months (p < 0.001). Estradiol levels remained below 2.7 pg/mL, confirming hormonal safety [89]. Similarly, a meta-analysis from Rowel et al. data from over 3000 women using transdermal testosterone, with or without concomitant estrogen and progestin hormone therapy, found that breast density did not change with transdermal testosterone treatment in several small trials with insufficient data to assess long-term breast cancer risk 45,46. [90]. Health Canada also reports that breast and gynecologic cancer survivors who used vaginal DHEA 6.5 mg for eight weeks experienced statistically significant improvements in GSM symptoms [91]. Although similar improvements were seen with personal moisturizers,DHEA showed specific promise for improving vaginal atrophy and sexual function [91].Furthermore, a retrospective comparative analysis involving 185 cancer survivors, including those with breast, endometrial, ovarian, cervical, and vulvar cancers, evaluated the efficacy and safety of vaginal estriol, vaginal DHEA, and ospemifene over a six-month period [92]. All three treatments were associated with significant improvements in GSM symptoms, including increased sexual activity, reduced vulvar pain and dyspareunia,and better scores on the FSFI and Female Sexual Distress Scale, which includes a sexual desire domain [92]. Notably,vaginal DHEA led to meaningful improvements in sexual desire, highlighting its potential value for BCS [92].However, continued evaluation is needed to confirm its long-term safety and effectiveness in this population.Additionally, an ongoing clinical trial, PROPOSE (NCT06611514), is evaluating the effect of intravaginal prasterone on moderate to severe GSM symptoms in individuals experiencing natural, surgical, or treatment-induced menopause following breast cancer [93].
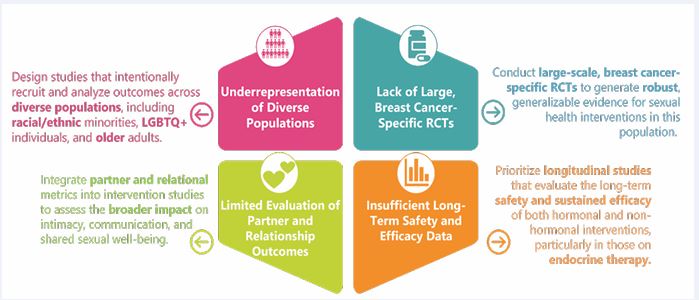
Research Gaps in Treatment Evidence
Figure 1 Current Research Gaps in Managing Low Libido in Breast Cancer Survivors.
Figure 1 visually demonstrates the current research gaps in managing low libido in BCS, as well as suggestions for addressing existing limitations.
While a growing body of evidence supports various interventions for managing low sexual desire in female cancer survivors, several gaps in the literature remain, as there is a clear need for more trials to be done within the cancer population. Many existing studies have small sample sizes, lack of control groups, limited diversity among participants, and short follow-up periods. These limitations affect the ability to have strong conclusions about long-term efficacy, safety, and generalizability. For instance, pharmacological interventions such as flibanserin and topical testosterone have shown promise, but they have not been largely studied in cancer-specific populations, particularly those undergoing endocrine therapy [79-84]. Similarly, for vaginal DHEA, additional research is needed to evaluate its safety in individuals on AIs and to compare its effectiveness with other non-hormonal therapies such as lubricants or moisturizers [88].There is also a need for more comprehensive research on psychosocial and behavioural interventions. While couple-based counseling and mindfulness-based therapies have shown short-term benefits, little is known about their long-term effectiveness, optimal delivery formats, or scalability across different clinical settings [34-94]. Furthermore, few studies explore intersectional factors such as age, race, sexual orientation, and cultural background, which may influence treatment access, preferences, and outcomes. Future research should focus on developing tailored,culturally sensitive interventions that address the unique needs of diverse survivor populations. Moreover, including partner-reported outcomes and exploring relational dynamics can offer a more comprehensive understanding of treatment impact [34-39]. Advancements in digital health also present opportunities to explore the feasibility and effectiveness of telehealth and app-based sexual health interventions [95,96]. Ultimately, evidence-based,longitudinal RCTs that evaluate both clinical and patient-reported outcomes are essential to establish evidence based, individualized care pathways for BCS with low sexual desire.
CONCLUSION
Low sexual desire is a prevalent and multifaceted concern among BCS, significantly impacting quality of life and intimate relationships. A range of non-pharmacological and pharmacological interventions show promise in addressing this issue, though current evidence is limited by small samples, short follow-up, and lack of cancer specific trials. Emerging therapies such as mindfulness based approaches, couple counseling, and hormonal agents offer potential benefits, yet further research is essential to establish their safety, long-term effectiveness, and applicability to diverse populations. Advancing this field requires patient-centered, culturally informed, and rigorously designed studies to guide personalized care in survivorship.
AUTHOR CONTRIBUTIONS
MA contributed to the concept and design. JB wrote the main body of the manuscript. JB prepared the figures and tables. MA provided significant comments on the manuscript and contributed to the final review and editing. All authors approved the final version of the manuscript.
REFERENCES
- Marzorati C, Masiero M, Pravettoni G. “Perspective: An integratedvision of the quality of life in breast cancer survivorship trajectory”. Breast. 2024; 77: 103785.
- Soldato D, Arecco L, Agostinetto E, Franzoi MA, Mariamidze E, Begijanashvili S, et al. The future of breast cancer research in the survivorship field. Oncol Ther. 2023; 11: 199-229.
- Vrancken Peeters NJMC, van der Meer DJ, Kok M, van Maaren MC, Peeters MTFDV, Siesling S, et al. Five-year conditional relative survival up to 10 years post-diagnosis among adolescent and young adult breast cancer patients by age, stage, and receptor subtype. J Natl Cancer Cent. 2025; 5: 297-305.
- Wagle NS, Nogueira L, Devasia TP, Mariotto AB, Yabroff KR, Islami F, et al. Cancer treatment and survivorship statistics, 2025. CA Cancer J Clin. 2025; 75: 308-340.
- Franzoi MA, Aupomerol M, Havas J, Soldato D, Lambertini M, Massarotti C, et al. Investigating sexual health after breast cancer by longitudinal assessment of patient-reported outcomes. ESMO Open. 2024; 9: 102236.
- Isanazar A, Akhlaghi Z, Nejatifar F, Mirfarhadi N. Sexual dysfunction in women with breast cancer: a forgotten aspect among survivors. BMC Womens Health. 2025; 25: 357.
- Kaufman R, Agrawal L, Teplinsky E, Kiel L, Abioye O, Florez N. From diagnosis to survivorship addressing the sexuality of women during cancer. Oncologist. 2024; 29: 1014-1023.
- Kennedy SKF, Mekhaeil S, Zhang E, Jolfaei NA, Wong HCY, Chan AW, et al. Sexual health after breast cancer: A clinical practice review. Ann Palliat Med. 2024; 13: 1281-1290.
- Bhinder JK, Kennedy SKF, Faouk Al Aadah C, Al-Khaifi M. 2024 SOGC, et al. A comparison of female cancer survivorship guidelines for the management of sexual health concerns. Support Care Cancer. 2025; 33: 584.
- Esmat Hosseini S, Ilkhani M, Rohani C, Nikbakht Nasrabadi A, Ghanei Gheshlagh R, Moini A. Prevalence of sexual dysfunction in women with cancer: A systematic review and meta-analysis. Int J Reprod Biomed. 2022; 20: 1-12.
- Robison K, Kulkarni A, Dizon DS. Sexual health in women affected by gynecologic or breast cancer. Obstet Gynecol. 2024; 143: 499-514.
- Canty J, Stabile C, Milli L, Seidel B, Goldfrank D, Carter J. Sexual function in women with colorectal/anal cancer. Sex Med Rev. 2019; 7: 202-222.
- Sousa Rodrigues Guedes T, Barbosa Otoni Gonçalves Guedes M, de Castro Santana R, Costa da Silva JF, Almeida Gomes Dantas A, Ochandorena-Acha M, et al. Sexual dysfunction in women with cancer: A systematic review of longitudinal studies. Int J Environ Res Public Health. 2022; 19: 11921.
- Cucciniello L, Miglietta F, Guarneri V, Puglisi F. Managing sexual health challenges in breast cancer survivors: A comprehensive review. Breast. 2024; 76: 103754.
- Rodrigues-Machado N, Bonfill-Cosp X, Quintana MJ, Santero M, Bártolo A, Olid AS. Sexual dysfunction in women with breast cancer: A systematic review. Support Care Cancer. 2025; 33: 332.
- Vitorino CN, Omodei MS, de Souza RC, Nahas GP, de Araujo Brito Buttros D, Carvalho-Pessoa E, et al. Assessment of sexual function in postmenopausal breast cancer survivors. Sex Med. 2024; 12: qfae035.
- Agrawal LS, Teplinsky E, Feygin YB, Kluthe T, Marcovici S, MennC. Women’s Insights on Sexual Health After Breast Cancer (WISH- BREAST) Survey. JCO Oncol Pract. 2025: OP2500043.
- Luo F, Link M, Grabenhorst C, Lynn B. Low sexual desire in breast cancer survivors and patients: A review. Sex Med Rev. 2022; 10: 367-375.
- Mishra N, Singh N, Sachdeva M, Ghatage P. Sexual dysfunction in cervical cancer survivors: A scoping review. Womens Health Rep (New Rochelle). 2021; 2: 594-607.
- Katz A, Agrawal LS, Sirohi B. Sexuality after cancer as an unmet need: Addressing disparities, achieving equality. Am Soc Clin Oncol Educ Book. 2022; 42: 1-7.
- Lustberg MB, Loprinzi CL, Streicher L. A pill for sexual desire in female cancer survivors: Too good to be true? J Clin Oncol. 2022; 40: 317-319.
- Pettigrew JA, Novick AM. Hypoactive sexual desire disorder in women: Physiology, Assessment, Diagnosis, and Treatment. J Midwifery Womens Health. 2021; 66: 740-748.
- French JE, McNulty JK, Makhanova A, Maner JK, Eckel LA, Nikonova L, et al. An empirical investigation of the roles of biological, relational, cognitive, and emotional factors in explaining sex differences in dyadic sexual desire. Biol Psychol. 2022; 174: 108421.
- Rossi A, Leone AG, Lambertini M, Sperti E, Cassani C, Vetromile A, et al. Sexual health in cancer care: A narrative review and position statement from the Italian Association of Medical Oncology (AIOM). ESMO Open. 2025; 10: 105311.
- Vegunta S, Kuhle CL, Vencill JA, Lucas PH, Mussallem DM. Sexual health after a breast cancer diagnosis: Addressing a forgotten aspect of survivorship. J Clin Med. 2022; 11: 6723.
- Hosseini FS, Khajavian N. Examination of the relationship between dimensions of sexual perfectionism and female sexual function and sexual performance anxiety among Iranian married women of reproductive age: A cross-sectional study. BMC Psychol. 2024; 12: 642.
- Goldfarb S. Endocrine therapy and its effect on sexual function. Am Soc Clin Oncol Educ Book. 2015: e575-81.
- Ribi K, Luo W, Walley BA, Burstein HJ, Chirgwin J, Ansari RH, et al. Treatment-induced symptoms, depression and age as predictors of sexual problems in premenopausal women with early breast cancer receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2020; 181: 347-359.
- Streicher L, Simon JA. Sexual function post-breast cancer. Cancer Treat Res. 2018; 173: 167-189.
- Bentsen L, Aagesen M, Bidstrup P, Hjerming M, Pappot H. Sexuality, intimacy, and body image among adolescents and young adults with cancer: A qualitative, explorative study. Support Care Cancer. 2024; 32: 219
- Favez N, Cairo Notari S. Body image, sexual activity, and side effects of treatments across the first year after surgery in women facing breast cancer: The influence of attachment insecurity. J Psychosoc Oncol. 2021; 39: 749-764.
- Merino M, Tornero-Aguilera JF, Rubio-Zarapuz A, Villanueva-Tobaldo CV, Martín-Rodríguez A, Clemente-Suárez VJ. Body perceptions and psychological well-being: A review of the impact of social media and physical measurements on self-esteem and mental health with a focus on body image satisfaction and its relationship with cultural and gender factors. Healthcare (Basel). 2024; 12: 1396.
- Raposo CF, Nobre PJ, Manão AA, Pascoal PM. Understanding sexual distress related to sexual function (SDRSF): A preliminary framework based on a qualitative study with clinical sexologists. Int J Clin Health Psychol. 2024; 24: 100473.
- Reese JB, Lepore SJ, Sorice KA, Zimmaro LA, Hasler J, Handorf E, et al. Efficacy of a couple-based intervention addressing sexual concerns for breast cancer survivors: Results of a randomized controlled trial. Cancer. 2025; 131: e35685.
- Seav SM, Dominick SA, Stepanyuk B, Gorman JR, Chingos DT, Ehren JL, et al. Management of sexual dysfunction in breast cancer survivors: a systematic review. Womens Midlife Health. 2015; 1: 9.
- Arring N, Barton DL, Reese JB. Clinical practice strategies to address sexual health in female cancer survivors. J Clin Oncol. 2023; 41: 4927- 4936.
- NCCN 2025. NCCN Guideline 2025.
- Zaider TI, Kissane DW. Couple Therapy for Sexual Dysfunction. In: Sexual Health, Fertility, and Relationships in Cancer Care [Internet]. Oxford University Press; 2020; 18: 139–58.
- Zhou J, Chen X, Wang Z, Li Q. Coping together: Couple-based communication interventions in cancer care. Asia Pac J Oncol Nurs. 2024; 11: 100379.
- Bindu Menon K, Gadiraju P. Psychological Distress and Sexual Dysfunction in Cancer Patients: Need for Psychological Intervention. J Psychosexual Health. 2025; 7: 107-14.
- Li M, Chan CWH, Chow KM, Xiao J, Choi KC. A systematic review and meta-analysis of couple-based intervention on sexuality and the quality of life of cancer patients and their partners. Support Care Cancer. 2020; 28: 1607-1630.
- Domar AD, Meyers AJ, Grill EA. How the medicalization of reproduction takes the fun out of the process. In: Psychological and Medical Perspectives on Fertility Care and Sexual Health. Elsevier; 2022. 2022; 22: 189-204.
- Smith T, Kingsberg SA, Faubion S. Sexual dysfunction in female cancer survivors: Addressing the problems and the remedies. Maturitas. 2022; 165: 52-57.
- Agrawal LS. Addressing sexual health in breast cancer survivors: Evidence-based practices and clinical considerations. Curr Treat Options Oncol. 2025; 26: 476-485.
- Dickenson JA, Tebbe E, Tellawi G. A Sexual Wellbeing Framework to Address Sexuality in Therapy with Transgender, Nonbinary, and Gender Expansive Clients. Women Ther. 2023; 46: 171-198.
- Shanahan J. Therapists’ use of feedback from clients about Sensate Focus exercises: An interpretive description of therapists’ perspectives. Sex Relatsh Ther. 2023; 39:1273-1291.
- Rowland DL, Kirana PS. A theoretical model for sexual performance anxiety (SPA) and a clinical approach for its remediation (SPA-R). Sex Med Rev. 2025; 13: 184-201.
- Titchener JL. Human sexual inadequacy. By W. H. Masters and V. E. Johnson. Little, Brown, Boston. 487 pp. 1970. Teratology. 1971; 4:465-467.
- Offerman C, Bourgeois J, Van Beurden J, Bozzon A. (Re)discovering Sexual Pleasure after Cancer: Understanding the Design Space. In: Proceedings of the 2025 CHI Conference on Human Factors in Computing Systems [Internet]. Yokohama Japan: ACM. 2025; 21: 1-17.
- Huang S, Li Z, Santtila P. The effectiveness of online sensate focus exercises in enhancing sexual function and intimacy among chinese heterosexual couples: A randomized controlled trial. J Sex Marital Ther. 2024; 50: 707-724.
- Jaderek I, Lew-Starowicz M. A systematic review on mindfulness meditation-based interventions for sexual dysfunctions. J Sex Med. 2019; 16: 1581-1596.
- Leavitt CE, Lefkowitz ES, Waterman EA. The role of sexual mindfulness in sexual wellbeing, Relational wellbeing, and self-esteem. J Sex Marital Ther. 2019; 45: 497-509.
- Stephenson KR, Kerth J. Effects of mindfulness-based therapies for female sexual dysfunction: A meta-analytic review. J Sex Res. 2017; 54: 832-849.
- Ciaurriz Larraz AM, Villena Moya A, Chiclana Actis C. Mindfulness- based intervention and sexuality: a systematic review. Trends Psychiatry Psychother. 2024; 46: e20210459.
- Banbury S, Chandler C, Lusher J. A Systematic Review Exploring the Effectiveness of Mindfulness for Sexual Functioning in Women with Cancer. Psych. 2023; 5: 194-208.
- Chang YC, Lin GM, Yeh TL, Chang YM, Yang CH, Lo C, et al. Impact of mindfulness-based stress reduction on female sexual function and mental health in patients with breast cancer. Support Care Cancer. 2022; 30: 4315-4325.
- Bagherzadeh R, Sohrabineghad R, Gharibi T, Mehboodi F, VahedparastH. Effect of Mindfulness-Based Stress Reduction Training on Revealing Sexual Function in Iranian Women with Breast Cancer. Sex Disabil. 2021; 39: 1-17.
- Gorman JR, Drizin JH, Smith E, Corey S, Temple M, Rendle KA. Feasibility of mindful after cancer: Pilot study of a virtual mindfulness- based intervention for sexual health in cancer survivorship. J Sex Med. 2022; 19: 1131-1146.
- Latifi M, Attarha M, Shayganfard M, Sharbafchi MR. The effect of cognitive-behavioral counseling on sexual function in women with breast cancer: An interventional study. Asian Pac J Cancer Prev. 2025; 26: 1745-1751.
- Sheikhzadeh M, Zanjani Z, Baari A. Efficacy of mindfulness-based cognitive therapy and cognitive behavioral therapy for Anxiety, Depression, and Fatigue in Cancer Patients: A randomized clinical trial. Iran J Psychiatry. 2021; 16: 271-280.
- Ko E, Lee Y. The effectiveness of cognitive behavioral therapy in women with gynecological cancer: A systematic review and meta- analysis of randomized controlled trials. Asia Pac J Oncol Nurs. 2024; 11: 100562.
- Carney LM, Schnur JB, Morgan O, Hyun C, Magin ZE, Martin L, et al. Psychosocial interventions to improve sexual functioning in women with cancer: a systematic review of randomized controlled trials. Sex Med Rev. 2024; 12: 142-153.
- Sopfe J, Pettigrew J, Afghahi A, Appiah LC, Coons HL. Interventions to improve sexual health in women living with and surviving cancer: Review and Recommendations. Cancers (Basel). 2021; 13: 3153.
- Carter J, Lacchetti C, Andersen BL, Barton DL, Bolte S, Damast S, et al. Interventions to address sexual problems in people with cancer: American society of clinical oncology clinical practice guideline adaptation of cancer care ontario guideline. J Clin Oncol. 2018; 36: 492-511.
- Rizzuto I, Oehler MK, Lalondrelle S. Sexual and Psychosexual Consequences of treatment for gynaecological cancers. Clin Oncol (R Coll Radiol). 2021; 33: 602-607.
- Huffman LB, Hartenbach EM, Carter J, Rash JK, Kushner DM. Maintaining sexual health throughout gynecologic cancer survivorship: A comprehensive review and clinical guide. Gynecol Oncol. 2016; 140: 359-68.
- Laan E, Rellini AH, Barnes T; International society for sexual medicine. Standard operating procedures for female orgasmic disorder: Consensus of the international society for sexual medicine. J Sex Med. 2013; 10: 74-82.
- Liu M, Juravic M, Mazza G, Krychman ML. Vaginal dilators: Issues and Answers. Sex Med Rev. 2021; 9: 212-220.
- Grosse-Rueschkamp MS, Dörfler C, Tissen-Diabaté T, Kronthaler S, Beier KM, Hatzler L. (116) SUBJECTIVE AND GENITAL SEXUAL RESPONSE IN FEMALE BREAST CANCER SURVIVORS: RESULTS FROM A PSYCHOPHYSIOLOGY STUDY. J Sex Med. 2024; 21:qdae054.110.
- Chambers LM, Herrmann A, Michener CM, Ferrando CA, Ricci S. Vaginal estrogen use for genitourinary symptoms in women with a history of uterine, cervical, or ovarian carcinoma. Int J Gynecol Cancer. 2020; 30: 515-524.
- Sakr HR, Ahmed YA, Kamel RM, Abdelhady RH, Elkalla RA, Georgui MA, et al. Clitoral therapy device for alleviating sexual dysfunction after female genital mutilation: Randomized controlled trial. JMIR Rehabil Assist Technol. 2023; 10: e43403.
- Latella D, Grimaldi A, Calabrò RS. Sexual functioning and sexual health in female patients following stroke: A scoping review with implications for rehabilitation. J Pers Med. 2024; 14: 267.
- Gupta N, Zebib L, Wittmann D, Nelson CJ, Salter CA, Mulhall JP, et al. Understanding the sexual health perceptions, concerns, and needs of female partners of prostate cancer survivors. J Sex Med. 2023; 20: 651-660.
- Barton DL, Pugh SL, Ganz PA, Plaxe SC, Koontz BF, Carter J, et al. Randomized controlled Phase II evaluation of two dose levels of bupropion versus placebo for sexual desire in female cancer survivors: NRG-CC004. J Clin Oncol. 2022; 40: 324-334.
- Adelman M, Nygaard IE. Time for a “Pause” on the Use of Vaginal Laser. JAMA. 2021; 326: 1378-1380.
- English C, Muhleisen A, Rey JA. Flibanserin (Addyi): The first FDA- Approved Treatment for Female Sexual Interest/Arousal Disorder in Premenopausal Women. P T. 2017; 42: 237-241.
- Kamrul-Hasan ABM, Hannan MA, Alam MS, Aalpona FTZ, Nagendra L, Selim S, et al. Role of flibanserin in managing hypoactive sexual desire disorder in women: A systematic review and meta-analysis. Medicine (Baltimore). 2024; 103: e38592.
- Simon JA, Clayton AH, Kim NN, Patel S. Clinically meaningful benefit in women with hypoactive sexual desire disorder treated with flibanserin. Sex Med. 2022; 10: 100476.
- Javaid S, Brown L. Evaluation of flibanserin’s benefits and tolerabilityin women with low libido and a history of breast cancer. J Clin Oncol. 2022; 40: e18761–e18761.
- Goldfarb S, Carter J, Toumbacaris N, Cheng M, Arkema A, Abdo N, et al.(017) FLIBANSERIN IMPROVES LIBIDO AND SEXUAL SATISFACTION IN WOMEN WITH BREAST CANCER ON ENDOCRINE THERAPY. J Sex Med. 2024; 21: qdae054.017.
- Dhillon S, Keam SJ. Bremelanotide: First Approval. Drugs. 2019; 79: 1599-1606.
- Goldman M, Abel MK. Oncology survivorship and sexual wellness for women. Urol Clin North Am. 2021; 48: 499-512.
- Suzuki S, Kitanaka C, Okada M. Melanocortin receptor agonist bremelanotide induces cell death and growth inhibition in glioblastoma cells via suppression of survivin expression. Anticancer Res. 2024; 44: 3875-3883.
- Kingsberg SA, Clayton AH, Portman D, Williams LA, Krop J, Jordan R, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: Two randomized Phase 3 Trials. Obstet Gynecol. 2019; 134: 899-908.
- Kingsberg S, Banks V, Caetano C, Janssenswillen C, Moeller C, Schoof N, et al. Real-world clinical evaluation of natural and induced vasomotor symptoms in the USA and Europe. Climacteric. 2024; 27: 364-372.
- Barakeh D, Mdaihly H, Karaoui LR. Pharmacotherapy of hypoactive sexual desire disorder in premenopausal women. Ann Pharmacother. 2025; 59: 148-161.
- Ni?? I, Ni?ipir C, Toma ?A, Limb?u AM, Pîrvu E, B?d?r?u IA. The importance of androgen receptors in breast cancer. Med Pharm Rep. 2021; 94: 273-281.
- Simon JA, Ohleth K. Testosterone for treating female sexual dysfunction. Clin Obstet Gynecol. 2025; 68: 60-67.
- Taranto P, de Brito Sales D, Maluf FC, Guendelmann RAK, de MeloPompei L, Leal A, et al. Safety and efficacy of topical testosterone in breast cancer patients receiving ovarian suppression and aromatase inhibitor therapy. Breast Cancer Res. 2024; 26: 133.
- Rowen TS, Simon JA. Sexual Desire and Pharmacologic Management. Obstet Gynecol Clin North Am. 2024; 51: 259-271.
- Jacobson M, Mills K, Graves G, Wolfman W, Fortier M. Guideline No. 422f: Menopause and Breast Cancer. J Obstet Gynaecol Can. 2021; 43: 1450-1456.
- Pennacchini E, Dall’Alba R, Iapaolo S, Marinelli M, Palazzetti PL, Zullo MA, et al. Treatment of genitourinary syndrome of menopause in breast cancer and gynecologic cancer survivors: Retrospective analysis of efficacy and safety of vaginal estriol, Vaginal Dehydroepiandrosterone and Ospemifene. J Menopausal Med. 2024; 30: 170-178.
- PROPOSE. Effect of intravaginal prasterone on symptoms of Genitourinary Syndrome of Menopause (GSM) in Women in Menopause with Previous Breast Cancer (PROPOSE).
- Dos Anjos GR, Machado GF, de Barros CP, de Andrade VP, Maciel RMB, Cunha LL. Cancer survivorship in low- and middle-income countries: Challenges, needs, and emerging support strategies. Front Public Health. 2025; 13: 1601483.
- Bergerot CD, Bergerot PG, Philip EJ, Ferrari R, Peixoto RM, Crane TE, et al. Enhancing cancer supportive care: Integrating Psychosocial Support, Nutrition, and Physical Activity Using Telehealth Solutions. JCO Glob Oncol. 2024; 10: e2400333.
- Muheriwa-Matemba SR, Alcena-Stiner DC, Glazier A, LeBlanc NM. Telehealth use for sexual and reproductive health promotion and care during the early phase of COVID-19 pandemic: A descriptive- interpretive qualitative study of healthcare providers’ perspectives and experiences in Western-Central New York State. PLOS Glob Public Health. 2024; 4: e0003259.